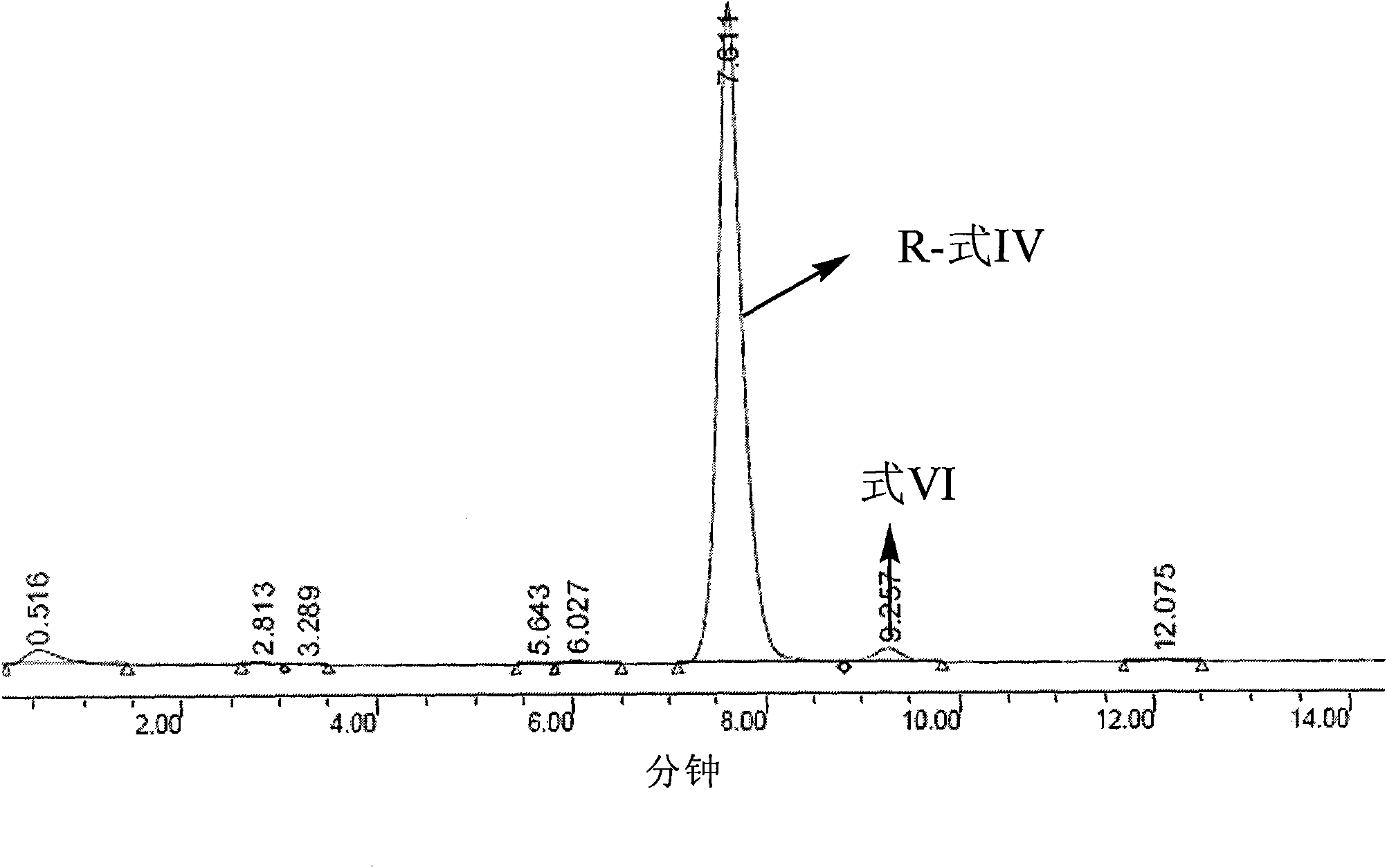
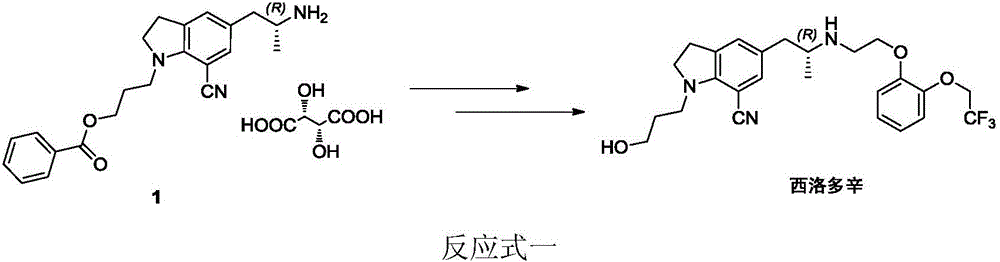
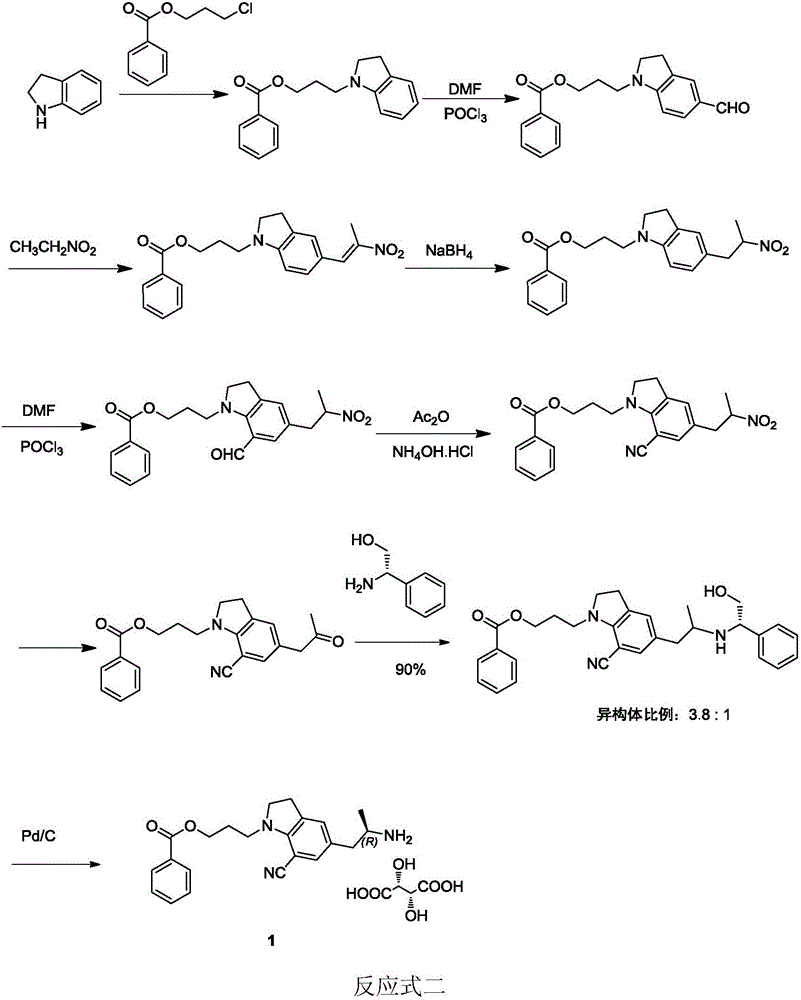
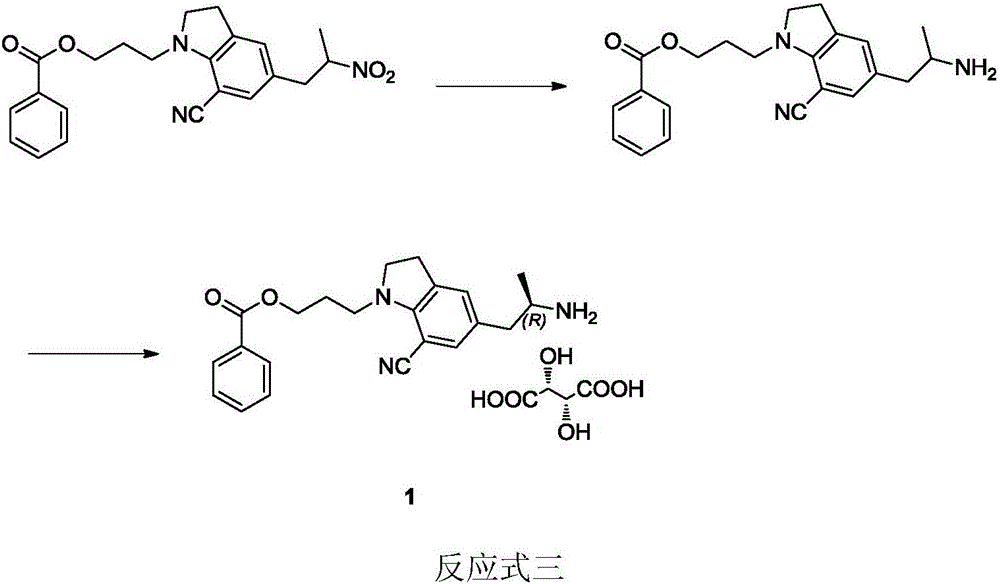
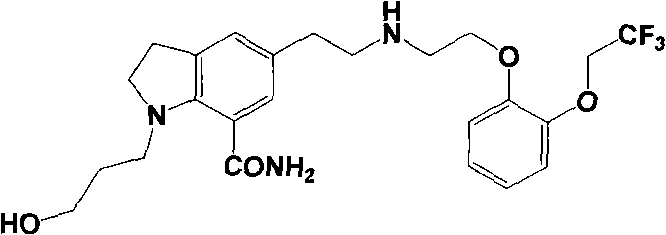
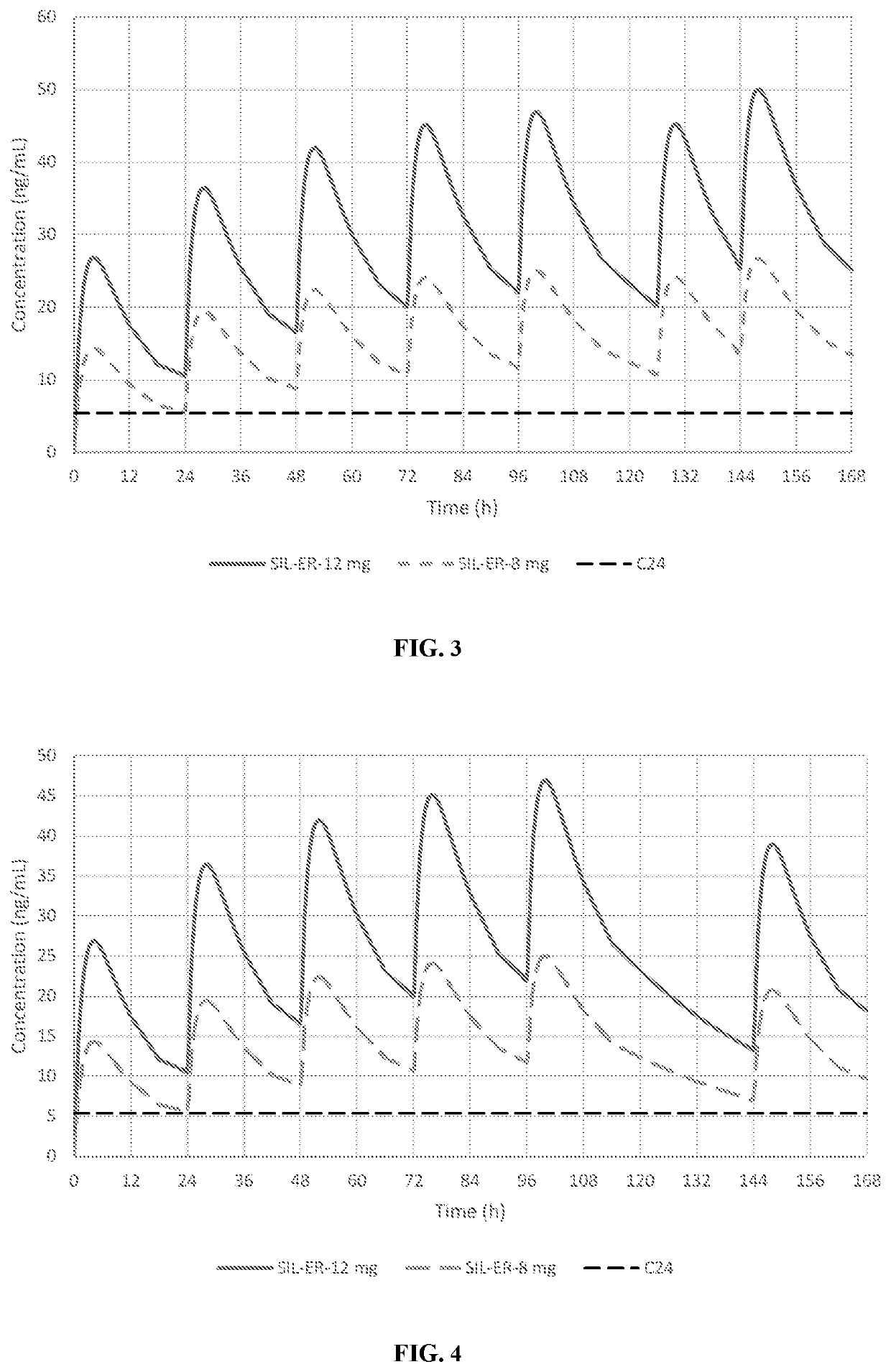
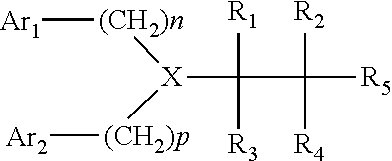
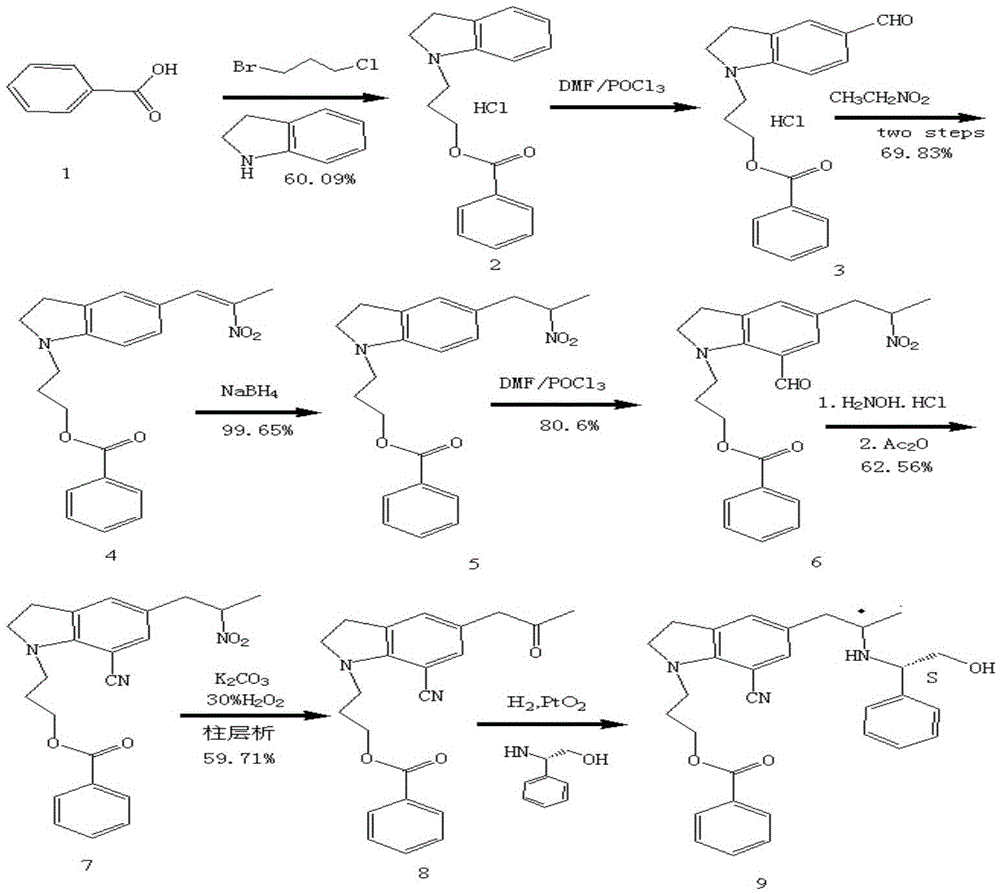
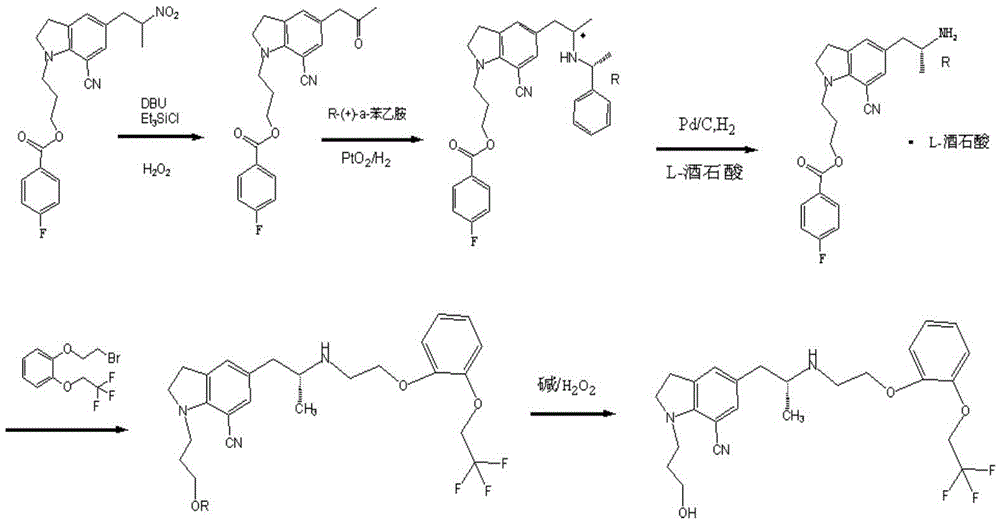
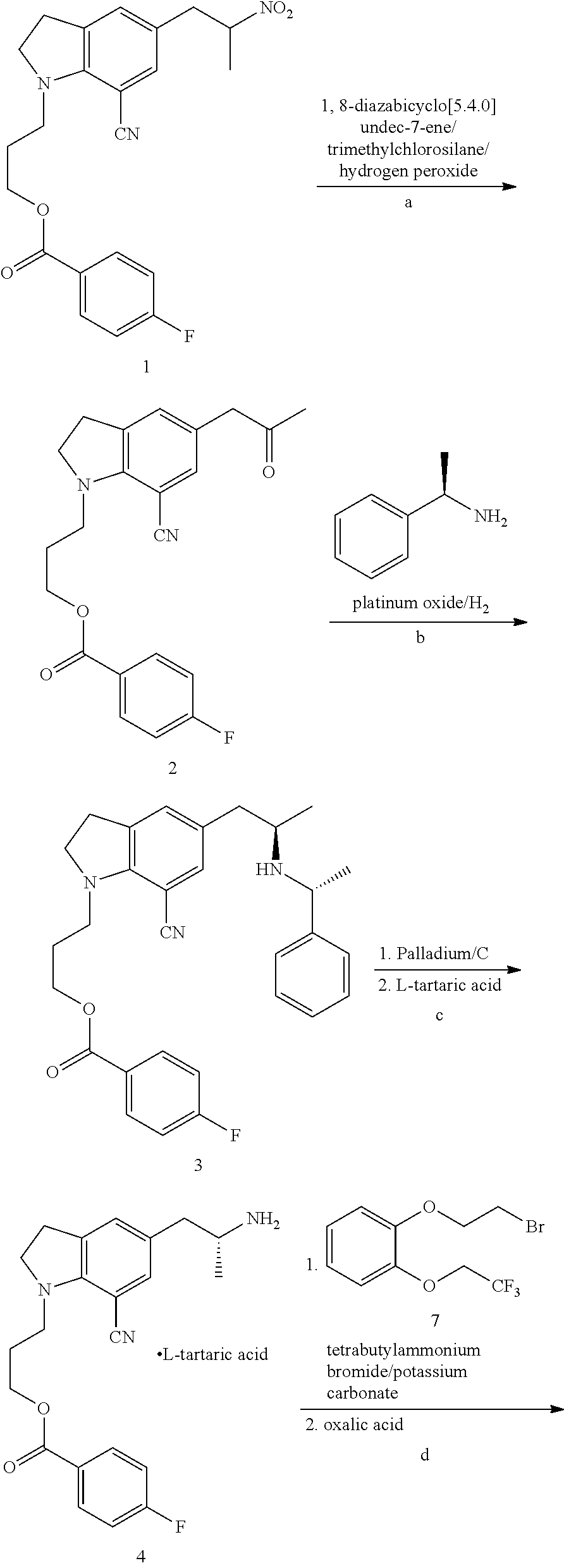
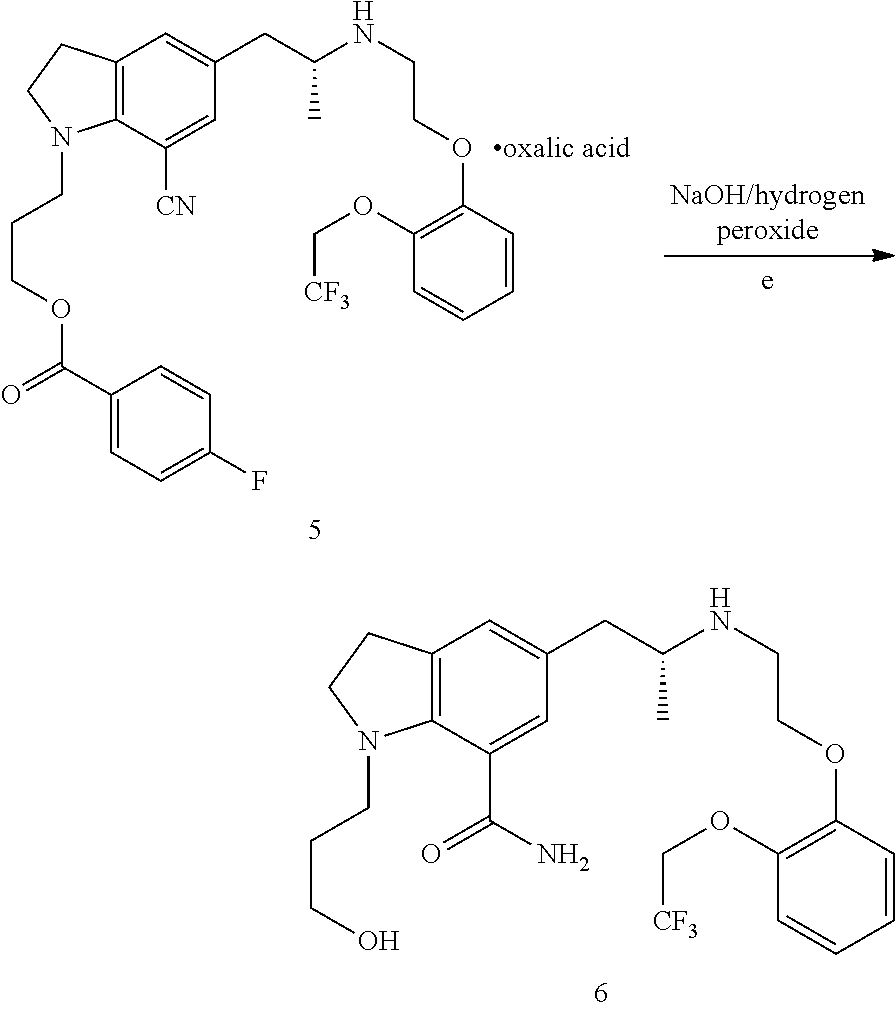
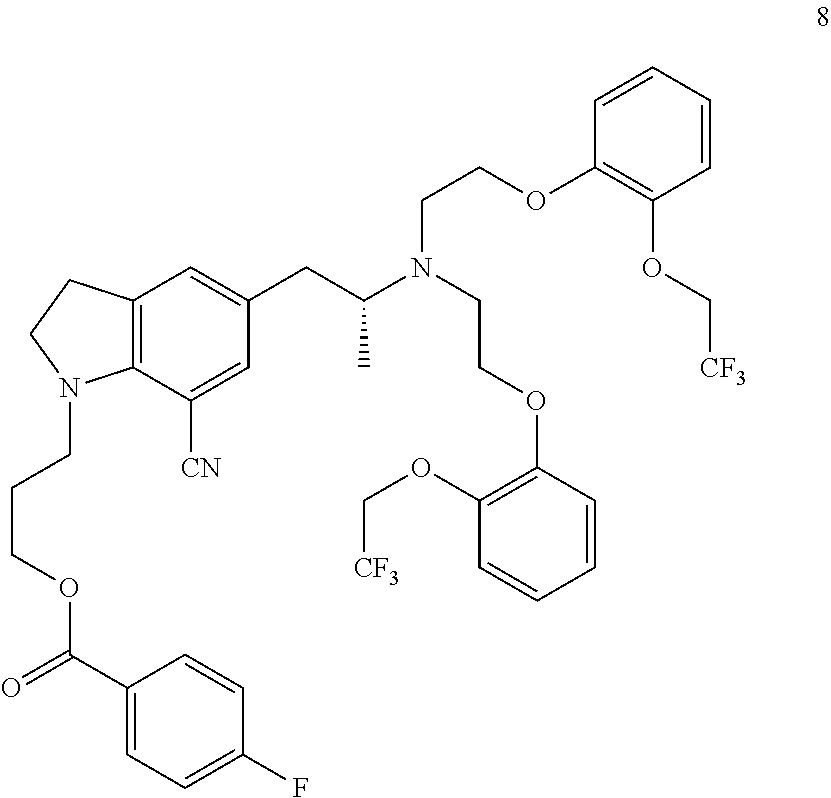
