Preparation method of silodosin intermediate
A technology of silodosin and intermediates, applied in the field of pharmaceutical preparation, can solve problems such as high production cost, serious production pollution, and potential safety hazards in industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
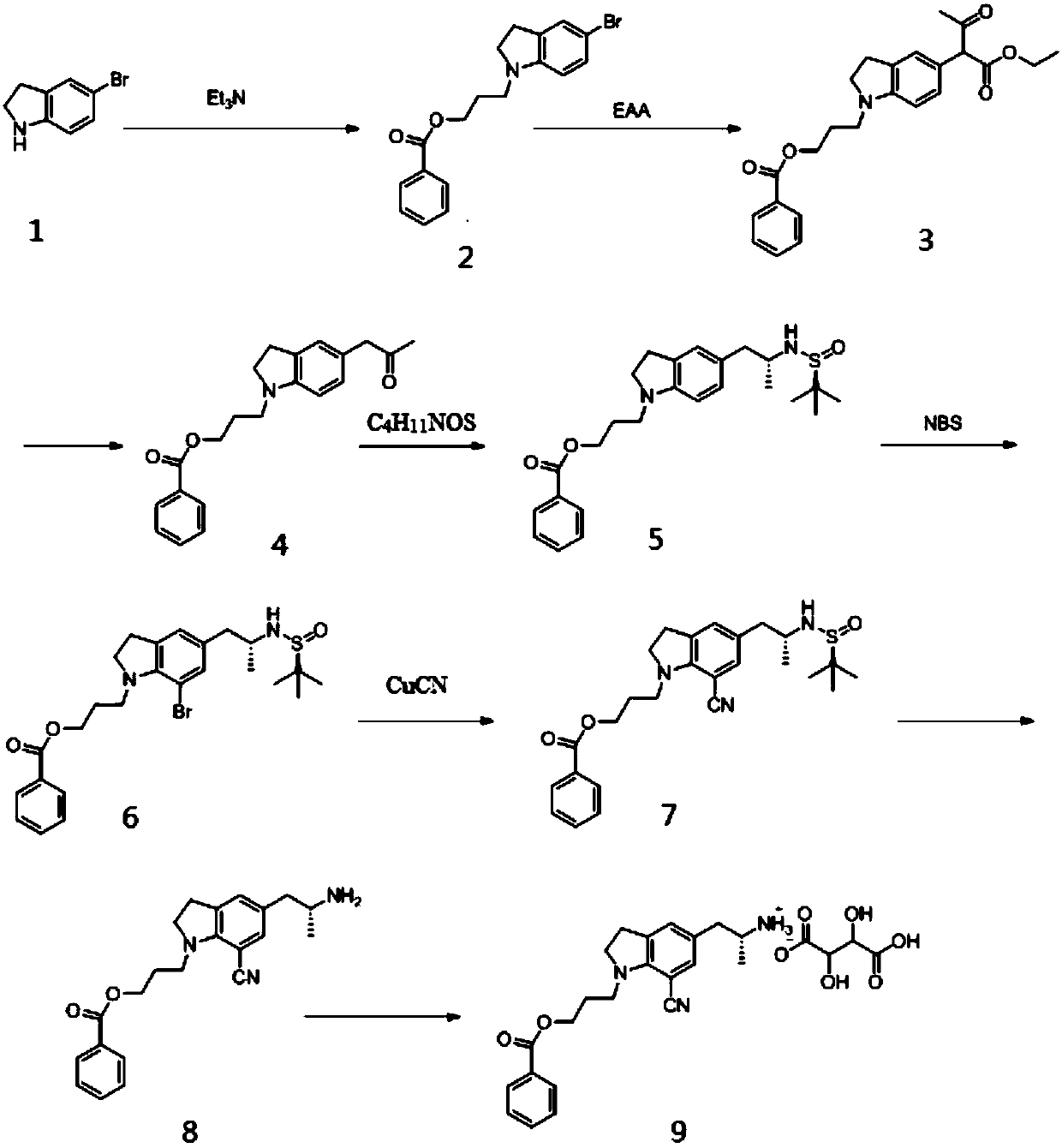
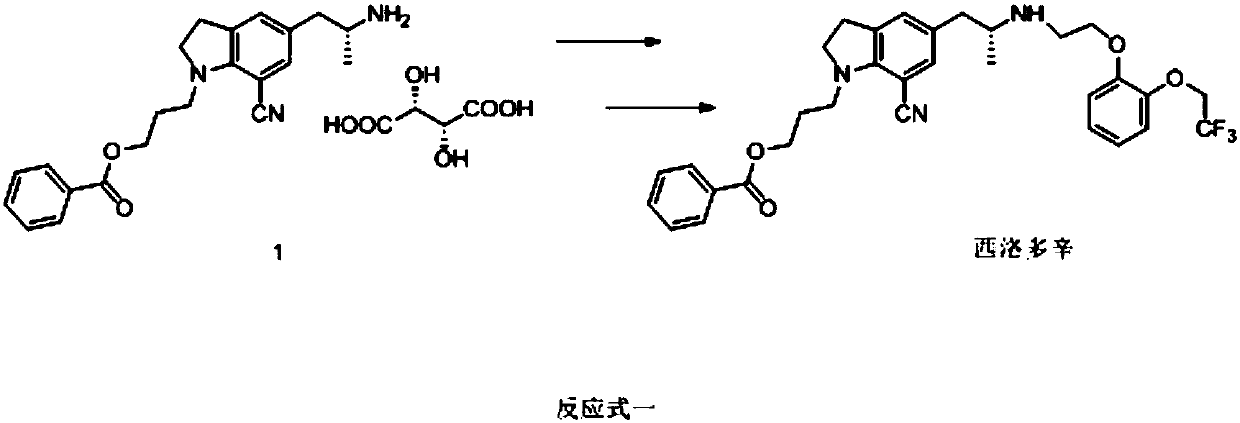
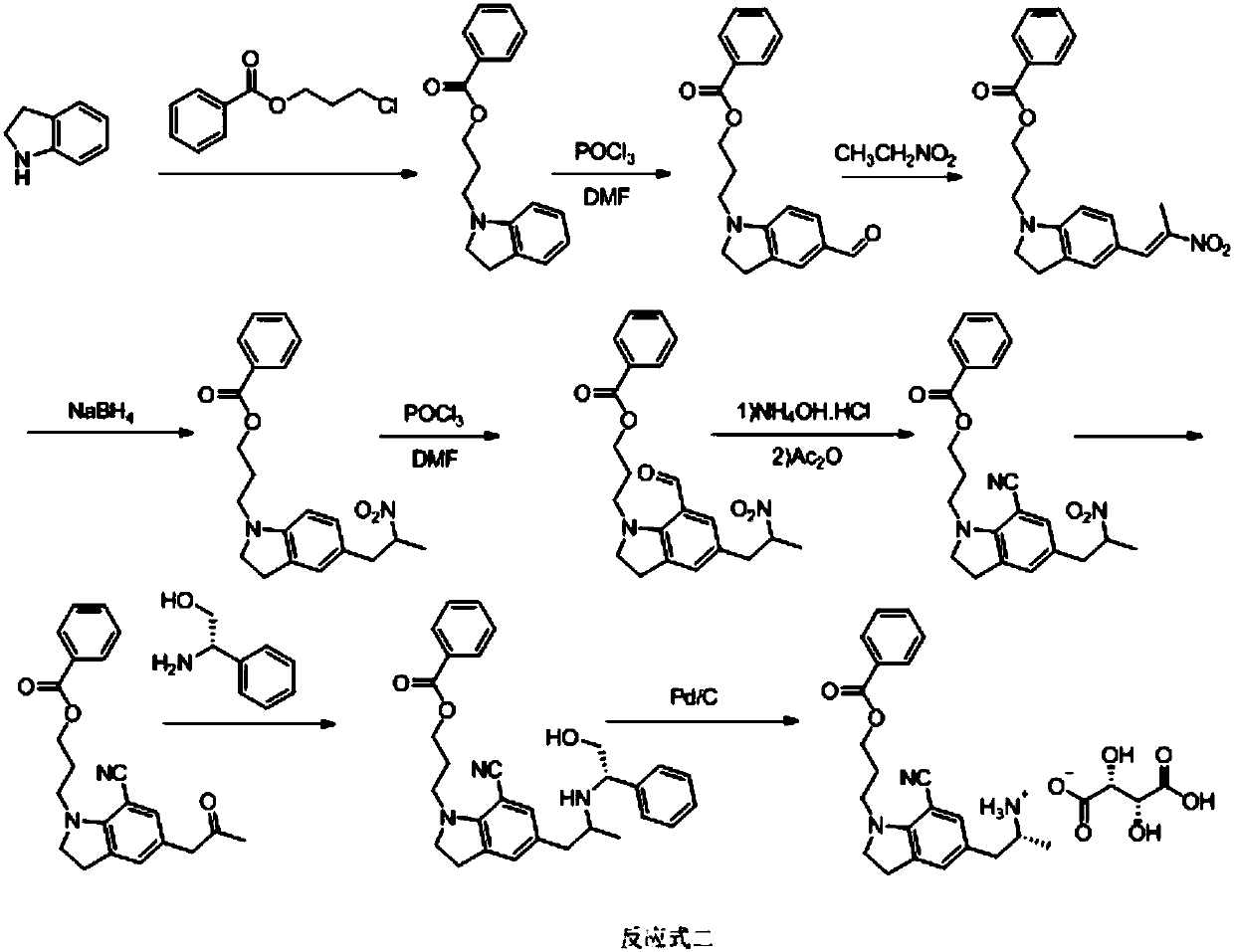
[0102] refer to figure 1 , the preparation method of a kind of silodosin intermediate provided by the invention, described method comprises the steps:
[0103] S1. Alkylation reaction of 5-bromoindoline solution and triethylamine to obtain substance A;
[0104] S2, performing a substitution reaction on the solution of substance A, ethyl acetoacetate, a catalyst, and an acid-binding agent to obtain substance B;
[0105] S3, performing a decarboxylation reaction on the solution of substance B and a Lewis acid to obtain substance C;
[0106] S4. Perform amination reaction of the solution of substance C, R-tert-butylsulfinamide, catalyst and reducing agent to obtain substance D;
