Preparation method of silodosin intermediate
A compound and reaction technology, applied in the field of medicine, can solve the problems of complex synthesis process, high cost and low yield, and achieve the effects of high industrial application value, low purchase cost and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] 1) Preparation of Compound 9-1
[0104]
[0105] Weigh 140g of benzoyl chloride (commercially available) into a 1L single-necked bottle, add 600mL of dichloromethane and 70g of triethylamine, cool to 0°C, slowly add 94g of 3-chloropropanol dropwise, and keep the temperature below 10°C. After the dropwise addition, the reaction was continued for 1 h.
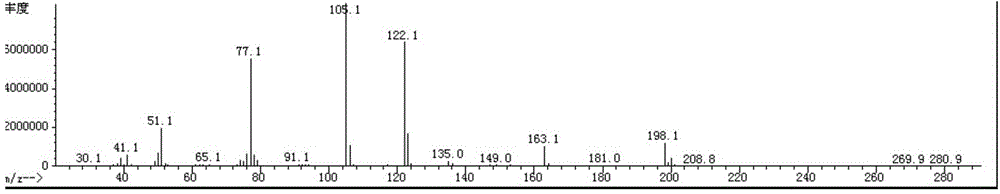
[0106] After the completion of the reaction was monitored by TLC, the organic layer was washed with 300 mL*3 water, dried over anhydrous sodium sulfate, and the solvent was concentrated to obtain 188 g of a colorless liquid (compound 9-1) with a purity of 99%. MS m / z: 198 (M + ), yield: 95%.
[0107] For the MS diagram of compound 9-1, see figure 1 .
[0108] 2) Preparation of compound 2
[0109]
[0110] Weigh 132g of compound 1 (commercially available) into a 2L three-neck flask, then add 1L of dichloromethane and 167g of triethylamine into it, cool to 0°C, slowly add acetyl chloride (130g, 1.5eq) dropwise, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com