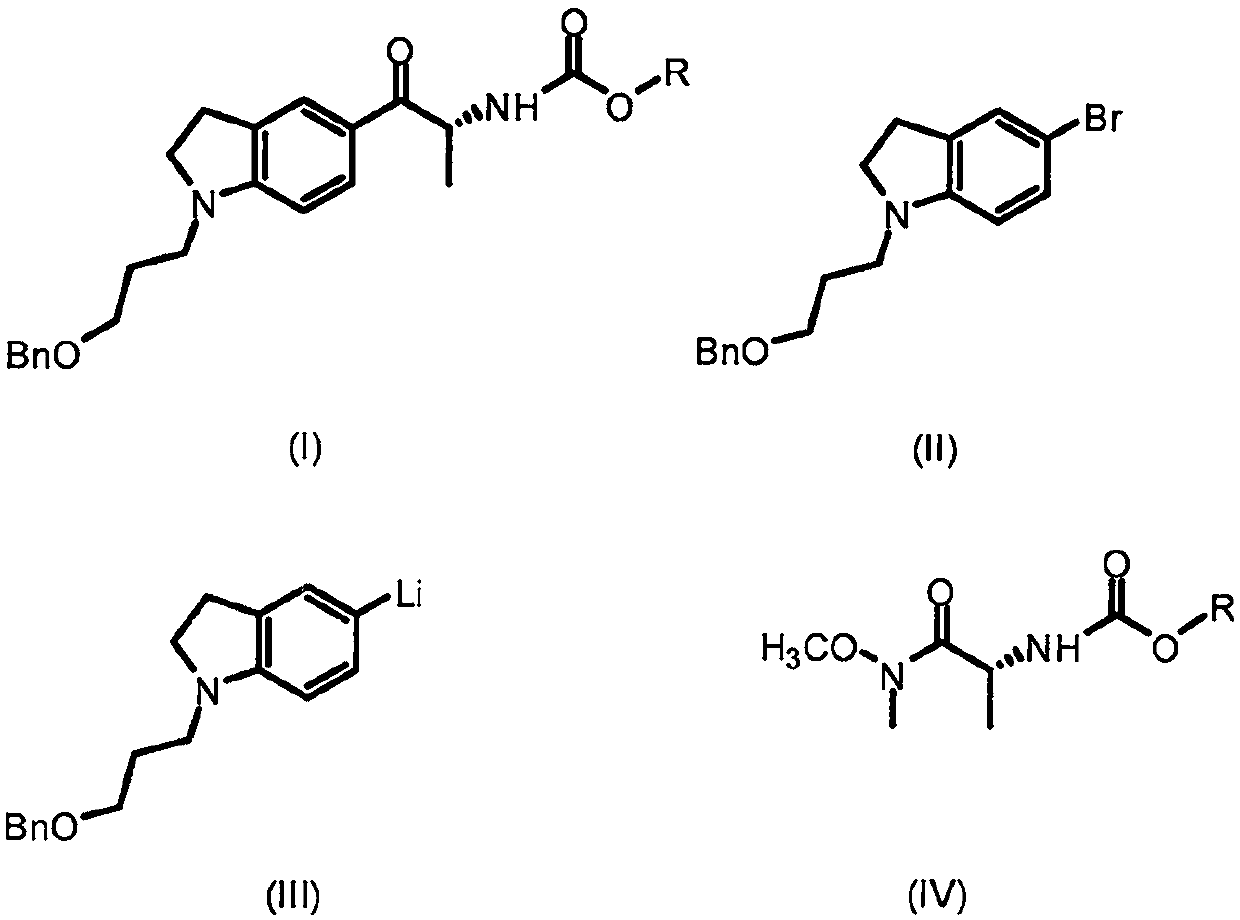
Patents
Literature
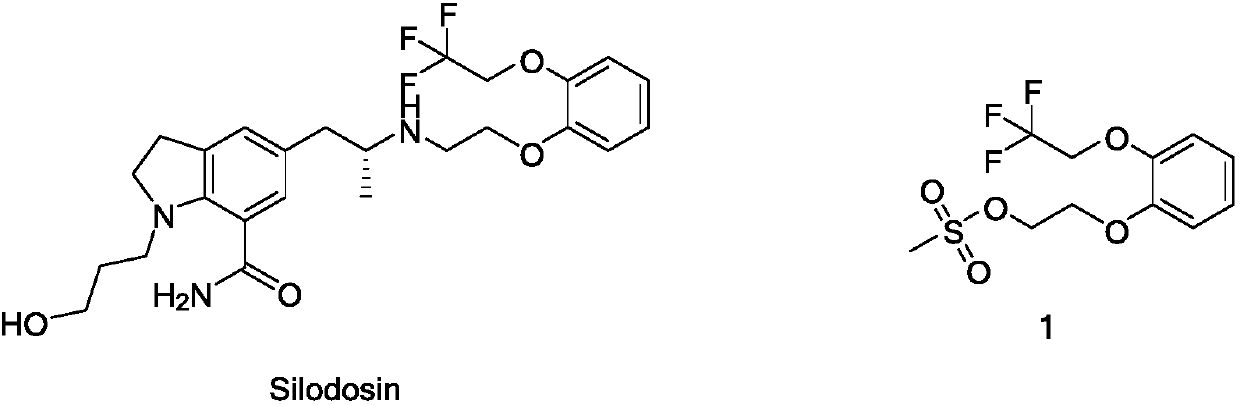
47 results about "Silodosin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
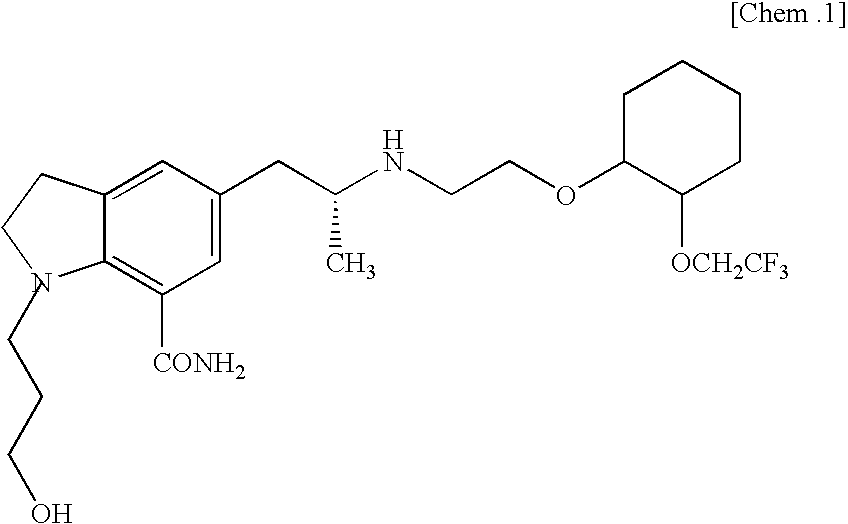
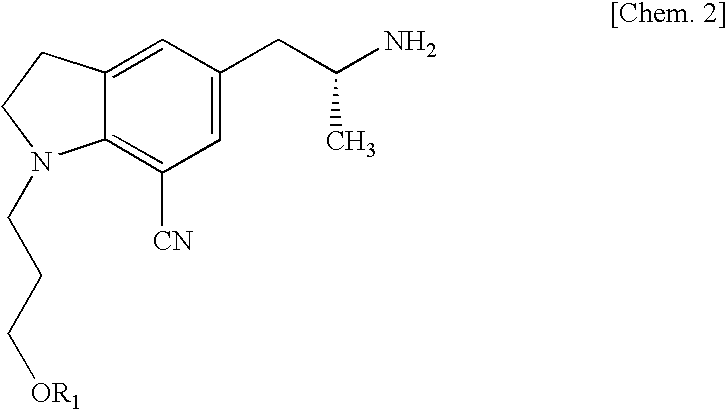
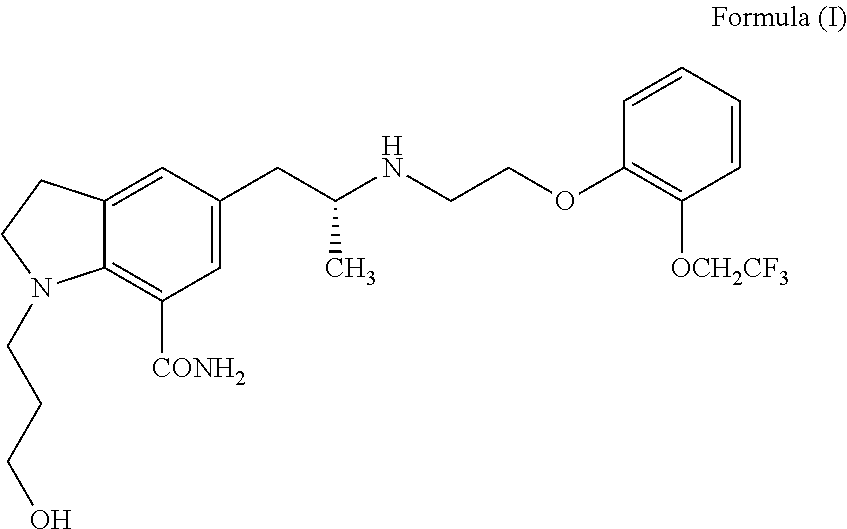
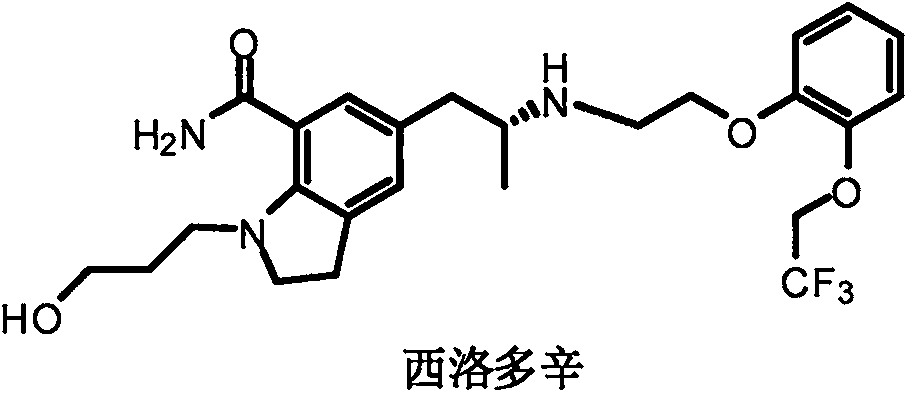
Silodosin is used by men to treat the symptoms of an enlarged prostate (benign prostatic hyperplasia-BPH).
