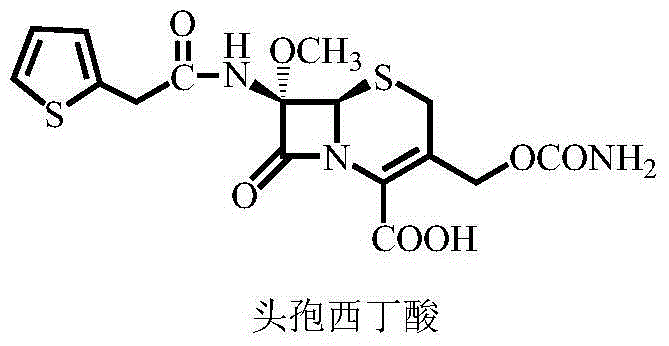
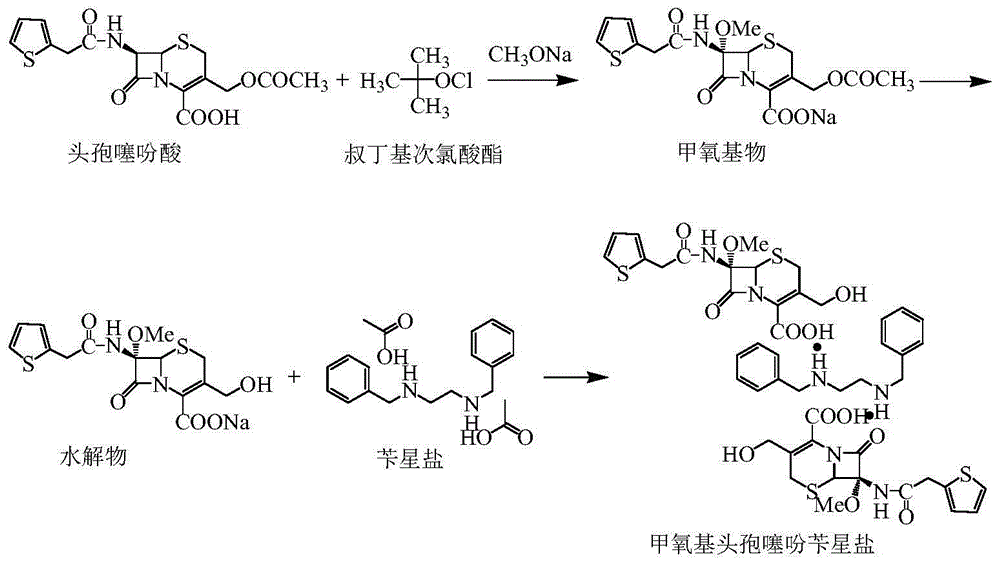
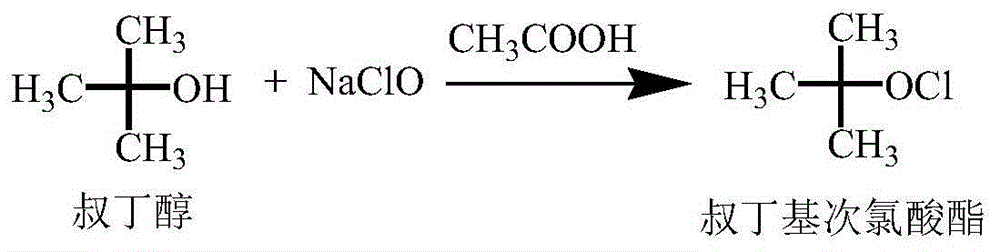Synthetic method of cefoxitin acid
A technology of cefoxitin acid and synthetic method, which is applied in the field of synthesis of cefoxitin acid, and achieves the effects of high yield, easy availability of raw materials, and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 tert-butyl hypochlorite
[0027] Add 37.0g (0.50mol) of tert-butanol and 33.0g (0.55mol) of glacial acetic acid into a 100mL beaker, stir and mix well for later use.
[0028] Add 405.8g (0.60mol) sodium hypochlorite to a 1000mL four-neck flask, dropwise add the prepared mixed solution of tert-butanol and glacial acetic acid, control the temperature during the dropwise addition at 20-25°C, and continue stirring for 2-4 hours after the addition is complete (GC detection tert-butanol<1%). After the reaction is complete, add about 100 mL of 5% sodium hydroxide aqueous solution dropwise, adjust the pH=6.5 to 7.0, stir at room temperature for 5 minutes, separate the liquids, wash the organic phase with 50 mL of 10% sodium chloride aqueous solution, separate the aqueous phase, and the organic phase is light 40.7 g of yellow liquid (tert-butyl hypochlorite), GC purity 96.3%, yield 72.2%.
Embodiment 2
[0029] The synthesis of embodiment 2 methoxy cephalothin benzathine salt
[0030] Put 150mL of methanol and 10.8g (0.20mol) of sodium methoxide into a 500mL four-neck flask, stir to dissolve, and cool down to -20--25°C for later use.
[0031] Put 600mL of dichloromethane, 39.6g (0.10mol) cephalothinic acid, 4.7g (0.01mol) trioctyl ammonium methyl ammonium bisulfate into a 2000mL four-neck flask, stir and cool down to -80~-90°C, dropwise to prepare The methanol solution of sodium methoxide, temperature control during the dropping process -80~-90°C, after the dropwise addition, 13.5g (0.12mol) tert-butyl hypochlorite prepared in the first step was added dropwise, and the temperature was controlled during the dropping process ‐80~‐90°C, after the dropwise addition is completed, control the temperature at ‐80~‐90°C and stir for 0.5~1h to obtain a methoxy compound solution (HPLC detection raw material cephalothinic acid<5%), the reaction is complete.
[0032] Add 9.5g (0.05mol) of...
Embodiment 3
[0049] The synthesis of embodiment 3 cefoxitin acid
[0050] Put 250mL of acetone and 50.5g (0.05mol) of methoxycefalotin benzathine salt into a 500mL four-neck flask, stir to dissolve, and cool down to -30--35°C for later use.
[0051] Add 28.3g (0.20mol) of CSI, control the temperature at ‐30~‐35°C and stir for 0.5~1h, then add 80.0g of 10% disodium hydrogen phosphate aqueous solution, control the temperature at 25~30°C and stir for 0.5~1h. Filter, transfer the filtrate to a 2L four-necked flask, and cool down to 0-5°C for later use.
[0052] Add 1500g of purified water dropwise, after dropping, control the temperature at 0-5°C to stir and crystallize for 1h, filter, beat the filter cake with 150g of purified water for 10 minutes, filter, put the filter cake (crude cefoxitin acid) into a 500mL four-necked flask for later use.
[0053] Add 300g of purified water, stir to cool down to 5-10°C, add about 8.0g of sodium phosphate in batches for alkali dissolution, control the pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com