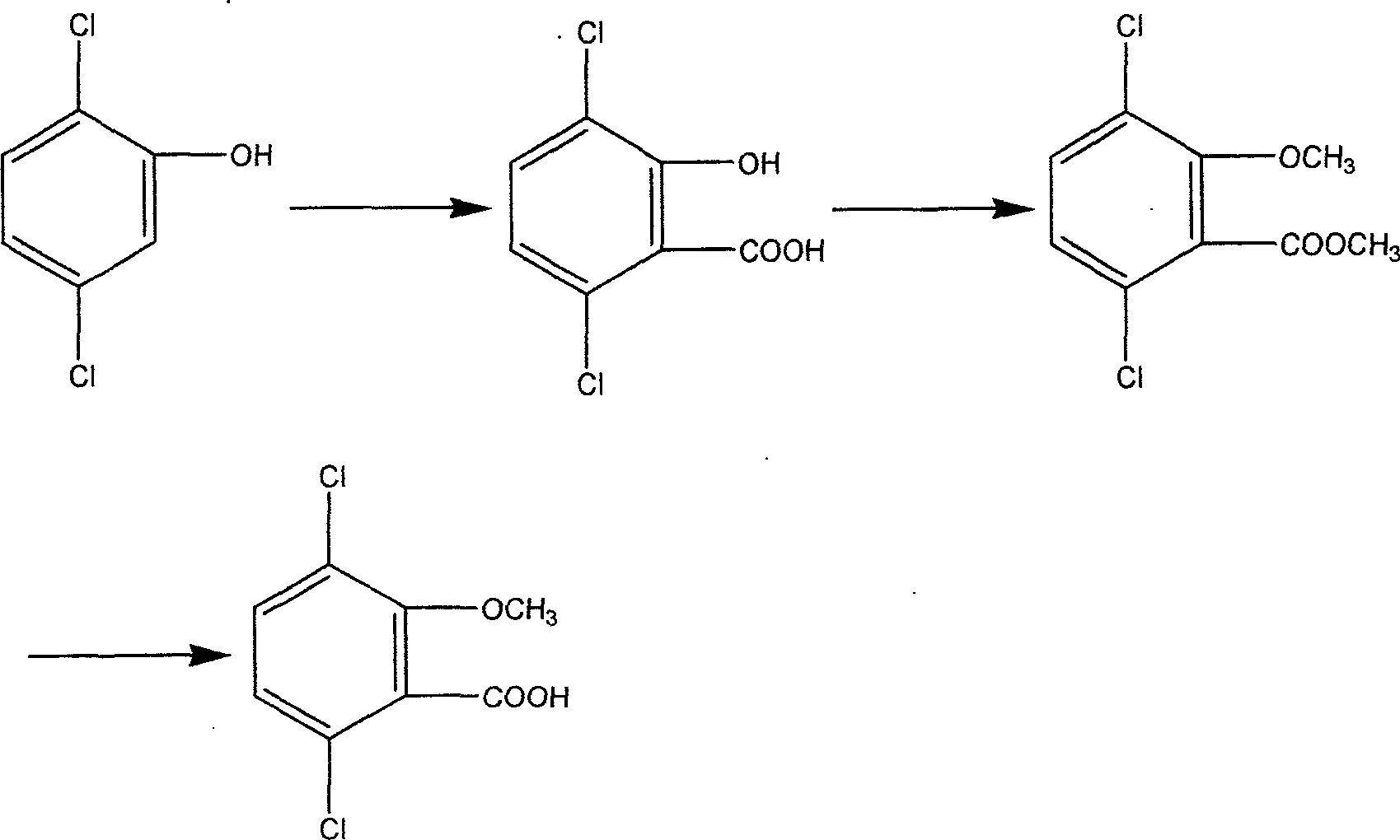
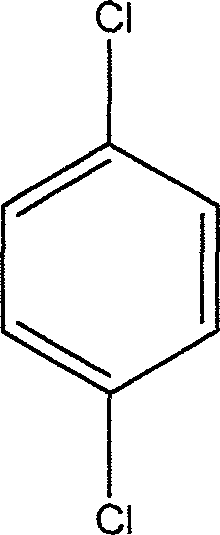
Process for catalyzing oxidating synthesizing 2,5-dichlorophenol
A technology for catalytic oxidation and dichlorophenol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as large environmental pollution, complicated process, long reaction route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention will be further described below in combination with specific embodiments.
[0026] 1) Preparation of heteropolyacid:
[0027] a)H 4 SiMo 12 o 40 preparation of
[0028] 50g Na 2 MoO 4 2H 2 O (0.21mol) was dissolved in 200ml of water, and the solution was heated to 80°C, 20ml of concentrated hydrochloric acid was added to the solution, and sodium metasilicate solution (0.045mol) was added dropwise within 30min under vigorous stirring, at this time the solution turned yellow, Continue to stir, add 60ml of concentrated hydrochloric acid dropwise with a dropping funnel, extract the filtrate with ether after cooling, volatilize the ether to obtain heteropolyacid H 4 SiMo 12 o 40 .
[0029] b)H 4 SiW 12 o 40 preparation of
[0030] 20gNa 2 WO 4 2H 2O (0.061mol) was dissolved in 40ml of water, then 0.0071mol of sodium metasilicate was added to the solution, the mixture was stirred and heated to boiling, 12ml of concentrated hydrochloric a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com