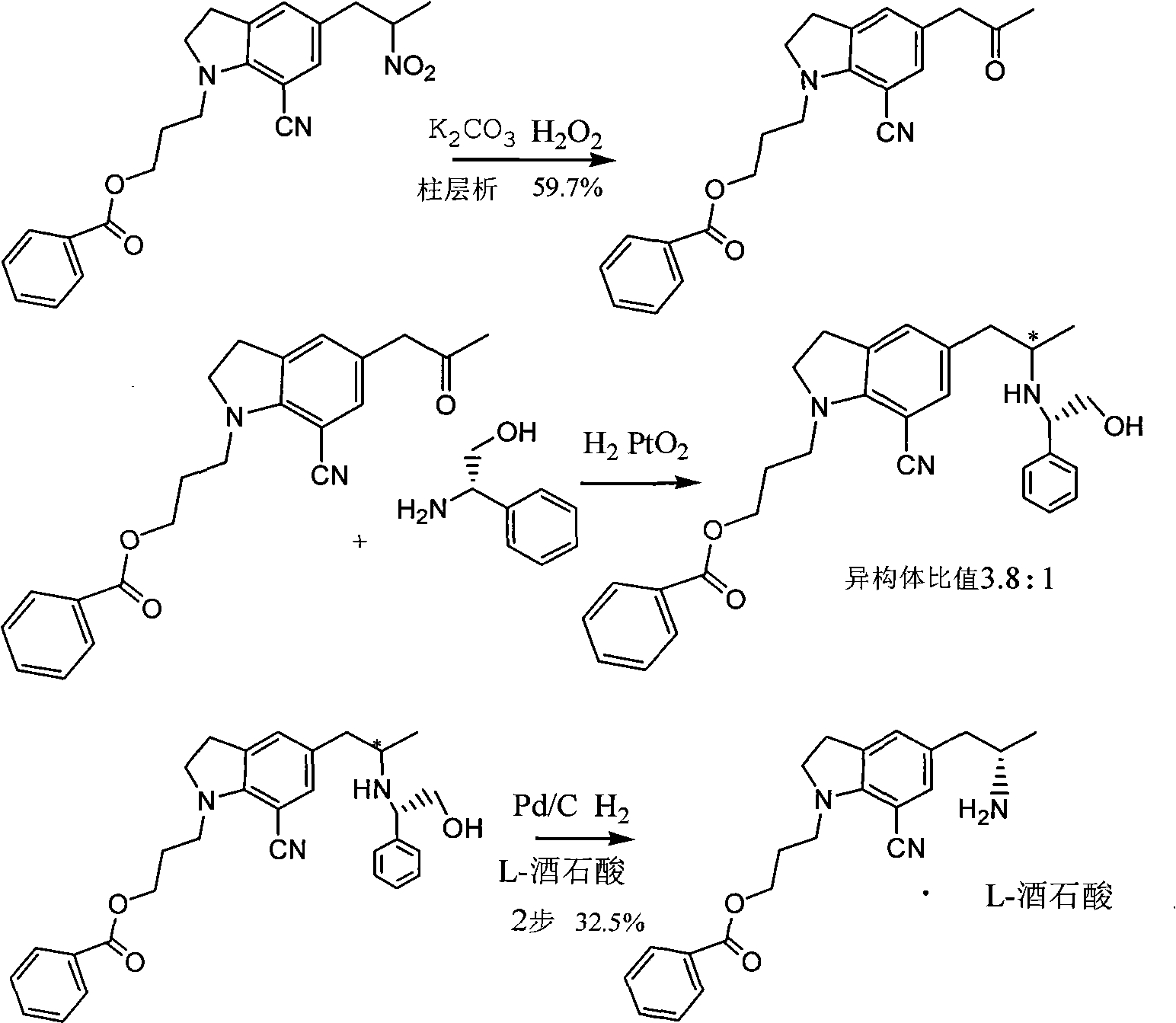
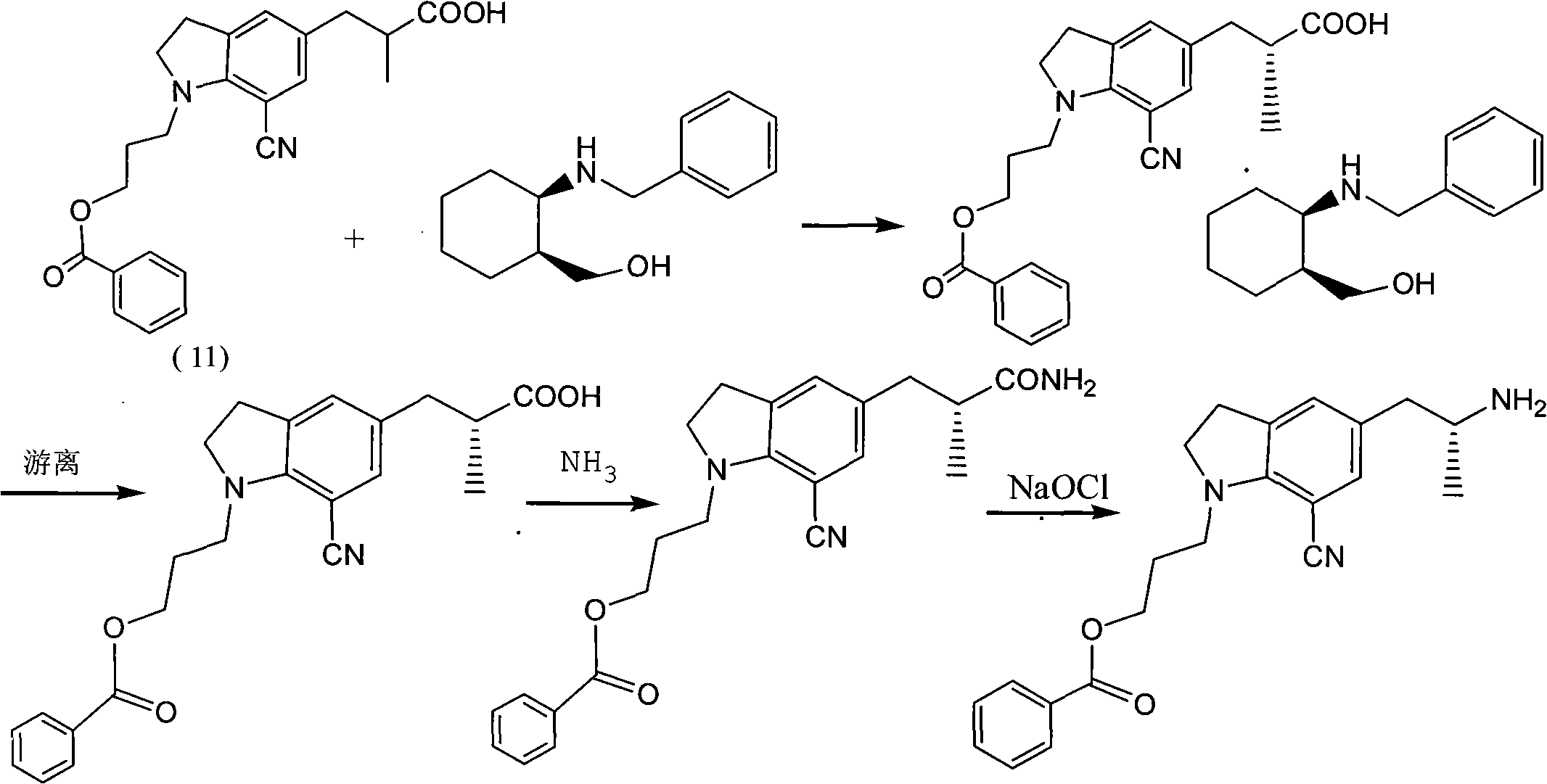
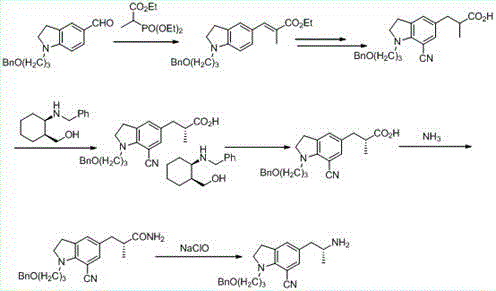
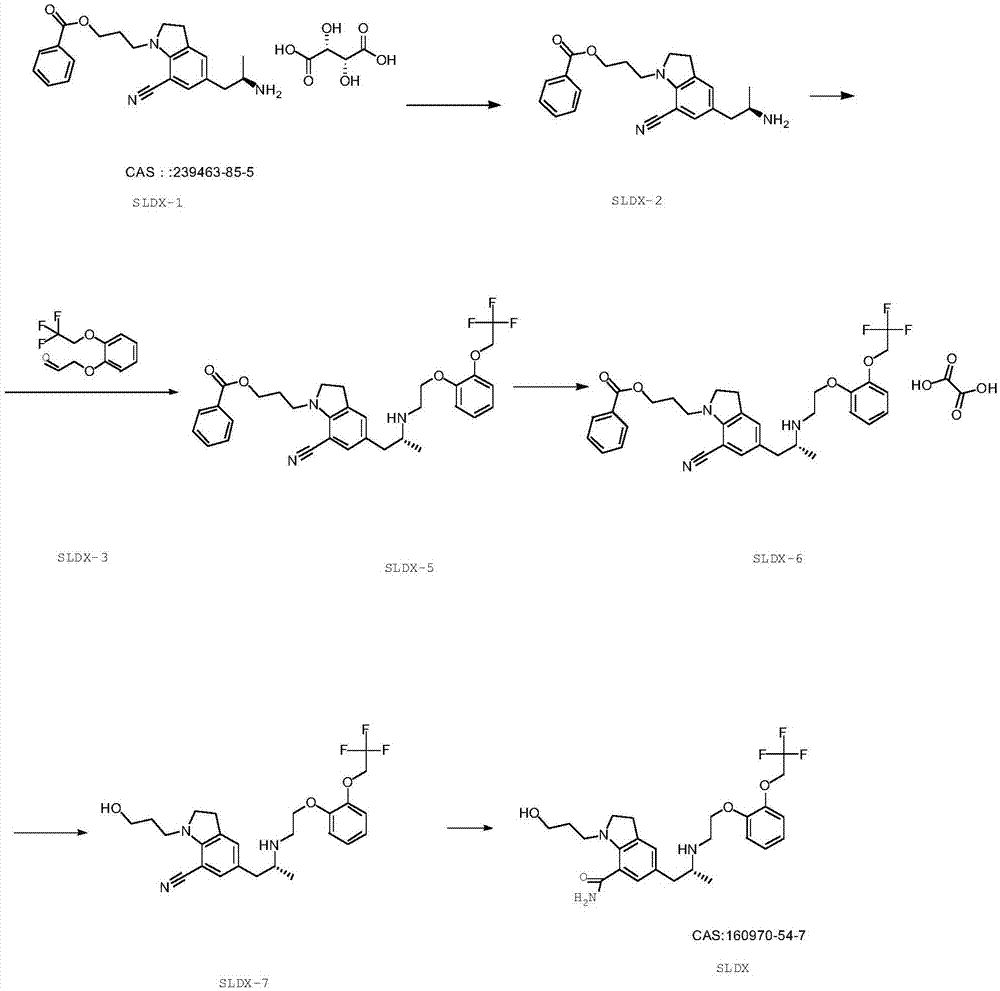
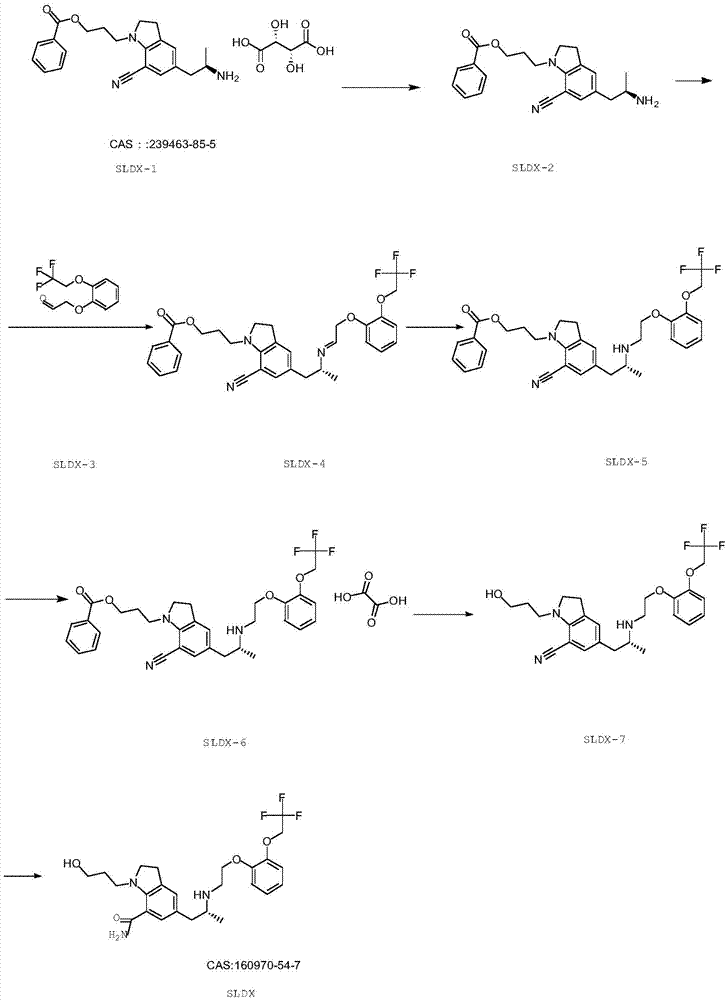
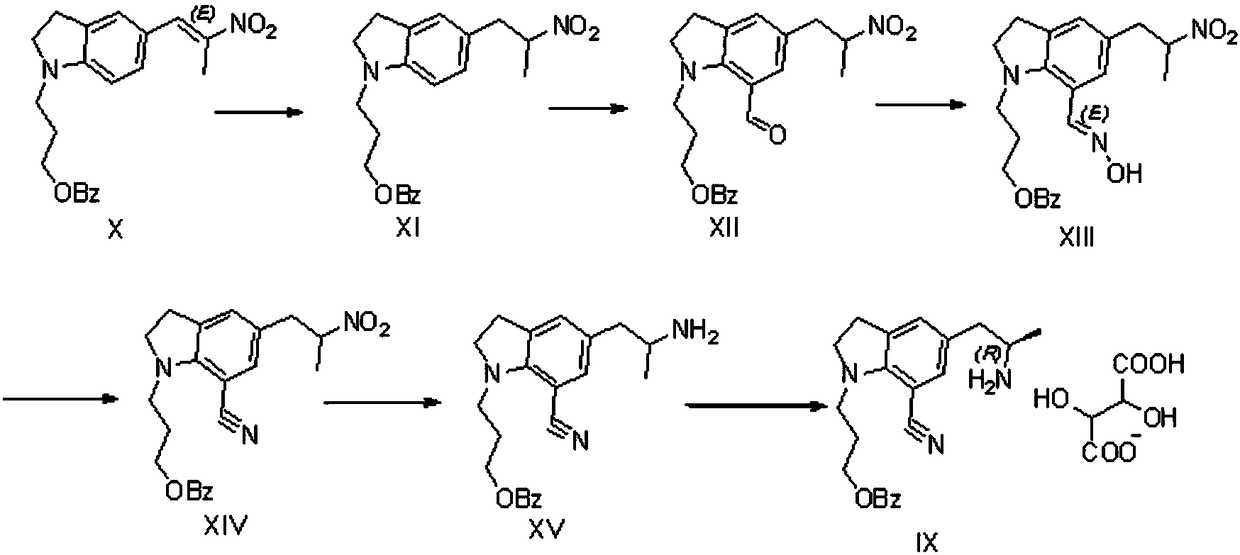
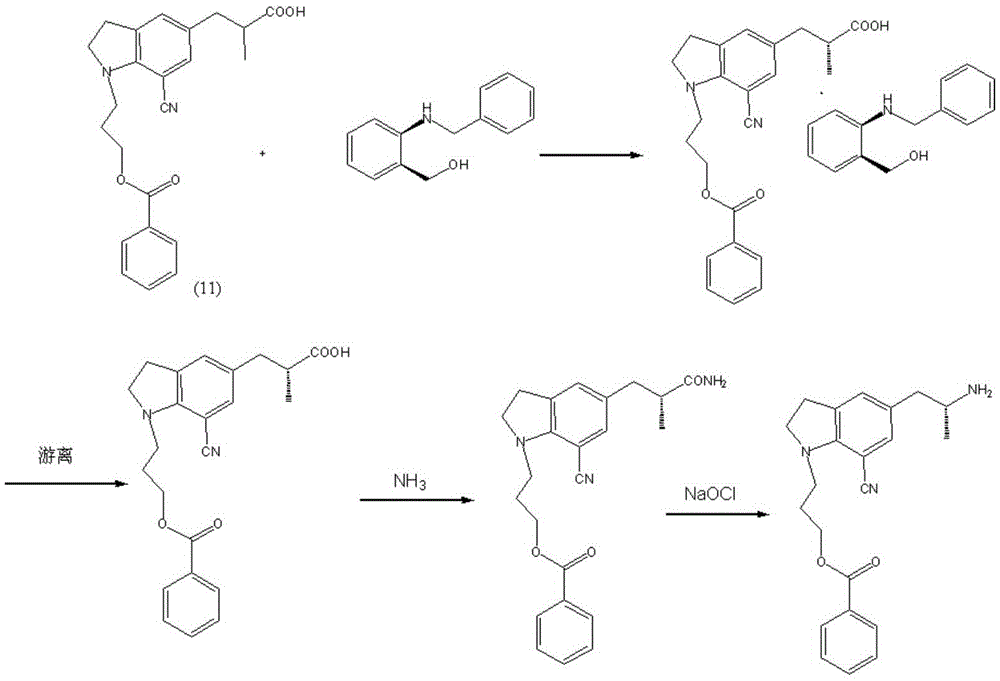
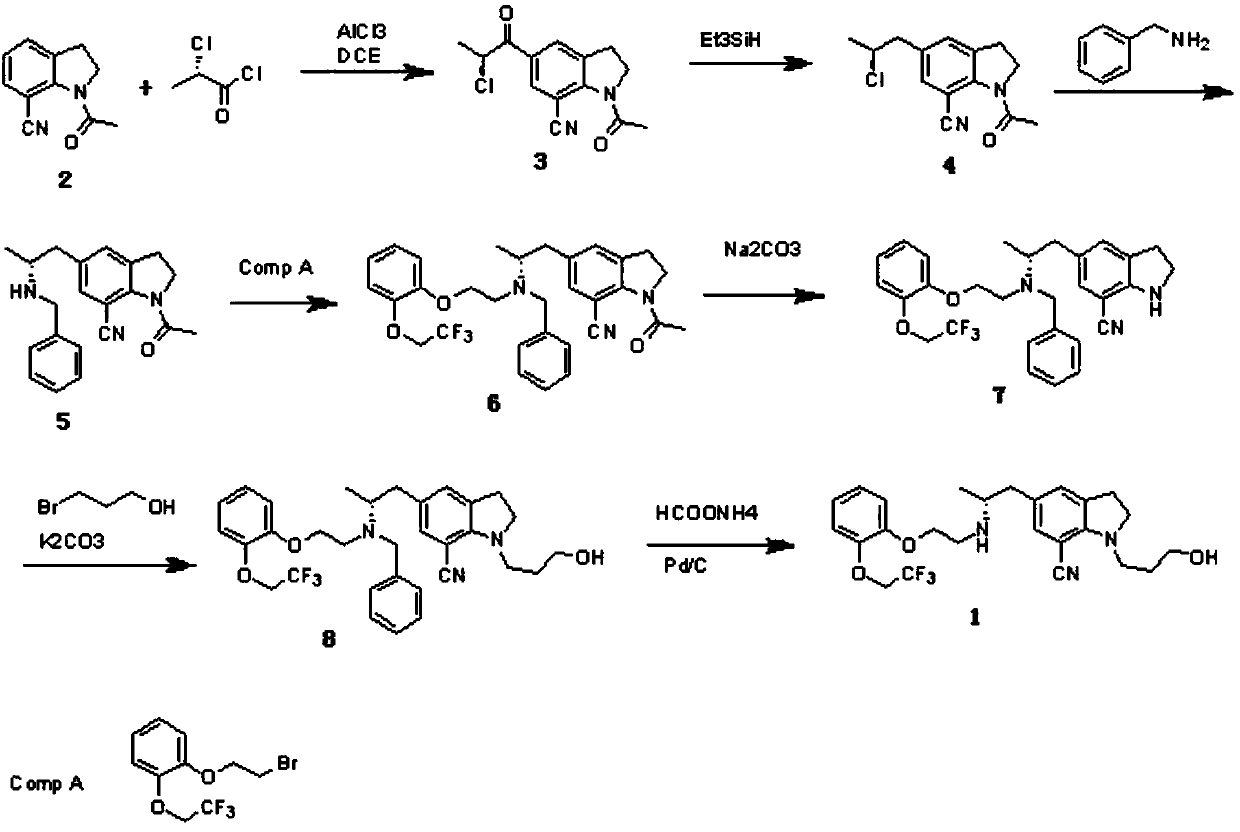
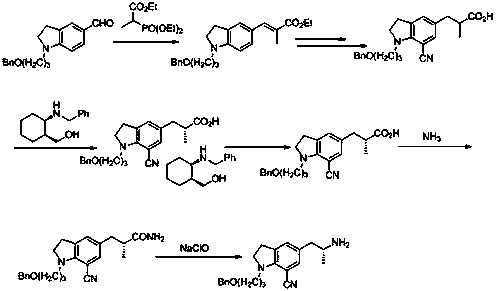
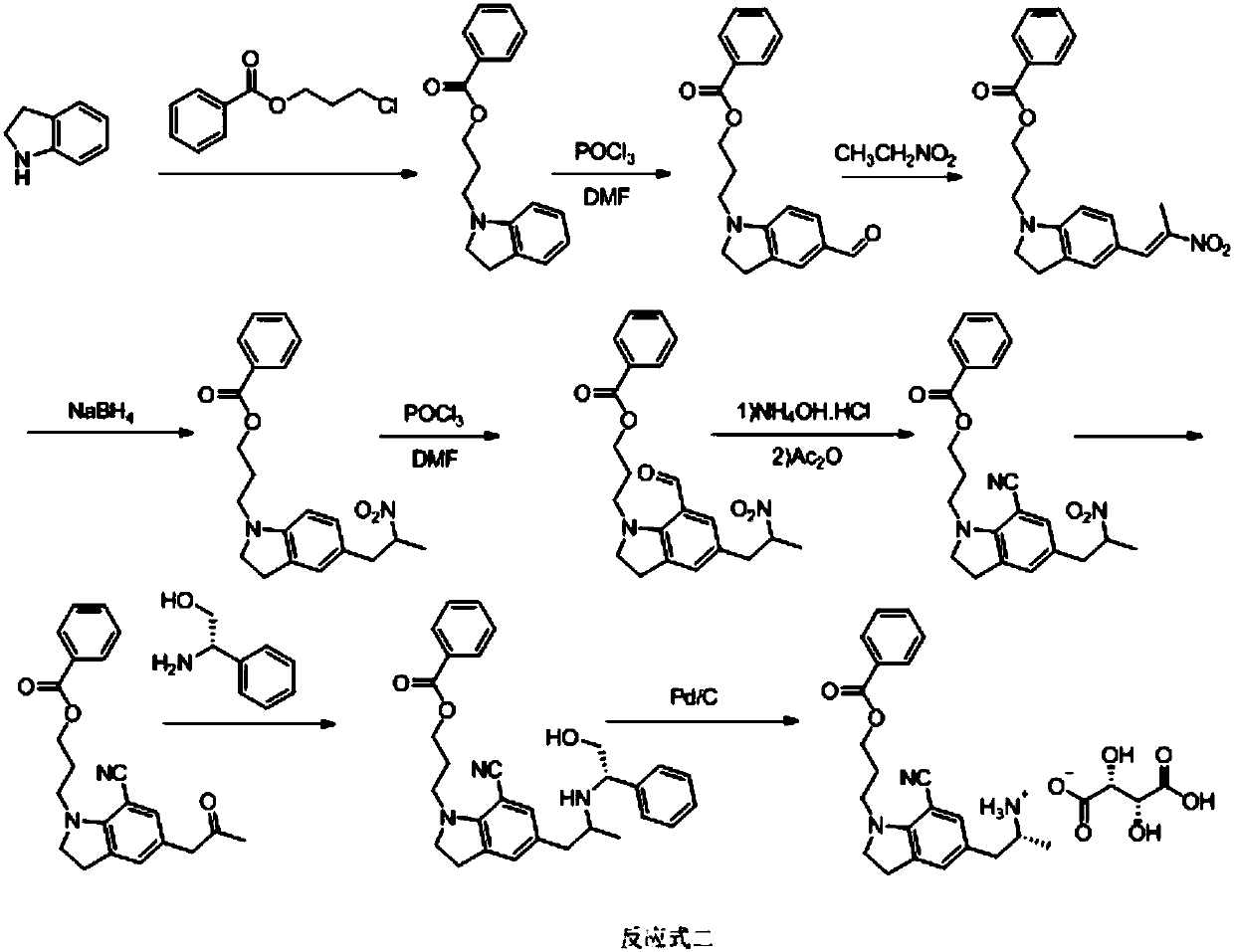
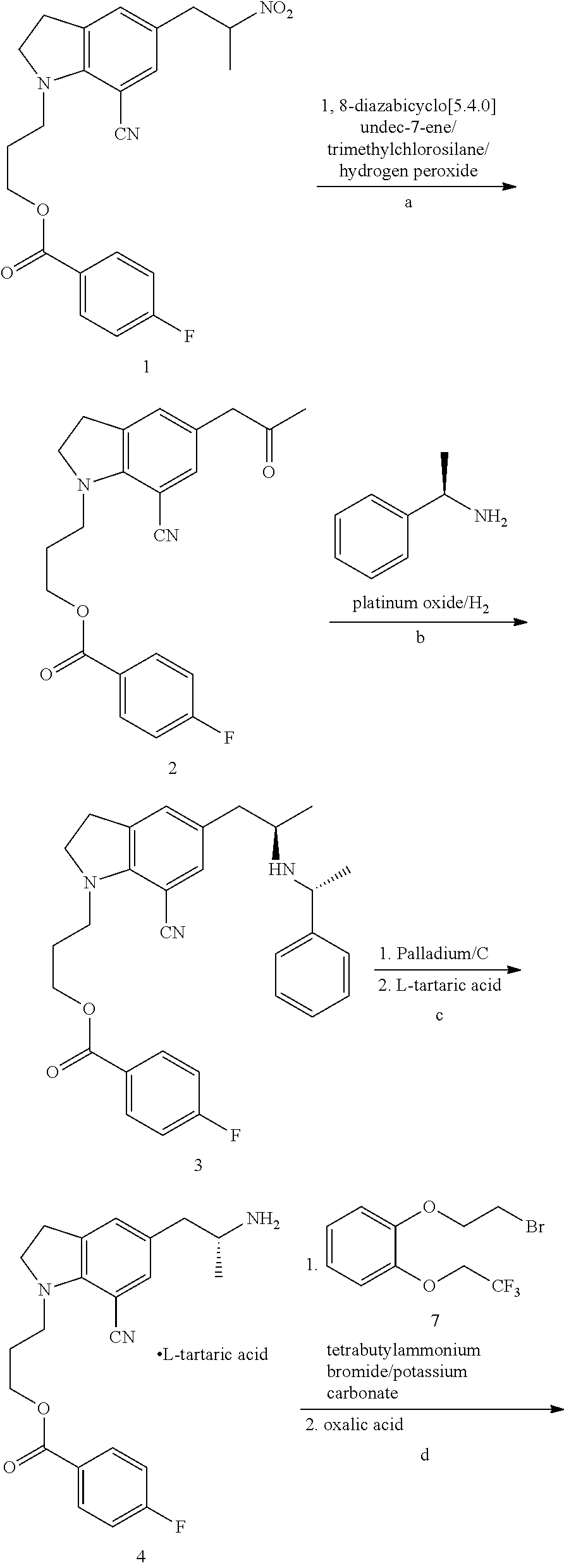
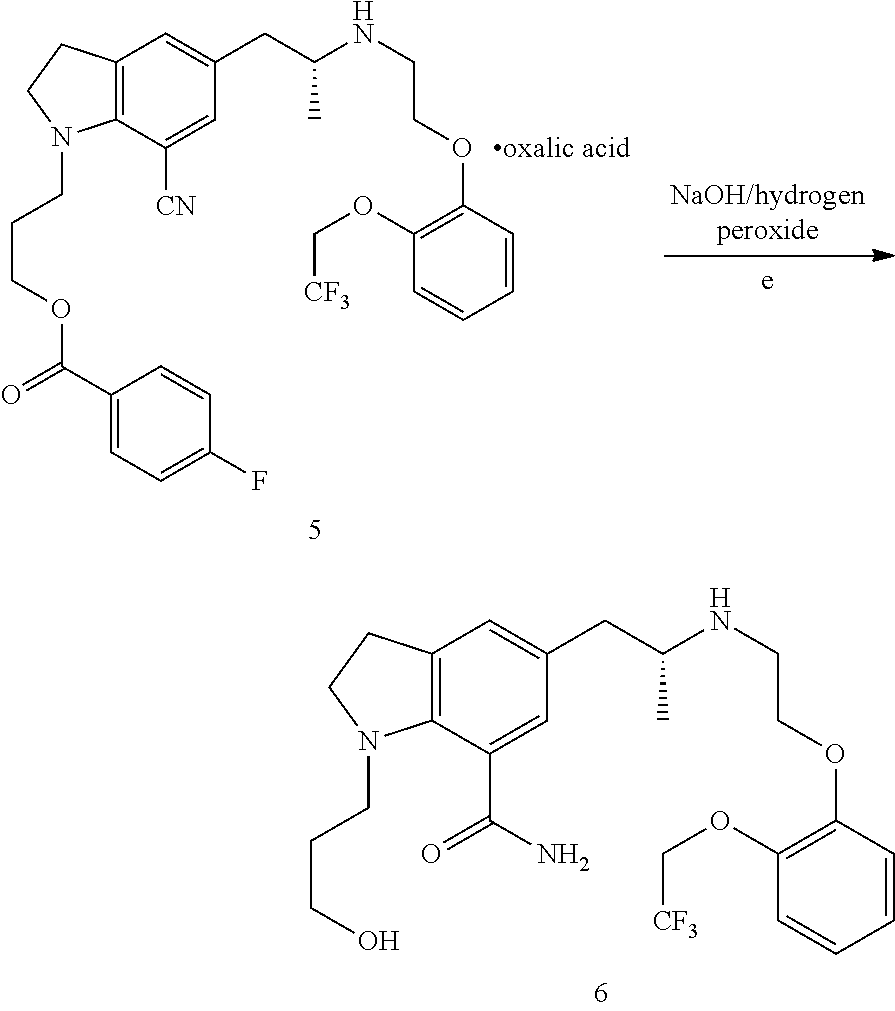
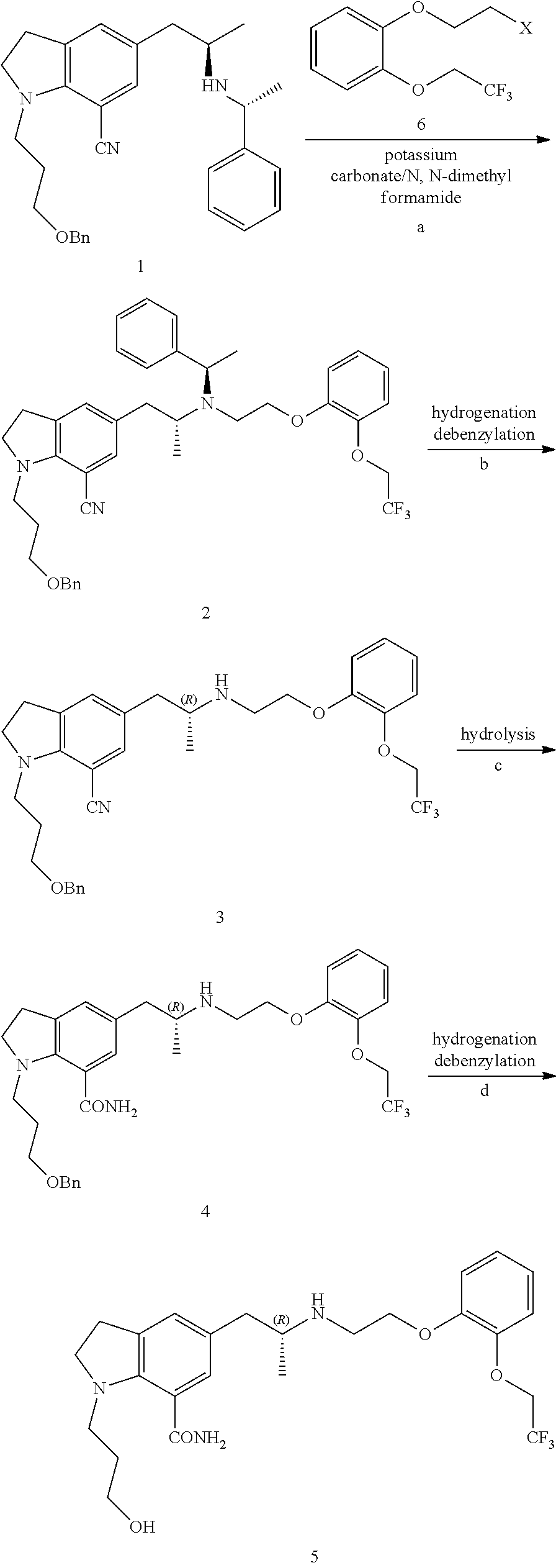
Preparation method of silodosin intermediate
ActiveCN104974072ALower purchase costReduce manufacturing costCarboxylic acid salt preparationIndolineCarbonyl reduction
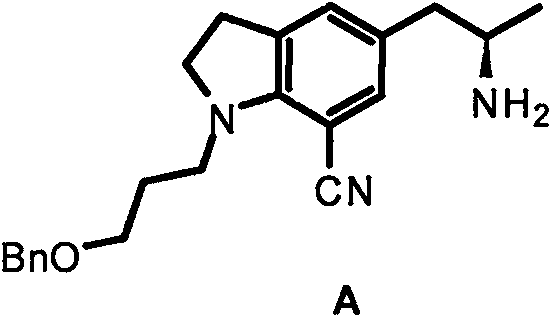
The invention discloses a preparation method of a silodosin intermediate, wherein the structure of the silodosin intermediate is represented as the formula A. The preparation method, wherein indoline is employed as a start raw material, includes the reactions of Friedel-Crafts acylation, carbonyl reduction, Gabriel reaction and chiral resolution and the like. The preparation method is simple in operation, is low in cost, is high in yield, allows the product to purify easily and is stable in processes, and is suitable for industrial production. The invention also discloses a new intermediate compound which is related in the method.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
Novel preparation method of silodosin
The invention discloses a novel method for preparing silodosin. In the invention, a complete silodosin synthesizing path is invented. By adopting brand new synthesis methods, intermediates synthesized in each step are brand new compounds, for example, if a Gabriel synthesis method is adopted, primary amine is introduced on the 2-site carbon atom, and chain extending reaction is carried out by utilizing benzyloxy-propyl, and the like. The invention provides the economic and safe path with high purity and high yield, and is suitable for industrial production.
Owner:傅军
Novel intermediate for synthesizing silodosin as well as preparation method and purpose of novel intermediate
ActiveCN102702067AAvoid generatingMild reaction conditionsOrganic chemistryIndolineCombinatorial chemistry
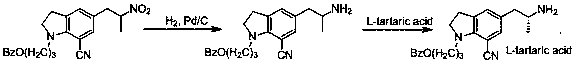
The invention provides a novel optical active compound 5-[(2R)-2-(benzyl amino) propyl]-1-[3-( benzoyloxy) propyl]-7-cyanogroup indoline and a preparation method of the novel optical active compound. The compound can be used as an intermediate for synthesizing silodosin.
Owner:北京联本医药化学技术有限公司 +2
Optical active compound of 1-(3-benzoyloxy-propyl)-5-(2-(1-phenyl ethyl amine) propyl-7-cyano indoline as well as preparation method and application thereof