[0107] S5, performing a bromination reaction on the solution of the substance D and the N-bromosuccinimide solution to obtain the substance E;
[0108] S6, subjecting the solution of substance E and cuprous cyanide to cyanation reaction to obtain substance F;
[0109] S7, subjecting the solution of s...
Embodiment 1
[0113] A kind of preparation method of silodosin intermediate, described method comprises the steps:
[0114] S1. Alkylation reaction of 5-bromoindoline solution and triethylamine to obtain substance A;
[0115] S2, performing a substitution reaction on the solution of substance A, ethyl acetoacetate, a catalyst, and an acid-binding agent to obtain substance B;
[0116] S3, performing a decarboxylation reaction on the solution of substance B and a Lewis acid to obtain substance C;
[0117] S4, performing amination reaction on the solution of substance C, R-tert-butylsulfinamide, catalyst, and sodium borohydride solution to obtain substance D;
[0118] S5, performing a bromination reaction on the solution of the substance D and the N-bromosuccinimide solution to obtain the substance E;
[0119] S6, subjecting the solution of substance E and cuprous cyanide to cyanation reaction to obtain substance F;
[0120] S7, subjecting the solution of substance F and acid to acidolysis ...
Embodiment 2
[0123] A kind of preparation method of silodosin intermediate, described method comprises the steps:
[0124] S1. Alkylation reaction of 5-bromoindoline solution and triethylamine to obtain substance A;
[0125] S2, performing a substitution reaction on the solution of substance A, ethyl acetoacetate, a catalyst, and an acid-binding agent to obtain substance B;
[0126] S3, performing a decarboxylation reaction on the solution of substance B and a Lewis acid to obtain substance C;
[0127] S4. Perform amination reaction of the solution of substance C, R-tert-butylsulfinamide, catalyst and reducing agent to obtain substance D;
[0128] S5, performing a bromination reaction on the solution of the substance D and the N-bromosuccinimide solution to obtain the substance E;
[0129] S6, subjecting the solution of substance E and cuprous cyanide to cyanation reaction to obtain substance F;
[0130] S7, subjecting the solution of substance F and acid to acidolysis reaction to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com