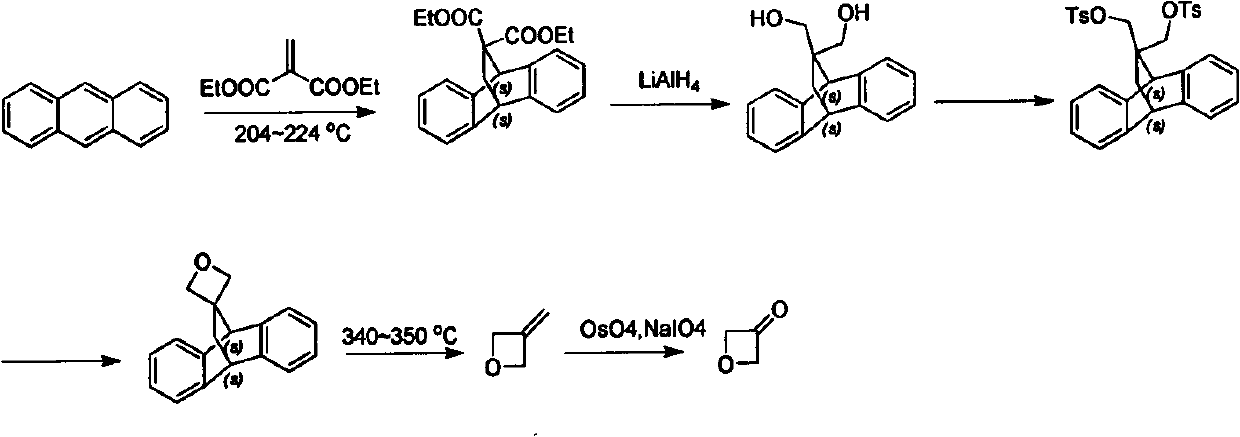
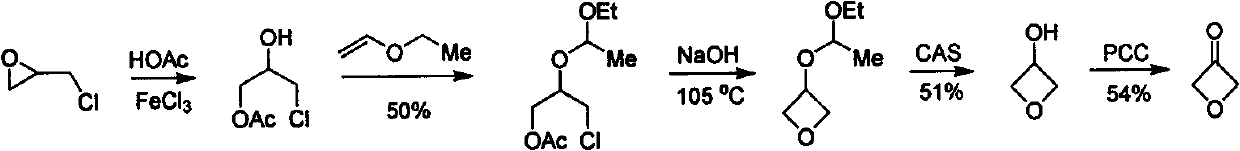
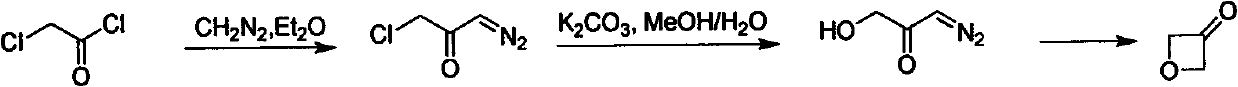Novel method for synthesizing 3-oxetanone
A technology of oxetanone and compound, which is applied in the field of synthesizing 3-oxetanone, can solve the problems of few 3-oxetanone manufacturers, hindering the development of downstream products, and low reaction yield. Achieve the effects of easy purification, reduced reaction cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] 1. Hydrolysis, ring closure reaction
[0016] 1,3-Dichloroacetone (50g) was dissolved in anhydrous methanol (350mL), and methanol solution of sodium methoxide (30g, 700mL) was slowly added dropwise at 0°C. After the addition was complete, it was stirred at room temperature for 30min. TCL tracked the end of the reaction, slowly added water (3.6g) dropwise to the reaction solution, and controlled the dropping temperature to be 0°C. After the dropwise addition was completed, the temperature was raised to reflux, and the reaction was carried out for 6 hours. The reaction solution was concentrated to a volume of (100 mL), extracted with ethyl acetate, and the organic phases were combined, washed with brine, dried over sodium sulfate, and concentrated and rectified product 2 (39.2 g), with a yield of 84%.
[0017] 1,3-Dichloroacetone (500g) was dissolved in anhydrous methanol (3.5L), and a methanol solution of sodium methoxide (300g, 7L) was slowly added dropwise at 0°C. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com