The invention provides an optical active compound of 1-(3-benzoyloxy-propyl)-5-(2-(1-phenyl ethyl amine) propyl-7-cyano indoline as well as a preparation method and the application thereof. The optical active compound is shown in a formula (1), comprises a (R,R) configuration and a (S,S) configuration and can be used as an intermediate for synthetizing silodosin. The optical active compound of the single formula (1) can be used for preparing an optical high-purity product and has good yield on the premise of ensuring good optical purity. The preparation method of the optical active compound uses low-cost chiral assistant agent of alpha-phenethylamine and derivatives thereof, has mild reaction conditions, low cost and controllable optical purity and is easy for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Preparation method of silodosin intermediate
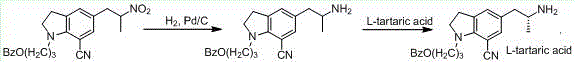
The invention discloses a preparation method of a silodosin intermediate. The preparation method comprises the following steps that in an organic solvent, under the action of lewis acid, friedel-crafts acylation reaction occurs between a compound 2 and a compound 3, so as to obtain a compound 4, wherein the lewis acid is one of or more of zinc trifluoromethanesulfonate, bismuth trifluoromethanesulfonate, scandium trifluoromethanesulfonate and aluminum trichloride; under the action of organic acid or boron trifluoride ether complex, the compound 4 reacts with triethyl silicane, so as to obtain a compound 5; the compound 5 reacts with sodium azide, so as to obtain a compound 6; under the action of catalysts, the compound 6 reacts with di-tert-butyl dicarbonate ester and hydrogen, so as to obtain a compound 7; under an acidic condition, the deamination protective reaction of the compound 7 occurs, so as to obtain a compound 8; the compound 8 reacts with L-tartaric acid, so as to obtain a compound 1, namely the silodosin intermediate. The preparation method of the silodosin intermediate has the advantages of simplicity, economy and mild reaction conditions, and chiral resolution is not needed.
Owner:ZHEJIANG TIANYU PHARMA
Indoline compound and process for producting the same
ActiveUS20070197627A1Simple methodEasy to handleBiocideOrganic active ingredientsPropyl benzoateOXALIC ACID DIHYDRATE
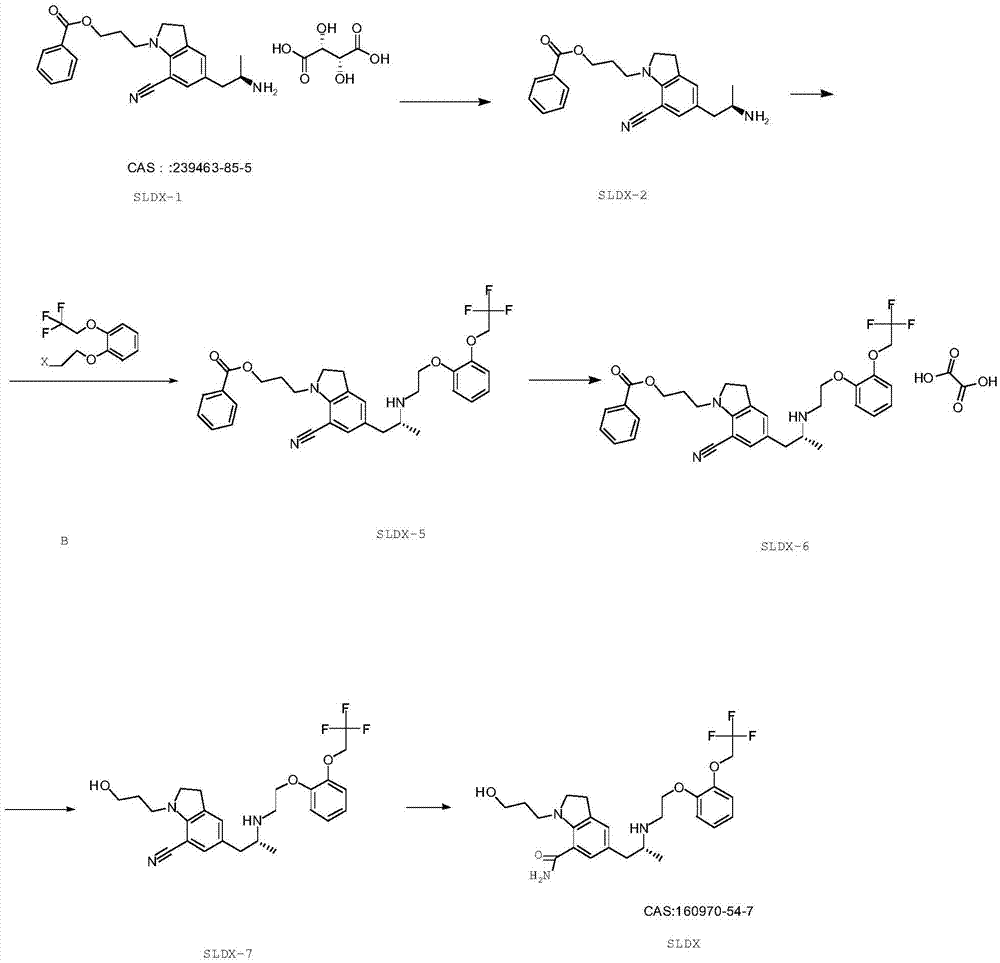
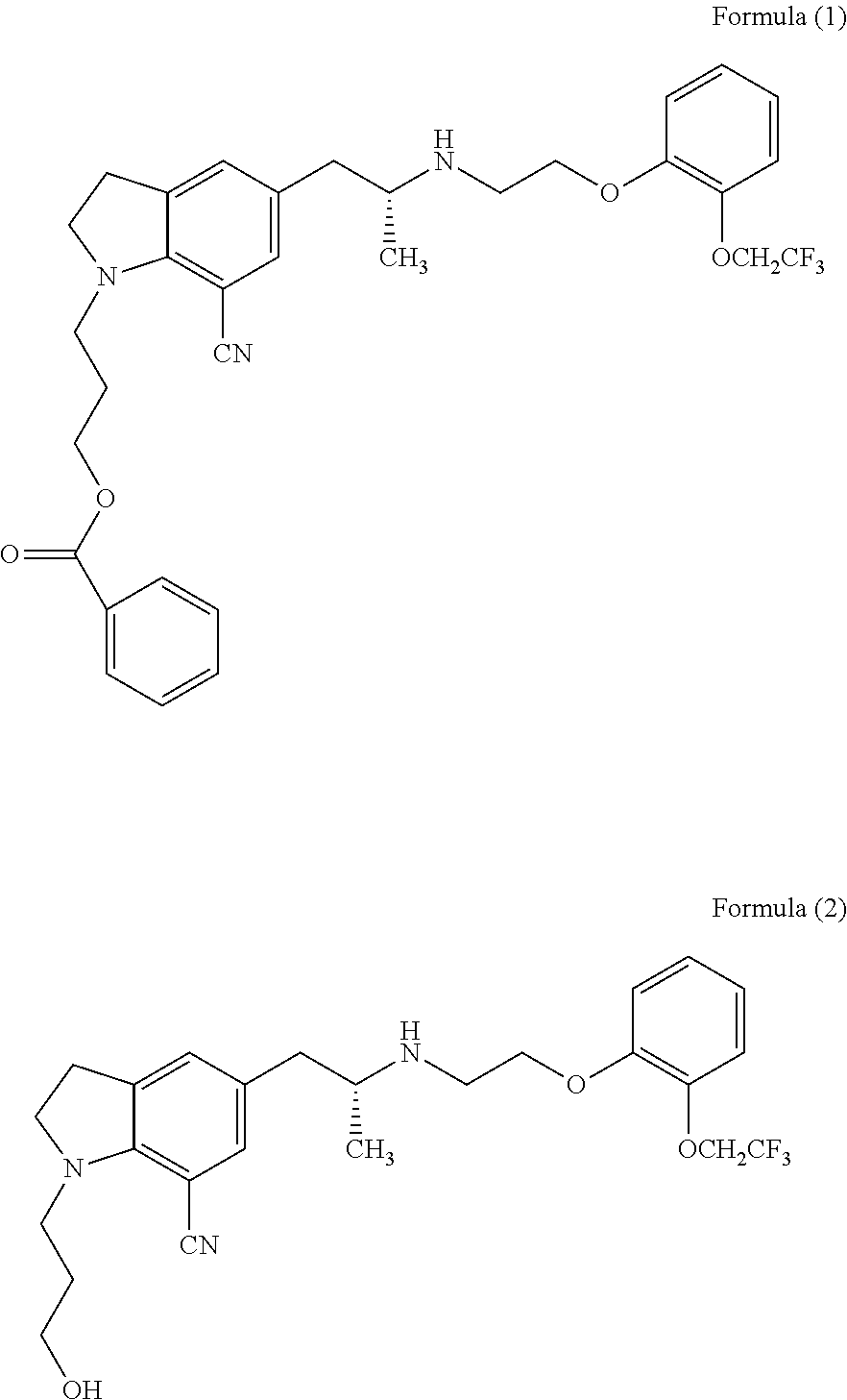
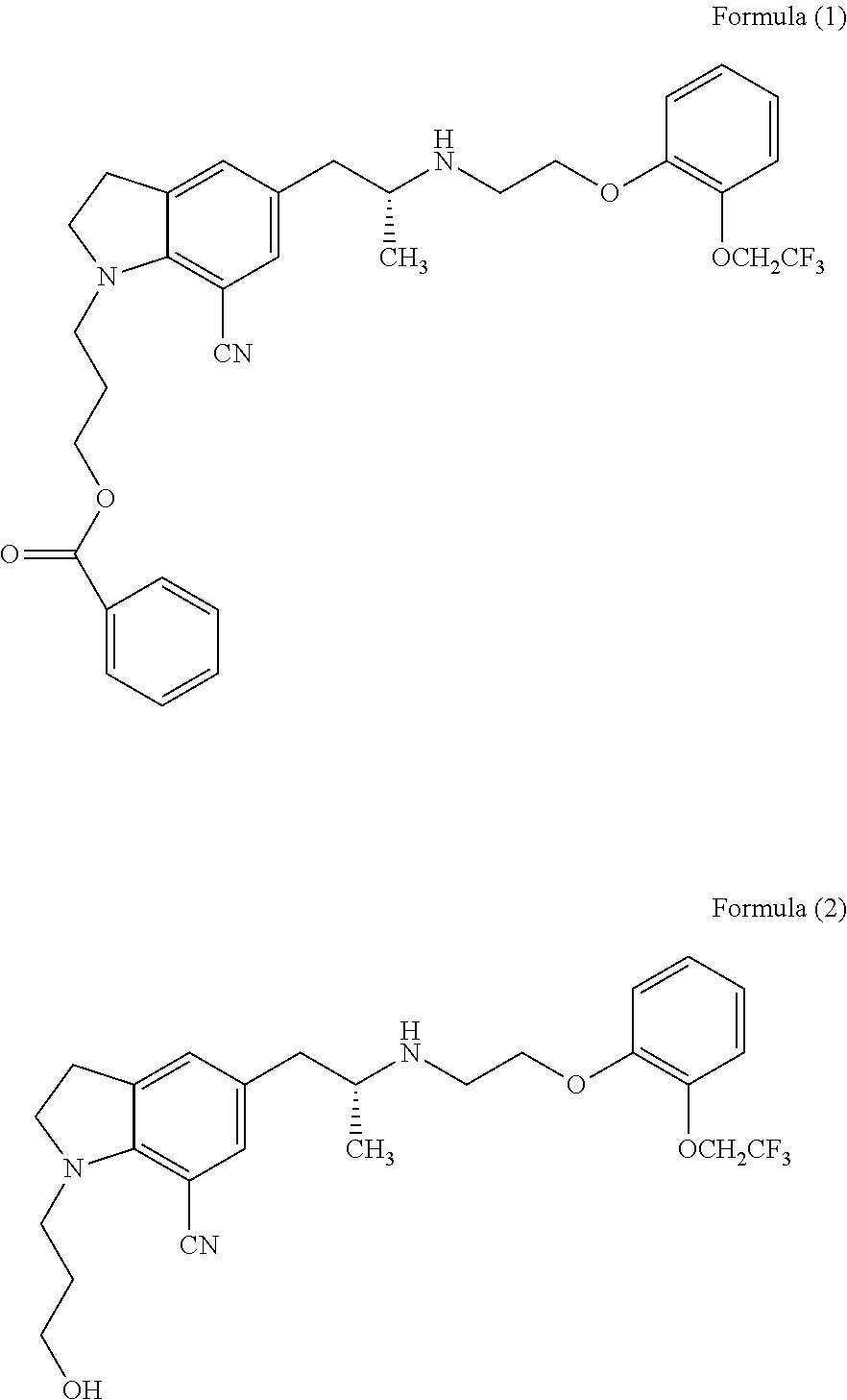
The present invention provides an industrial method production of silodosin, which is useful for a therapeutic agent for dysuria associated with benign prostatic hyperplasia. The production of silodosine is characterized by mixing 3-{7-cyano-5-[(2R)-2-({(2-[2-(2,2,2-trifluoroethoxy)-phenoxy]ethyl}amino]propyl]-2,3-dihydro-1H-indol-1-yl}-propyl benzoate and oxalic acid to yield the oxalate, subsequently hydrolyzing the oxalate salt to yield 1-(3-hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino]propyl]-2,3-dihydro-1H-indole-7-carbonitrile and hydrolyzing the same, and manufacturing intermediates used therefore.
Owner:KISSEI PHARMA
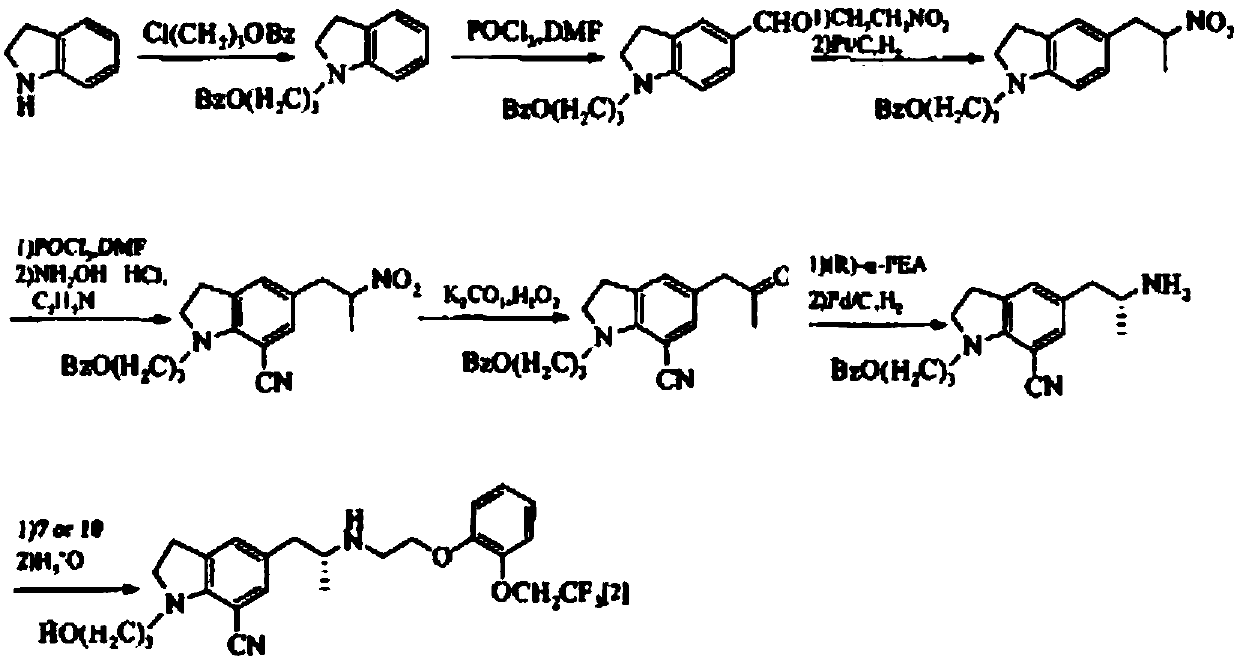
Preparation method for 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline
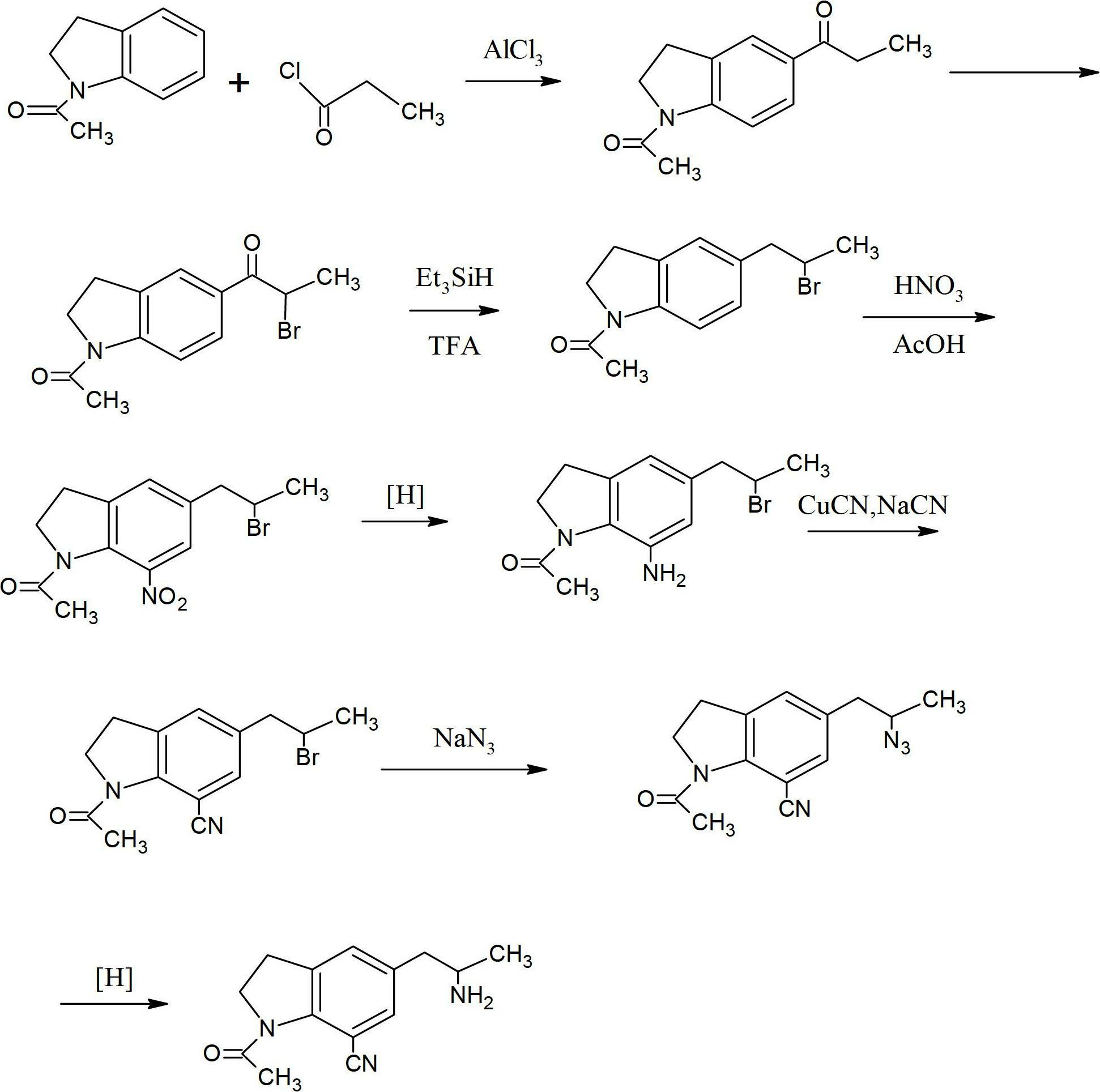
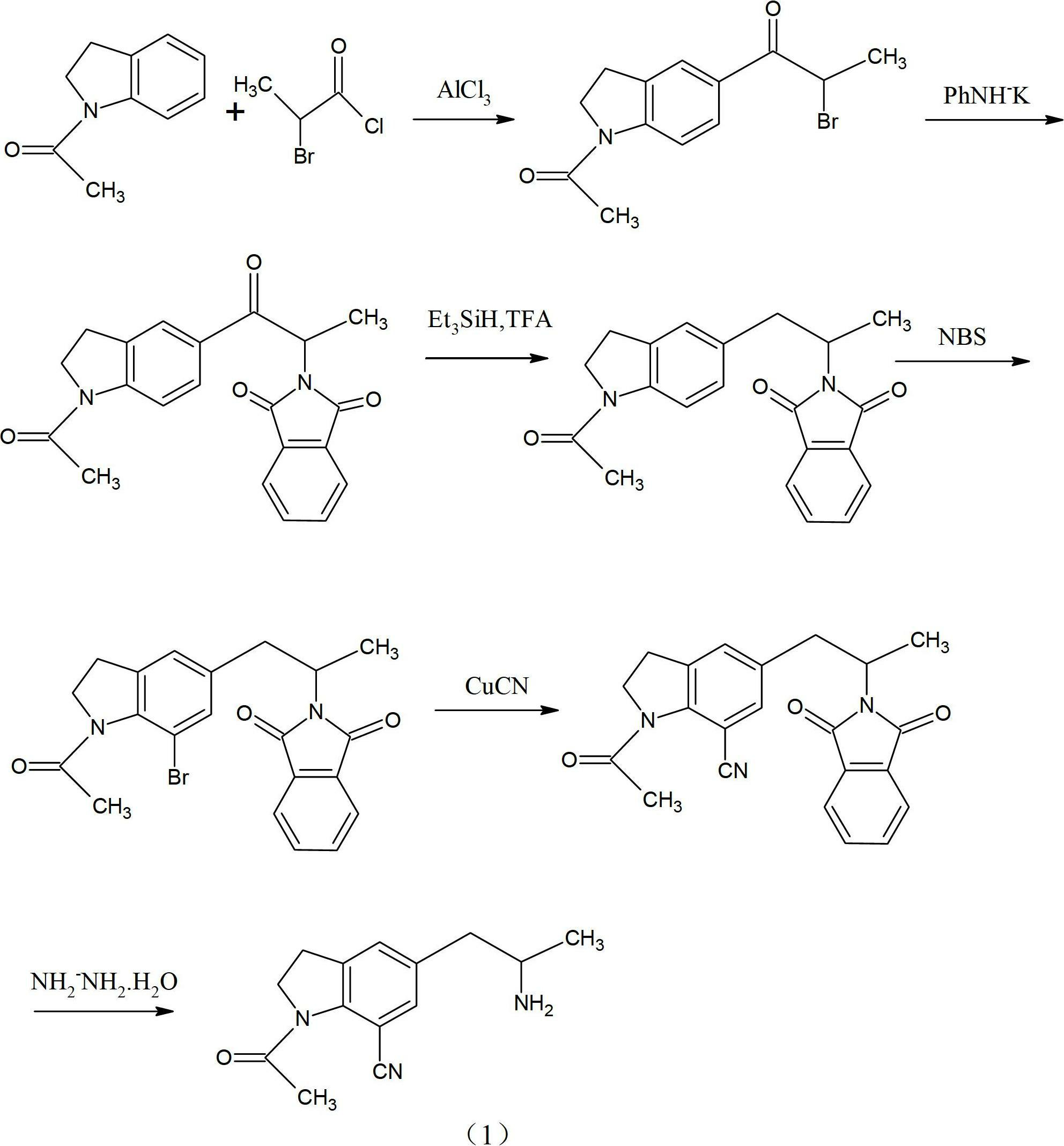
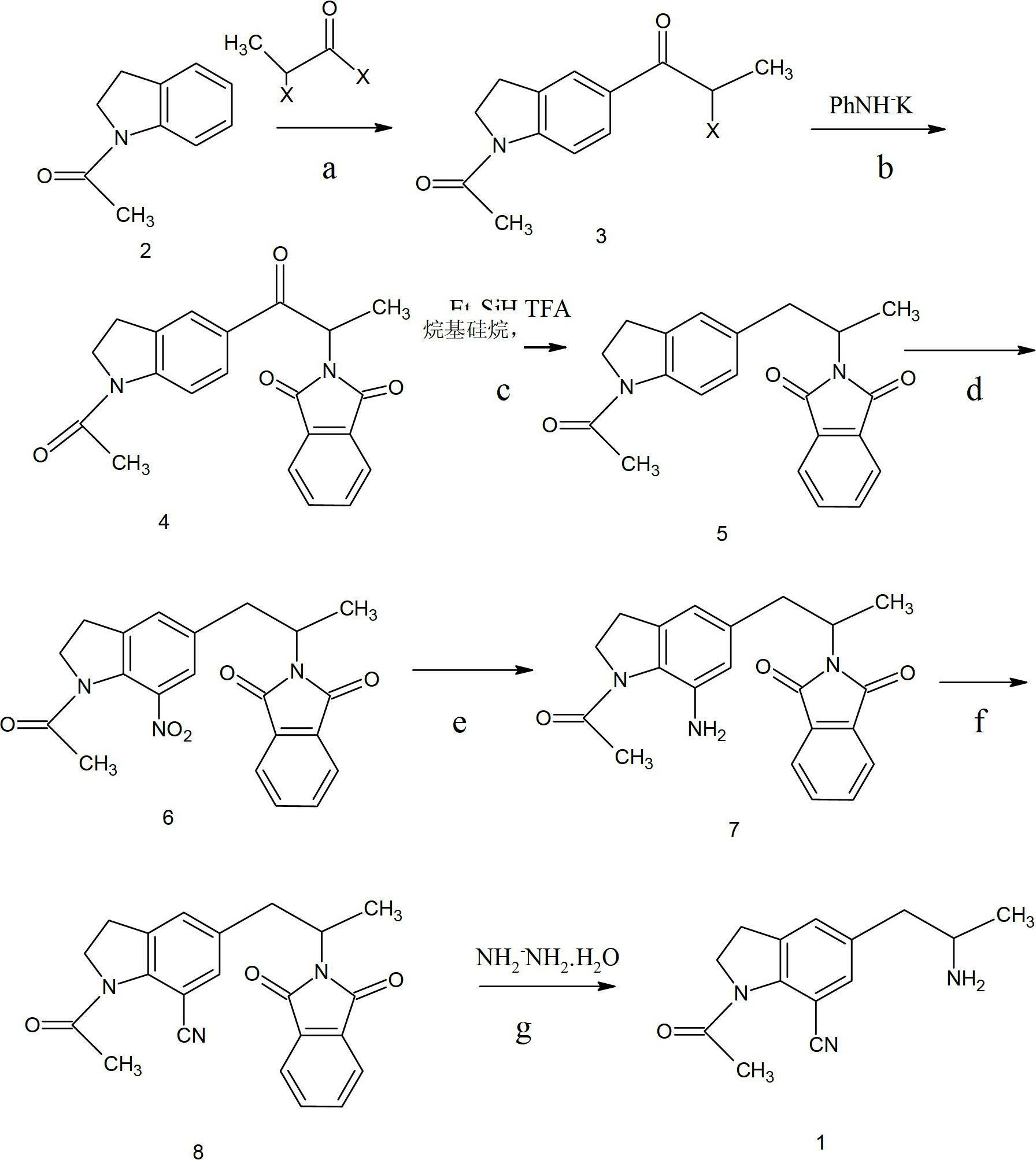
The invention provides a preparation method for synthesizing 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline which is a key intermediate of silodosin. The method comprises the following steps: 1-acetylindoline is adopted to serve as the material, and 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline can be obtained through the such 7 steps as friedel-crafts acylation reaction, phthalimide amination, alkylsilane reduction, nitrification, reduction, heavy nitrogen cyaniding and deprotection. According to the method, the materials in the whole synthesizing line are cost-saving and easy to obtain, the operation is simple and the operation cost is low. Moreover, the yield rate of each step of reaction is higher than that in the prior art. Therefore, the method provided by the invention is very suitable for industrial production and has larger industrial application value.
Owner:LINHAI TIANYU PHARMA
Preparation method of beta type silodosin crystal
InactiveCN103360298AHigh purityHigh yieldOrganic active ingredientsOrganic chemistryAlkaneState of art
The invention discloses a preparation method of a beta type silodosin crystal. Silodosin is a medicament which is clinically used for treating benign prostatic hyperplasia, and three crystal forms, namely alpha, beta and gamma exist. Although the beta crystal form silodosin can be used as an active ingredient of an oral medicament, the preparation method in the prior art has the shortcomings of more process steps, low yield, low purity and the like. According to the preparation method disclosed by the invention, the beta crystal form silodosin is obtained by selecting an appropriate halogenated alkane type solvent and adopting a mild crystallization way. The method has the advantages of stable process, simplicity in operation, convenience, quickness, higher yield and higher purity, the used solvent is very easy to remove, and the method can be used for industrial production.
Owner:KUNMING JIDA PHARMA
Preparation method of silodosin intermediate
ActiveCN104974073AShort reaction pathReduce manufacturing costOrganic chemistryPhotochemistrySilodosin
A method for preparing silodosin intermediate is disclosed. Said intermediate has the structure of (M), wherein R is benzyl or benzoyl. Said preparation method has the characteristics of short reaction route, simple operation, low cost, high yield, stable process, etc. The method is suitable for industrial production, and has extra-high industrial application value. Intermediate compounds for preparing the intermediate represented by M are also disclosed.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
Preparation method of indoline derivative for synthesizing silodosin
ActiveCN106380438AHigh optical purityChemical cost reductionOrganic chemistryBenzoic acidPalladium on carbon
The invention provides a preparation method of an indoline derivative for synthesizing silodosin. Indoline is taken as a starting raw material and reacts with benzoic acid and 1-chloro-3-bromopropane to prepare a compound (1); a reaction is carried out in a Vilsmeier agent, and a formyl group in introduced to the 5th position to prepare a compound (2); the compound (2) and nitroethane undergoes an asymmetric Henry reaction in the catalysis of quinidine-copper acetate to prepare a compound (3); the compound (3) is subjected to acetylation through acetic anhydride to prepare a compound (4); a cyano group is introduced to the 7th position to prepare a compound (6); and two functional groups are reduced through palladium-on-carbon hydrogenation in one step to obtain a high-chiral-purity target compound: (R)-1-[1-(3-benzoyloxypropyl)-5-(2-aminopropyl)-7-cyano]indoline. In the early stage of the method, cheap quinidine is used to perform an asymmetric Henry reaction for introduction of chiral centers, thereby avoiding resolution in the latter stage. The method is reasonable in design, simple to operate, effectively improved in yield and reduced in cost, and therefore is suitable for massive production.
Owner:江苏宇田医药有限公司
Method for preparing silodosin
InactiveCN106995399AReduce the impactSimplified purification procedureOrganic chemistryBenzoic acidChemical synthesis
The invention relates to a method for preparing silodosin, especially to an industrial preparation method of a silodosin compound, and belongs to the field of pharmaceutical chemical synthesis. The method includes: carrying out salt hydrolysis on 5-[(2R)-2-aminopropyl]-2,3-dihydro-1-[3-(benzoyloxy)propyl]-1H-indole-7-nitrile tartrate to obtain 5-[(2R)-2-aminopropyl]-2,3-dihydro-1-[3-(benzoyloxy)propyl]-1H-indole-7-nitrile, preparing benzoic acid-R-3-[7-cyano-5-(2-{2-[2-(2,2,2-trifluoro-ethoxy)-phenoxyl]-ethylamino}-propyl)-2,3-dihydro-indole-1-yl]-propylester which is an intermediate, and finally performing a hydrolysis reaction to produce silodosin. According to the provided industrial production method of silodosin, the yield is high, purification becomes easy and the impurity content is low.
Owner:北京天泰恒华医药技术有限公司
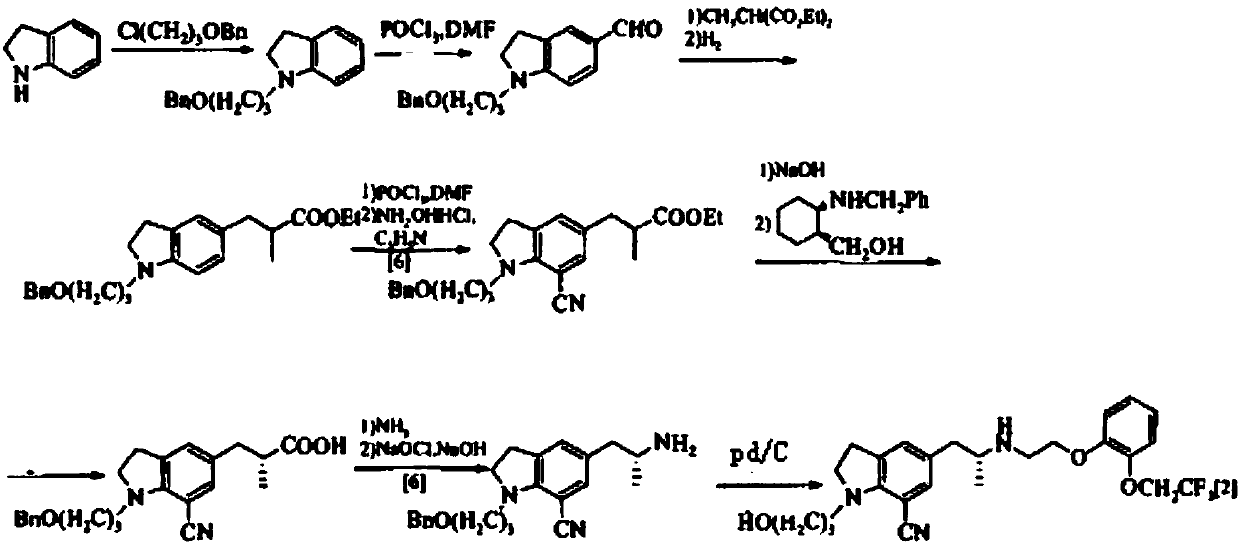
Process for the preparation of indoline derivatives and their intermediates thereof
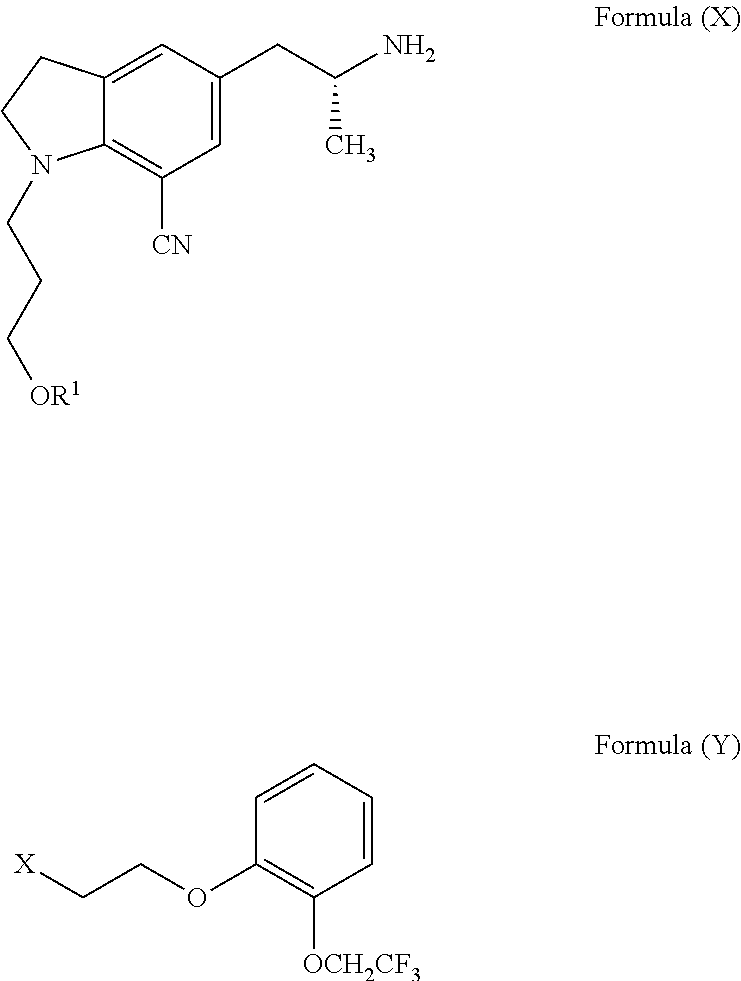
Processes for the preparation of Silodosin and its intermediates comprising reductive amination of compound of Formula (VIII) with a compound of Formula (VII) or a compound of Formula (XV) in a suitable solvent using a reducing agent.
Owner:SANDOZ AG
Method for preparing and purifying Silodosin intermediates
InactiveCN102320996AEasy to operateHigh yieldSulfonic acid esters preparationEther preparation by ester reactionsIodidePhenol
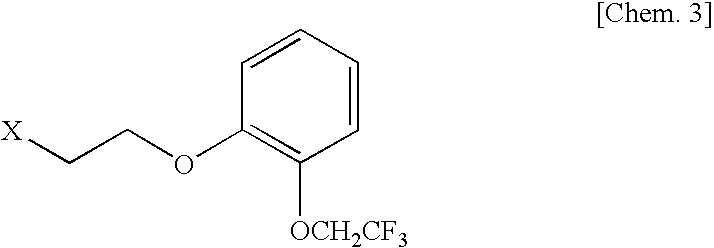
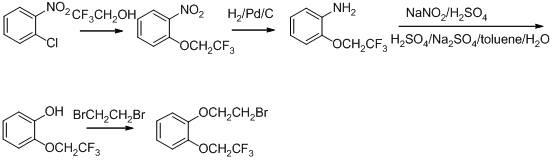
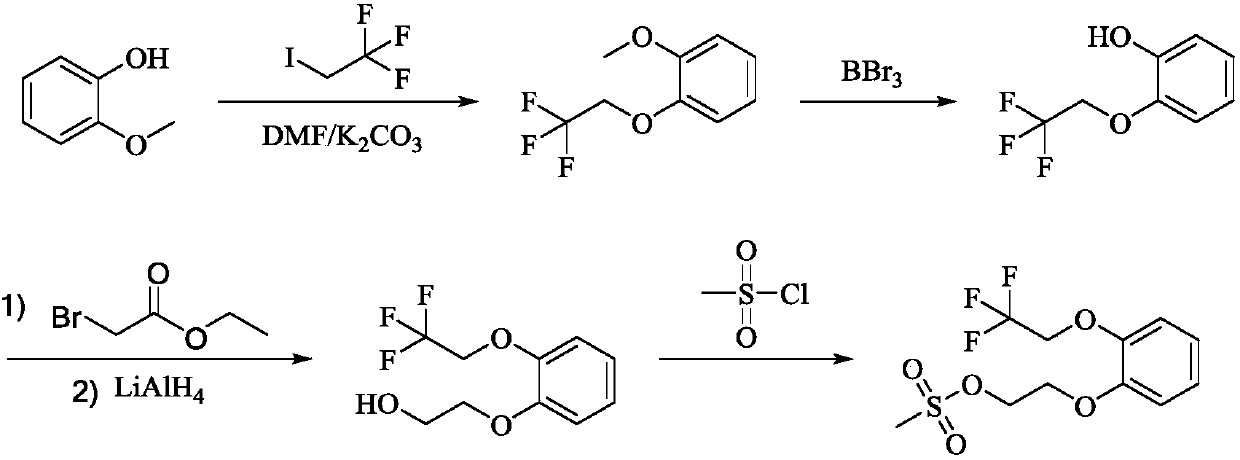
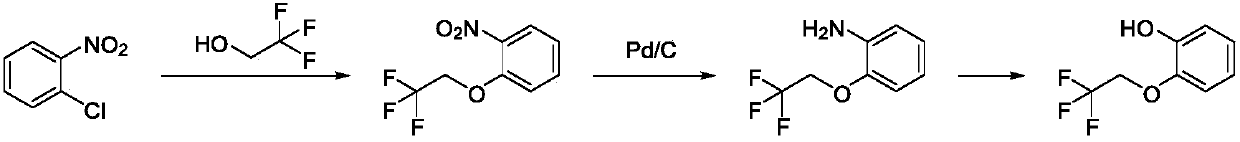
The invention discloses a method for preparing and purifying Silodosin intermediates namely 2-(2,2,2-tirfluoroethyoxy) phenol and 2-(2,2,2-tirfluoroethyoxy) phenoxy ethyl methanesulfonate. The method comprises the steps of: A. reacting catechol or a salt thereof, 2-halogenated trifluoroethane or 2,2,2-trifluoroethyl sulfonate as raw materials with alkali metal iodide in an aprotic solvent under the action of basic compound to obtain 2-(2,2,2-trifluoroethoxy) phenol (II), and carrying out steam distillation and purification; and B. reacting 2-(2,2,2-trifluoroethoxy) phenol (II) obtained in the step A with ethylene dimethanesulfonate in the aprotic solvent to obtain 2-(2,2,2-trifluoroethoxy) phenoxy ethyl methanesulfonate (I). The method has the advantages that the starting raw materials are inexpensive and easily available, and the production cost is lowered; complicated column chromatography is not used; and the method is suitable for large-scale industrial production.
Owner:SICHUAN UNIV
Oral solution
InactiveCN101108182AStable in natureImprove bioavailabilityOrganic active ingredientsUrinary disorderDysuriaSolvent
The invention provides an oral solution for curing dysuria, which takes silodosin as main active ingredient or silodosin prodrug or pharmaceutically acceptable salt. The oral solution, which is made of water, ethanol or the mixture of the two, overcomes the defects of difficult taking, slow dissolving out and low biological utilization rate in tablet, capsule and other solid preparations. With stable fluid quality, the oral solution has excellent compliance and biologic utilization rate and can be best taken by elder patients.
Owner:李献阳
Methods for treating prostatitis
It has been discovered that silodosin is effective in treating patients having symptoms associated with prostatitis with silodosin or a pharmaceutically acceptable salt thereof. In a preferred embodiment, patients are treated with 4 mg once daily.
Owner:ALLERGAN SALES LLC +1
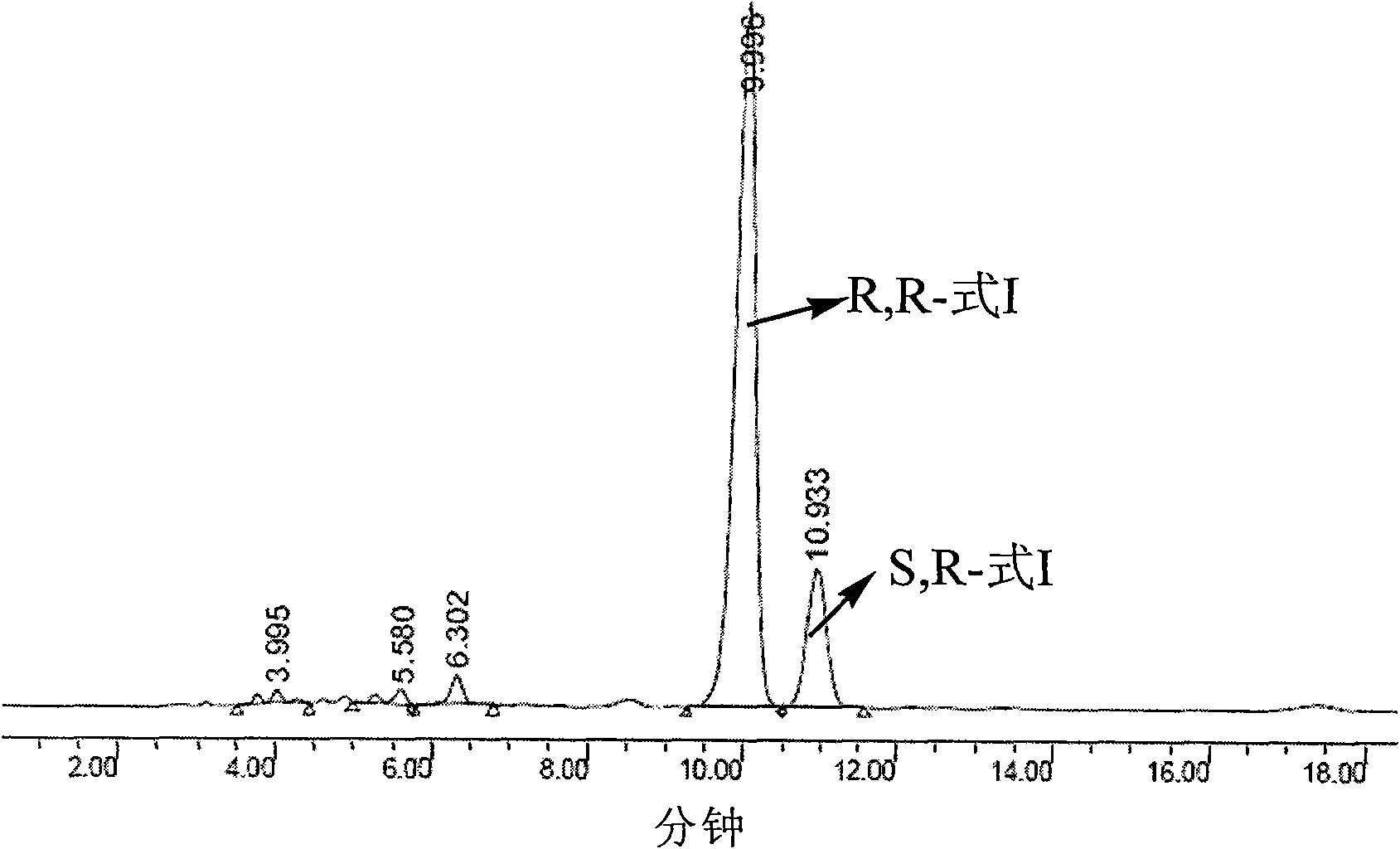
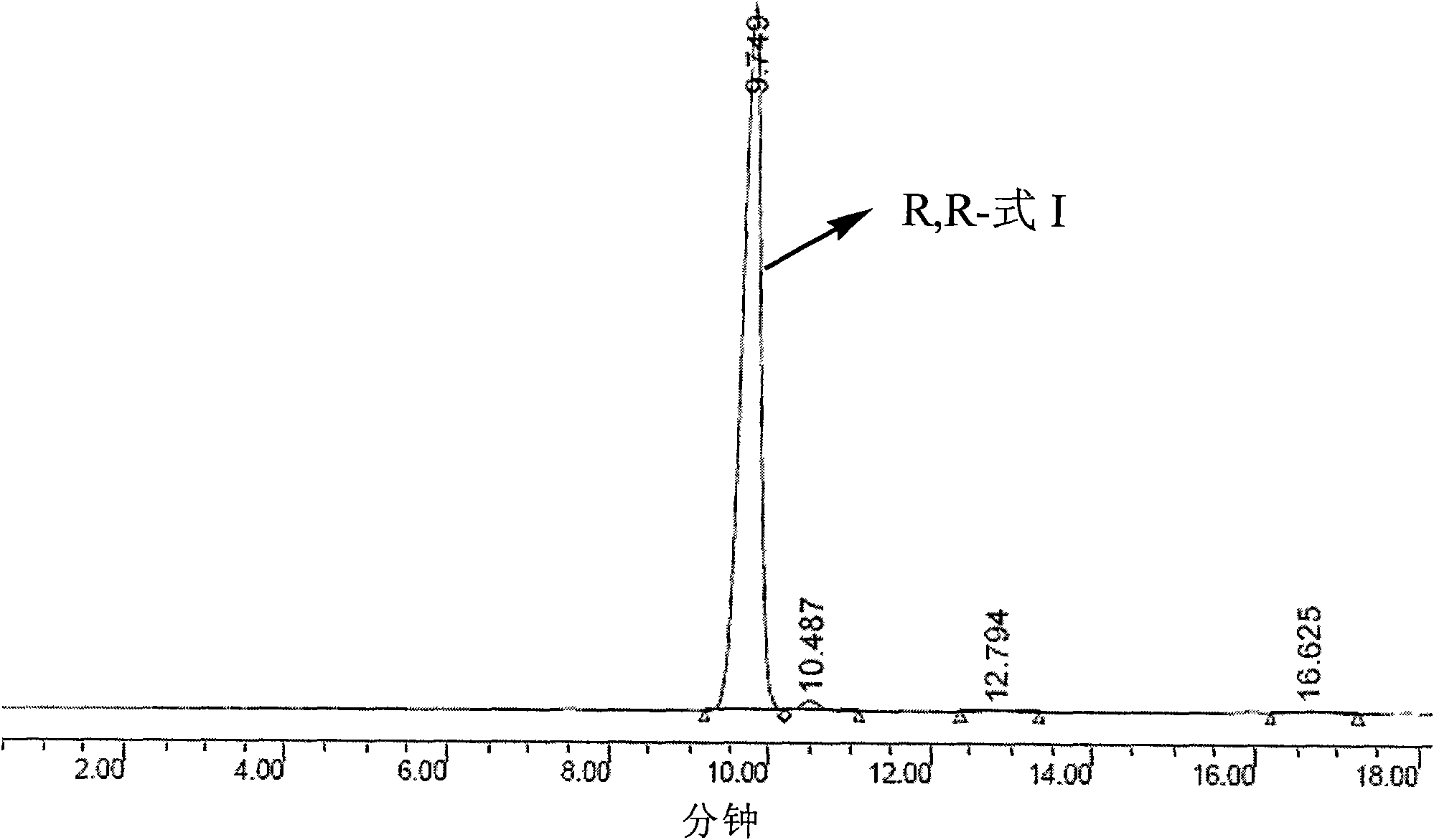
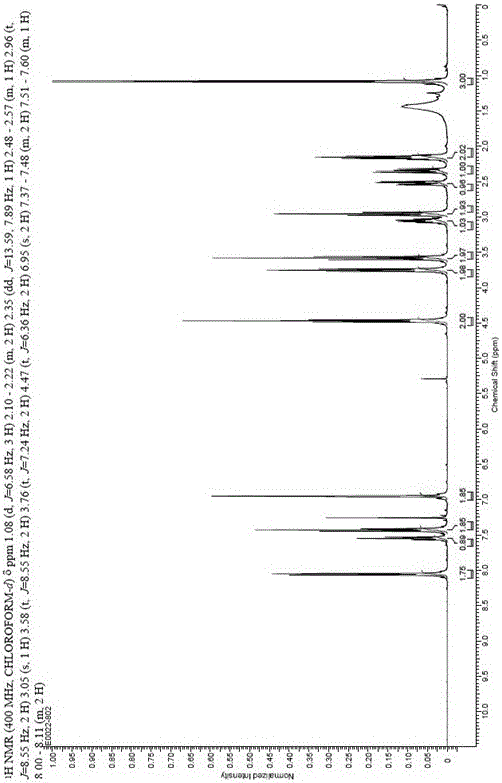
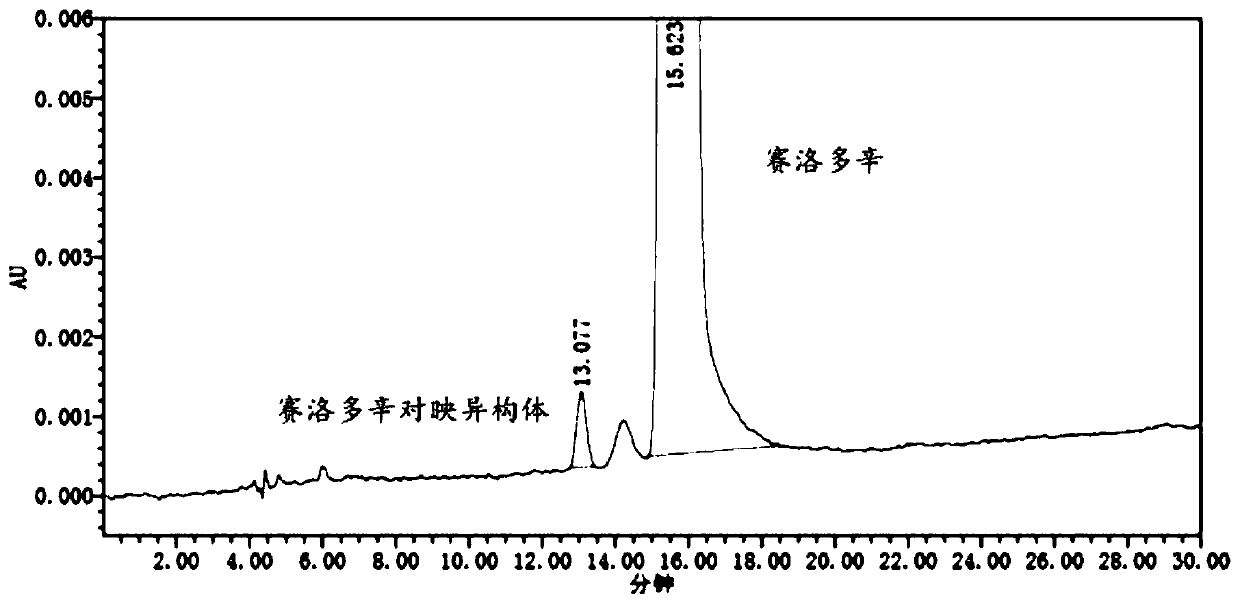
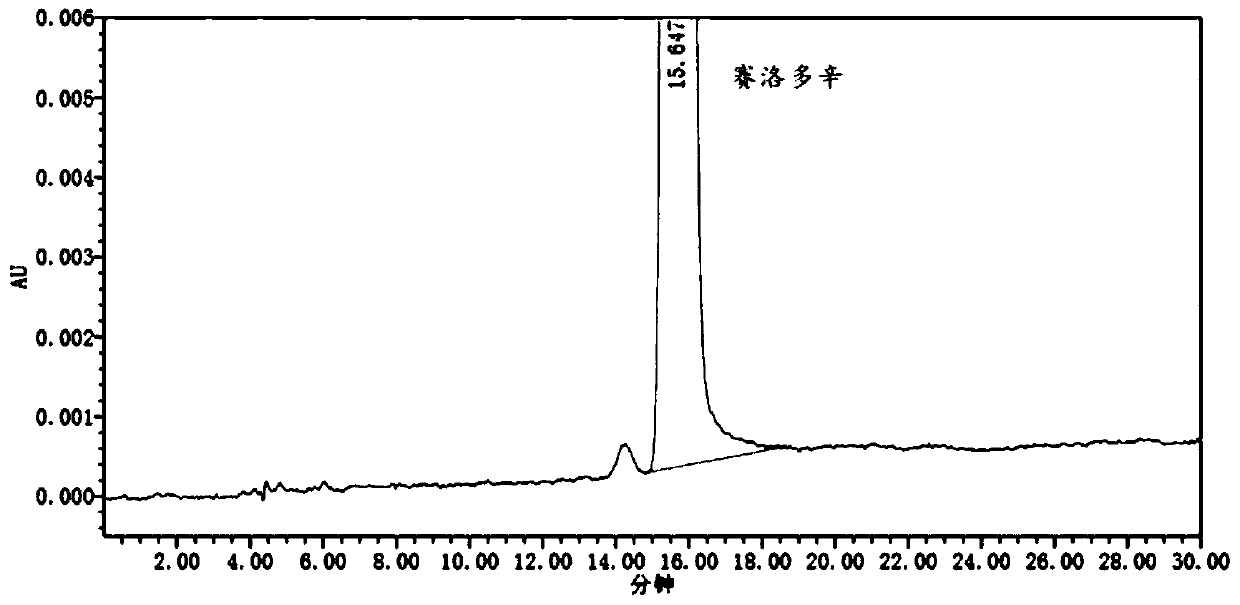
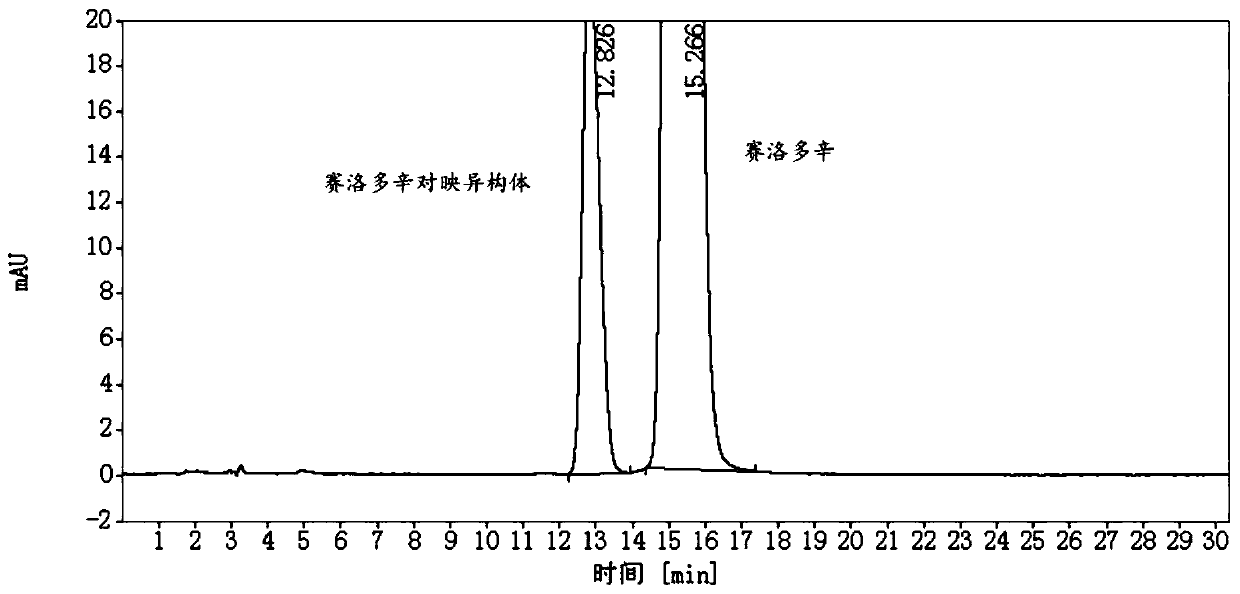
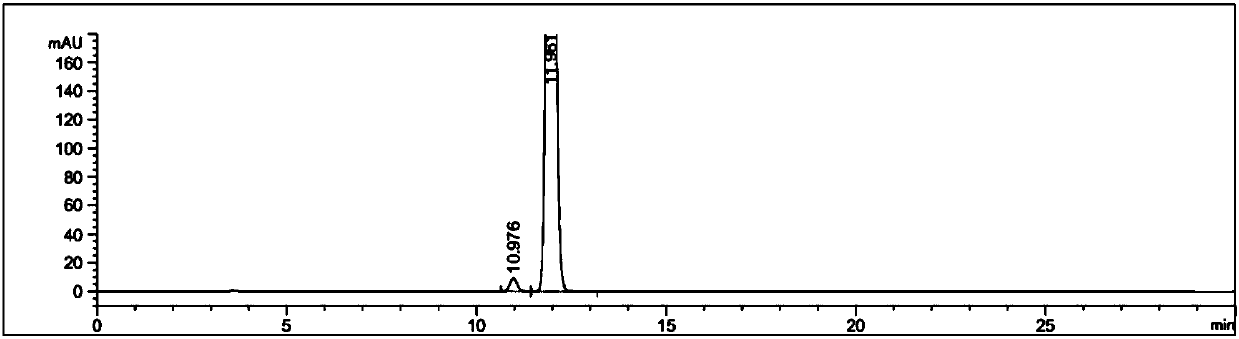
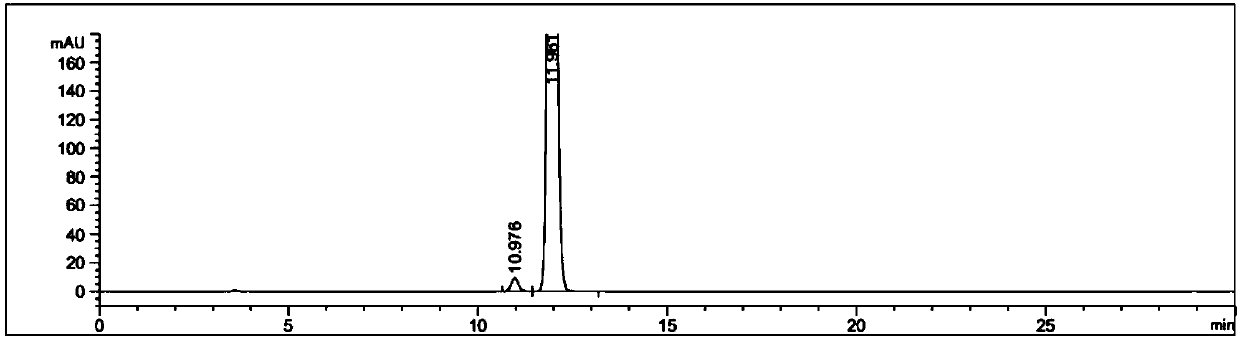
Detection method and application of silodosin enantiomer
The invention belongs to the field of medicines, and particularly relates to detection method and application of silodosin enantiomer. The method comprises the steps of preparing a silodosin test solution and a contrast solution; preparing a system adaptability solution of silodosin and the silodosin enantiomer; and performing reversed-phase high-performance liquid chromatography on the test solution, the contrast solution and the system adaptability solution, wherein the detection condition is as follows: a chromatographic column comprises a chiral chromatographic column taking silica gel surface covalently bonded with amylase-tri(3-chlorine-5-methyl phenyl carbamate) as a filling agent, a mobile phase comprises diammonium phosphate solution-acetonitrile, the flowing speed of the mobile phase is 0.7mL / min, the detection wavelength is 270 nanometers, and the column temperature of the chromatographic column is not larger than 30 DEG C. The detection method of the silodosin enantiomer, provided by the invention, is relatively good in sensitivity, relatively short in washing time, relatively small in balancing difficulty, favorable in reproducibility and favorable in durability, is convenient to operate and is of important significance to effective quality control of the silodosin.
Owner:北京华氏康源医药科技有限公司
Method for separating and determining silodosin and optical isomer thereof by virtue of liquid chromatography
The invention discloses a method for separating and determining silodosin and an optical isomer thereof by virtue of liquid chromatography. The method comprises the following steps: preparing system suitability test solution; preparing reference solution; preparing test solution; and performing chromatography, namely taking system suitability solution, injecting into a liquid chromatograph, and recording a chromatogram map I; taking the reference solution, injecting into the liquid chromatograph, and regulating detection sensitivity; and taking the test solution and the reference solution, respectively injecting into the liquid chromatograph, recording chromatogram maps II and III, and analyzing and calculating according to the chromatogram maps I, II and III. The method disclosed by the invention can simply, rapidly and accurately separate and detect the silodosin and the optical isomer thereof.
Owner:ANHUI QINGYUN PHARMA & CHEM
Preparation method of silodosin intermediate
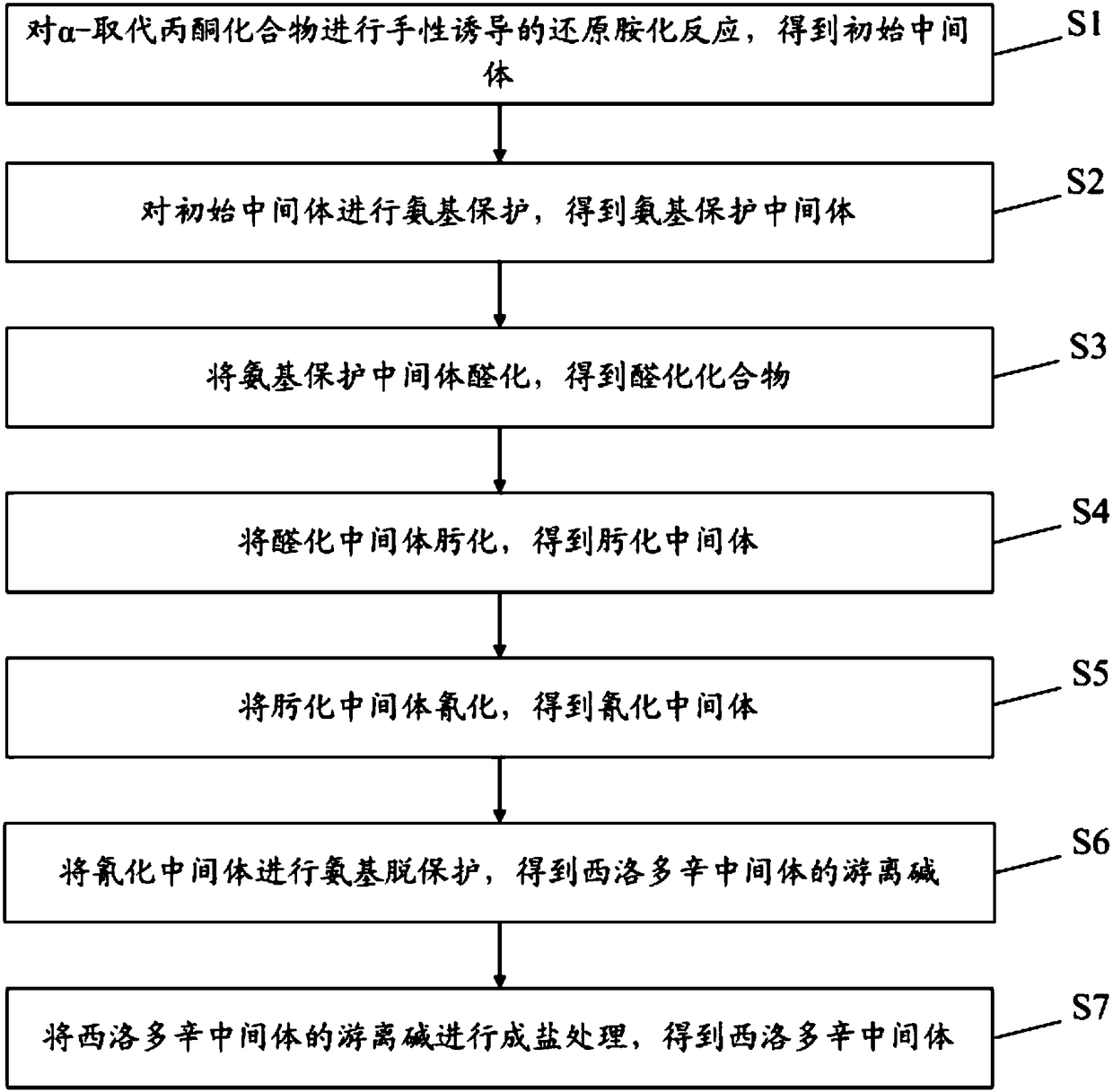
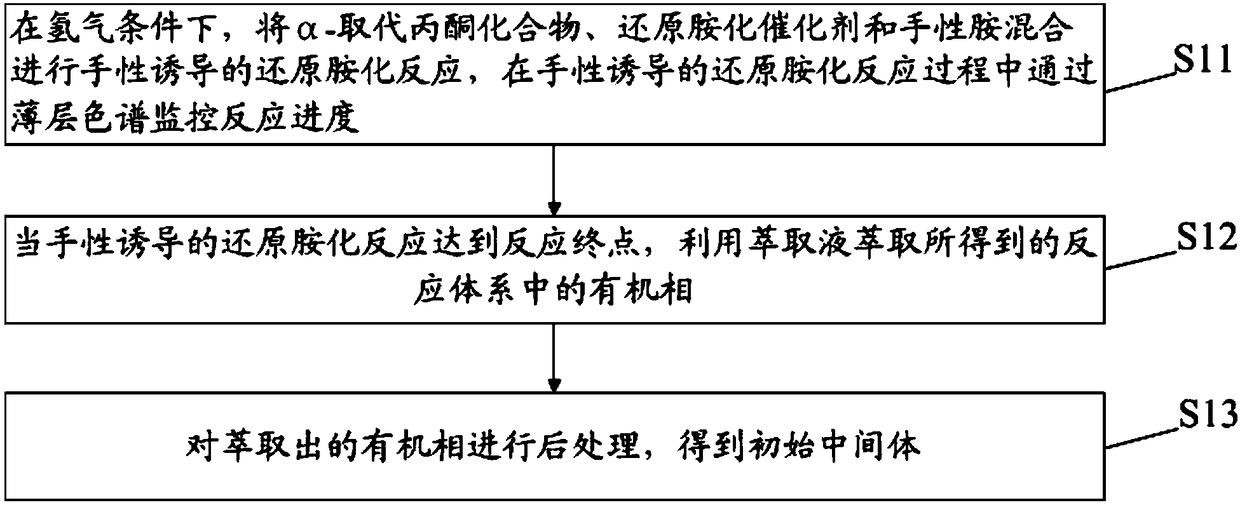
ActiveCN109305932AHigh yieldAtom economy is highOrganic chemistry methodsCarboxylic acid salt preparationFree baseChemistry
The invention discloses a preparation method of a silodosin intermediate and relates to the technical field of medical synthesis, aiming at solving the problem in an existing preparation method that the yield of the silodosin intermediate is low. The preparation method of the silodosin intermediate comprises the following steps: carrying out chirally-induced reductive amination on an alpha-substituted acetone compound to obtain an initial intermediate; carrying out amino protection on the initial intermediate to obtain an amino protected intermediate; carrying out hydroformylation on the aminoprotected intermediate to obtain a hydroformylated intermediate; carrying out oximation on the hydroformylated intermediate to obtain an oximated intermediate; carrying out cyaniding on the oximatedintermediate to obtain a cyanated intermediate; carrying out amino deprotection on the cyanated intermediate to obtain free alkali of the silodosin intermediate; carrying out salt forming treatment onthe free alkali of the silodosin intermediate to obtain the silodosin intermediate. The preparation method of the silodosin intermediate, provided by the invention, is used for preparing silodosin.
Owner:FUYANG XINYIHUA MATERIAL TECH
Non-hormonal compositions and methods for male contraception
ActiveUS10912762B2Ensure convenienceEnsures reversibilityOrganic active ingredientsGranular deliverySide effectPhysiology
Owner:PHARMAJOR INT
A kind of synthetic method of silodosin
ActiveCN103554003BAvoid generatingAvoid splittingOrganic chemistryTriethylsilaneUltimate tensile strength
Owner:LIANYUNGANG GUIKE PHARMA
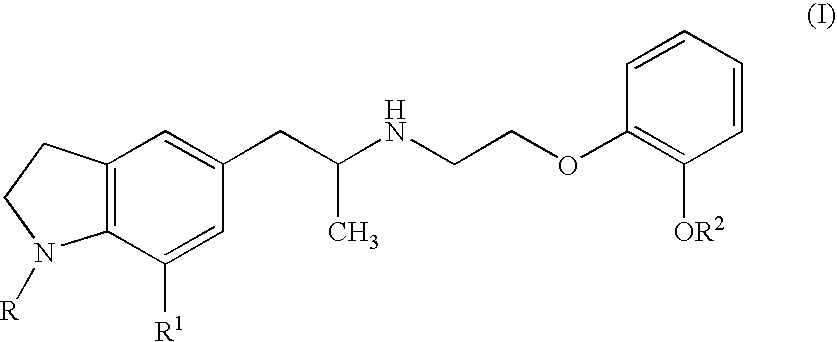
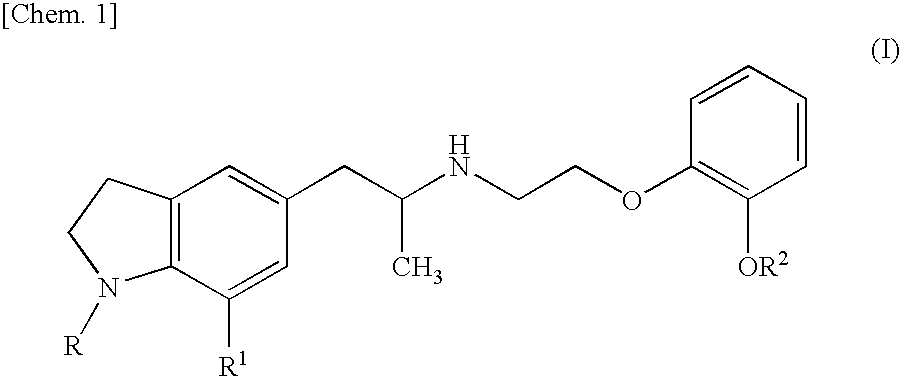
Preventive and/or therapeutic agent for urine collection disorder accompanying lower urinary tract obstruction
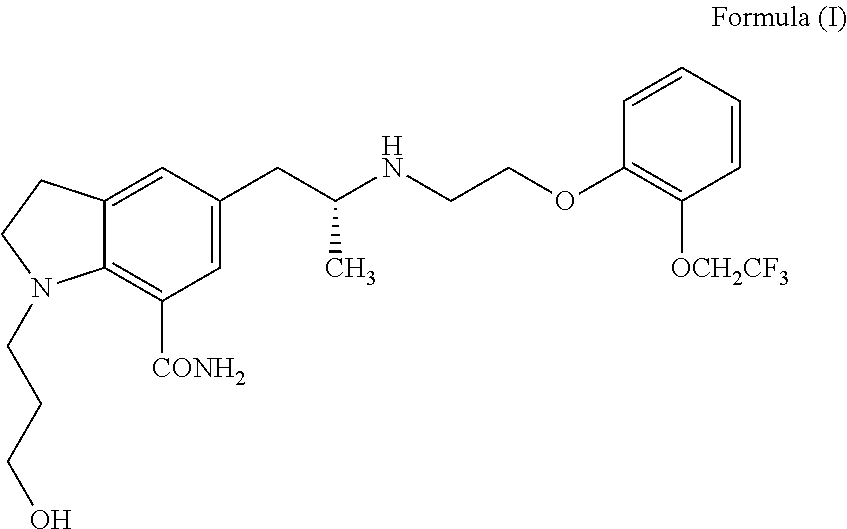
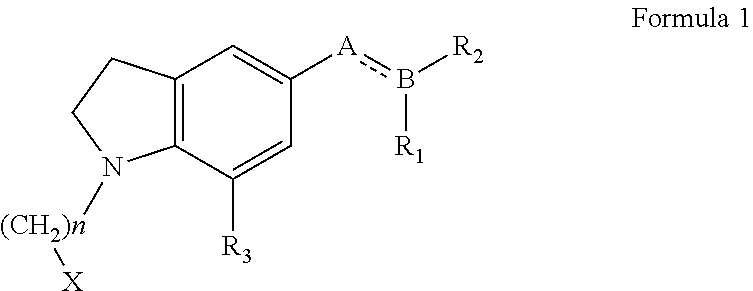
An agent for the prevention and / or treatment of urine collection disorders associated with lower urinary tract obstructive disease, characterized by containing an indoline derivative represented by the following general formula (I):wherein R; R1; and R2 are defined in the specification. The derivative and salt are usable as an agent for the prevention and / or treatment of urine collection disorders associated with lower urinary tract obstructive disease. Silodosin is the preferred indoline derivative.
Owner:KISSEI PHARMA
Preparation method of silodosin intermediate
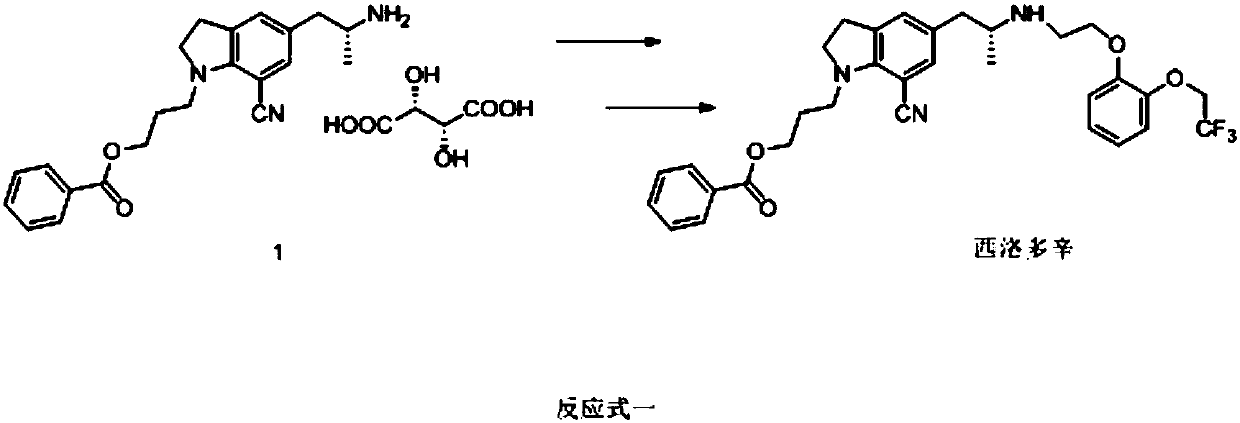
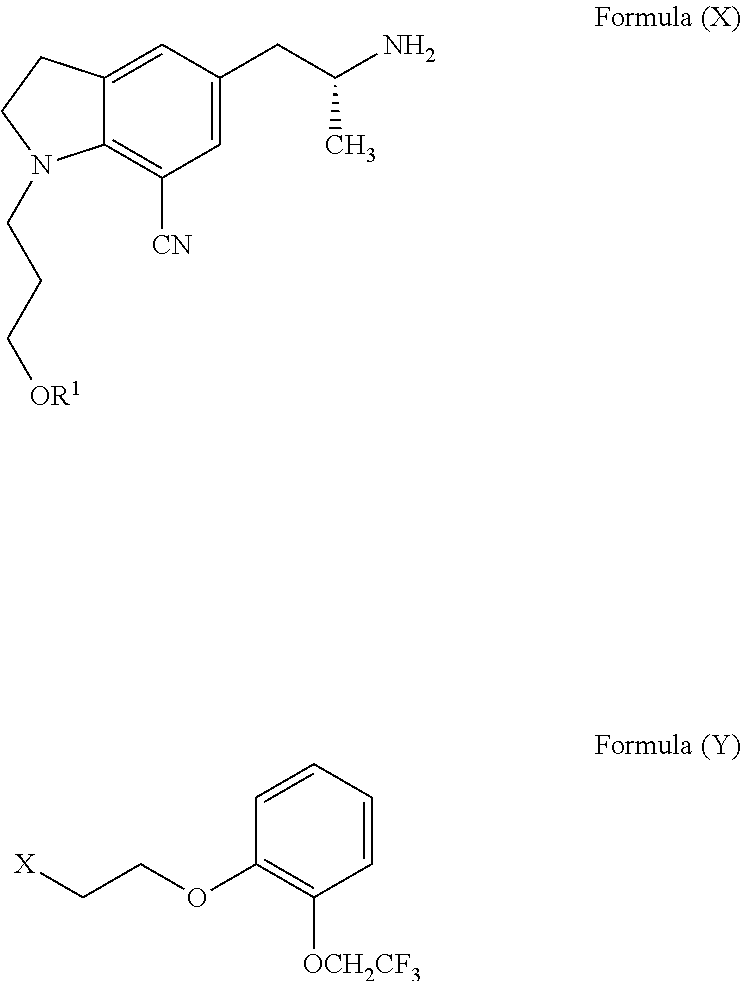
The invention relates to a preparation method of a silodosin intermediate. The preparation method is characterized in that: the intermediate is 1-(3- hydroxypropyl)-5-[(2R)-2-[2-[2-(2,2,2-trifluoroethoxyl] phenoxyl] ethylamino] propyl]-7-nitrile-1H-indoline. The preparation method is shown in the description. The raw materials used in the method are sold on the market, are cheap, and are low in cost. The yield in various step is relatively high, and the post-processing is simple. The preparation method does not use poisonous or hazardous agent, and is convenient for industrialization. The product quality is high, and the optical purity is high.
Owner:CHANGZHOU RUIMING PHARMA
A kind of preparation method for the indoline derivative of synthetic silodosin
ActiveCN106380438BHigh optical purityChemical cost reductionOrganic chemistryBenzoic acidPalladium on carbon
Owner:江苏宇田医药有限公司
Preparation method of silodosin intermediate
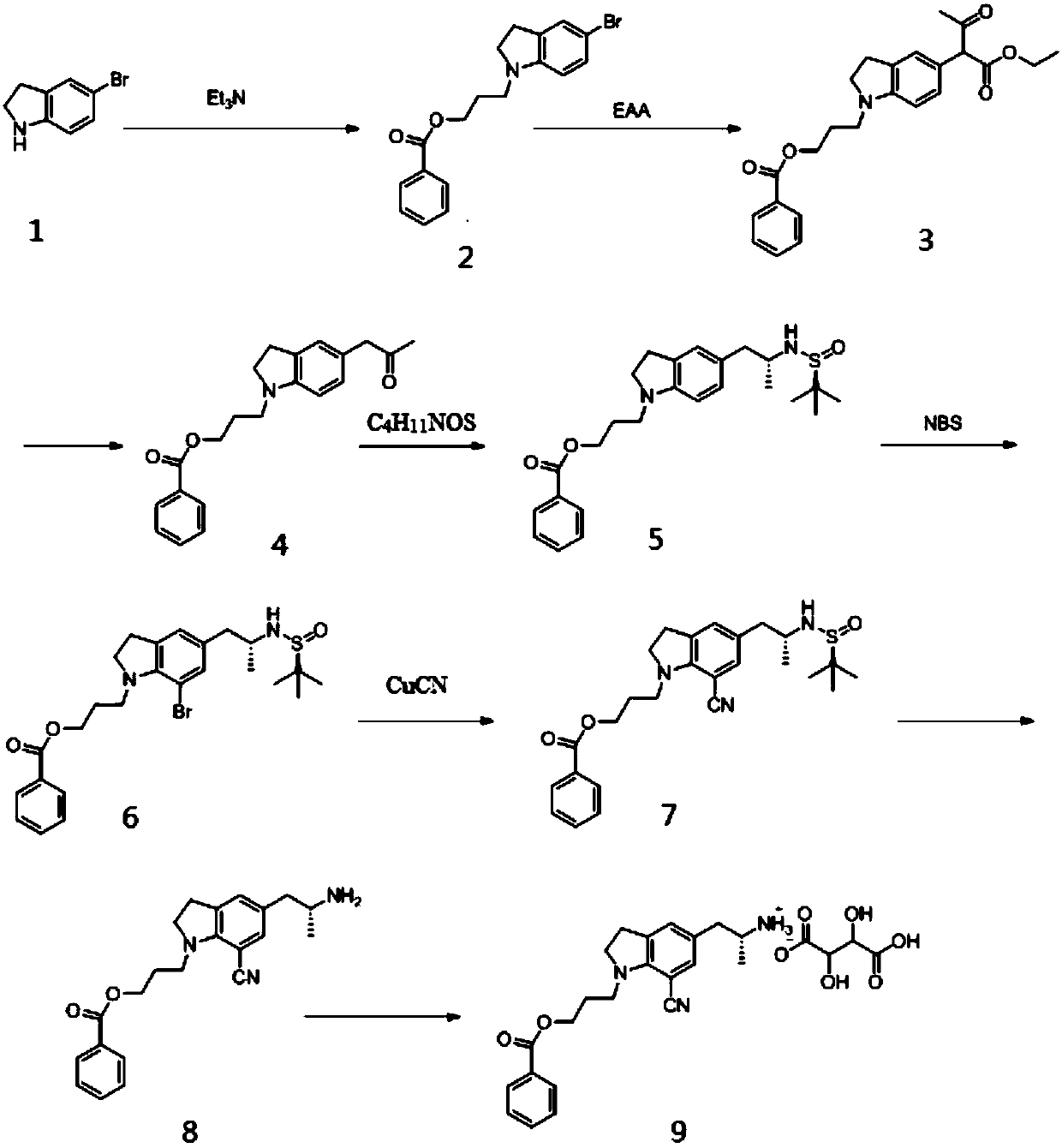
The invention discloses a preparation method of a silodosin intermediate. The preparation method includes: allowing 5-bromoindoline solution and triethylamine to be in alkylation reaction to obtain asubstance A; allowing a solution of the substance A, ethyl acetoacetate, catalyst and acid-binding agent to be in substitution reaction to obtain a substance B; allowing a solution of the substance Band Lewis acid to be in decarboxylic reaction to obtain a substance C; allowing a solution of the substance C, R-tert butyl sulfonamide, catalyst and reducer to be in amination reaction to obtain a substance D; allowing a solution of the substance D and N-bromosuccinimide solution to be in bromination reaction to obtain a substance E; allowing a solution of the substance E and cuprous cyanide to be in cyanation reaction to obtain a substance F; allowing a solution of the substance F and acid to be in acidolysis reaction to obtain a substance G; preparing the substance G into tartrate to obtainthe silodosin intermediate. The preparation method is simple, high in yield, low in energy consumption and environment-friendly; the silodosin intermediate prepared by the method is high in quality and suitable for large-scale industrial production.
Owner:ANHUI QINGYUN PHARMA & CHEM
Process for the preparation of indoline derivatives and their intermediates thereof
InactiveUS20120165548A1Organic compound preparationCarbonyl compound separation/purificationIndolineSolvent
Processes for the preparation of Silodosin and its intermediates comprising reductive amination of compound of Formula (VIII) with a compound of Formula (VII) or a compound of Formula (XV) in a suitable solvent using a reducing agent.
Owner:SANDOZ AG
Method for preparing silodosin and intermediate thereof
ActiveUS9745264B2Increase valueHigh yieldSulfonic acids salts preparationCarboxylic acid salt preparationOrganic acidAlcohol
Provided is a method for preparing silodosin. Also provided is a method for preparing an organic acid salt of a new intermediate 3-(7-cyano-5-((R)-2-((R)-1-phenylethylamino)propyl)-1-hydrogen-indolyl) propyl alcohol (ester or ether), and a new intermediate 3-(7-cyano-5-((R)-2-(((R)-1-phenethyl)(2-(2-(trifluoroethoxy)phenoxy) ethyl)amino)propyl)1-hydrogen-indolyl)propyl alcohol (ester or ether) and a salt thereof. The method has the following advantages: raw materials are cheap and easy to obtain, the operation is simple, the intermediate and product are easy to purify, the yield is high, and the method is applicable to industrial production.
Owner:SHANGHAI SYNCORES TECH INC +1
Method for preparing silodosin chiral intermediate
The present invention provides a method for synthesizing a silodosin intermediate with a high enantiomeric purity. The method comprises the steps of using 1-[3-(benzyloxy)propyl]-5-bromoindoline as the raw material, performing a bromine lithium exchange reaction with an organolithium reagent to obtain 1-[3-(benzyloxy)propyl]-5-lithiumindoline, performing a Weinreb amidization reaction with (R)-N-(alkoxycarbonyl)alanine to obtain the silodosin intermediate with a good yield and enantioselectivity. The method has the advantages of simple operation, cheap and easily-available raw materials, highenantiomeric purity of the product and no resolution step, and has extremely high application value for industrial preparation of silodosin.
Owner:SUN YAT SEN UNIV
N-haloalkylindoline intermediates, their process and use in preparation of Silodosin and its derivatives
Owner:MANKIND PHARMA LTD
Preparation method of silodosin intermediate
ActiveCN109516933AHigh purityNew routeOrganic compound preparationSulfonic acid esters preparationChemical synthesisBenzaldehyde
The invention discloses a preparation method of a silodosin intermediate and relates to the technical field of chemical synthesis of drugs. The preparation method comprises the following steps: subjecting salicyaldehyde and ethylene carbonate to transesterification to obtain 2-(2-hydroxyethoxy)benzaldehyde; then, carrying out a Dakin oxidation reaction to obtain sodium 2-(2-hydroxyethoxy) phenol;then, subjecting sodium 2-(2-hydroxyethoxy) phenol and trifluoroethanol to an etherification reaction to obtain 2-[2-(2,2,2-trifluoroethyoxy)phenoxy]ethyl alcohol; and finally, subjecting 2-[2-(2,2,2-trifluoroethyoxy)phenoxy]ethyl alcohol and methanesulfonyl chloride to an esterfication reaction to obtain the silodosin intermediate 2-[2-(2,2,2-trifluoroethyoxy)phenoxy]ethyl methanesulfonate. The preparation method is novel and short in synthesis route, and a target product can be prepared by only four-step reaction. Both raw materials and reagents used for preparation are cheap, available andenvironment-friendly, all the reaction conditions are mild and controllable, the preparation method is convenient and simple in operation, and the prepared silodosin intermediate is high in purity andyield, suitable for industrial production and wide in prospect and industrial application value.
Owner:ANHUI QINGYUN PHARMA & CHEM
Method for preparing silodosin and intermediate thereof
ActiveUS20160304452A1Low costIncrease valueOrganic compound preparationSulfonic acids salts preparationOrganic acidAlcohol
Provided is a method for preparing silodosin. Also provided is a method for preparing an organic acid salt of a new intermediate 3-(7-cyano-5-((R)-2-((R)-1-phenylethylamino)propyl)-1-hydrogen-indolyl) propyl alcohol (ester or ether), and a new intermediate 3-(7-cyano-5-((R)-2-(((R)-1-phenethyl)(2-(2-(trifluoroethoxy)phenoxy) ethyl)amino)propyl)1-hydrogen-indolyl)propyl alcohol (ester or ether) and a salt thereof. The method has the following advantages: raw materials are cheap and easy to obtain, the operation is simple, the intermediate and product are easy to purify, the yield is high, and the method is applicable to industrial production.
Owner:SHANGHAI SYNCORES TECH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com