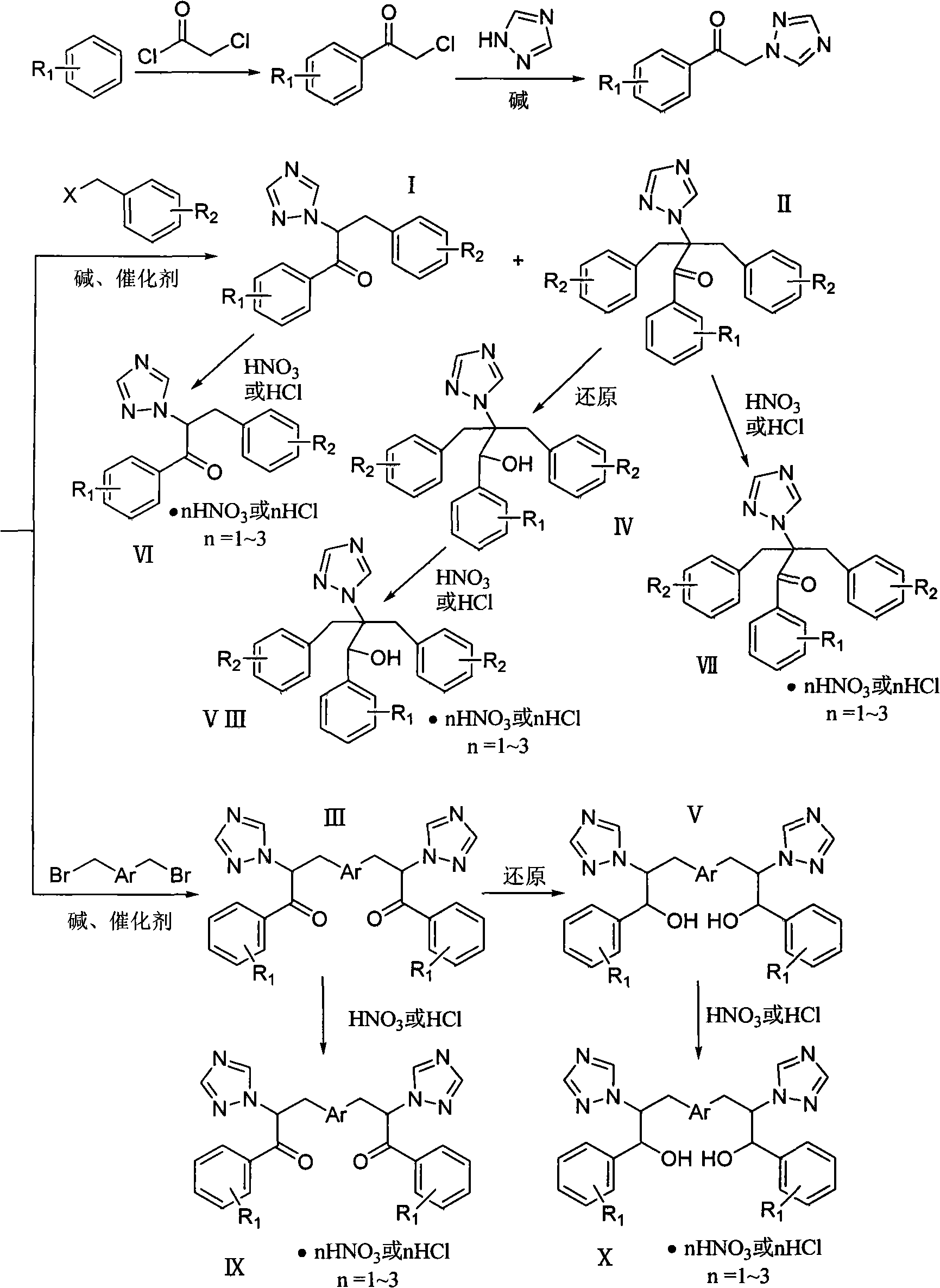
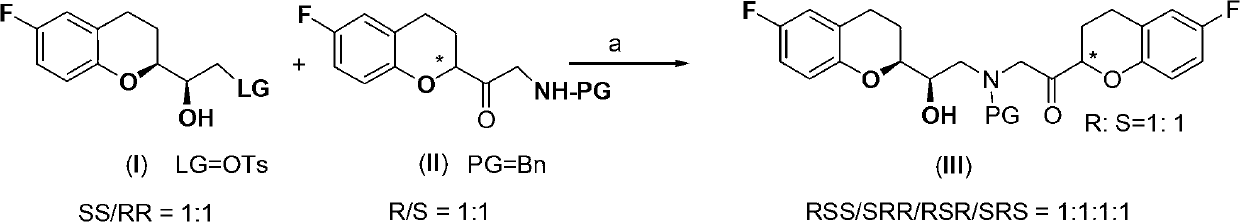
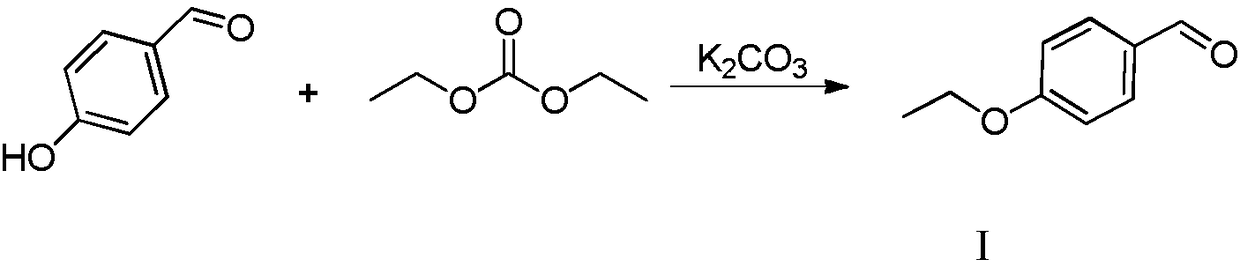
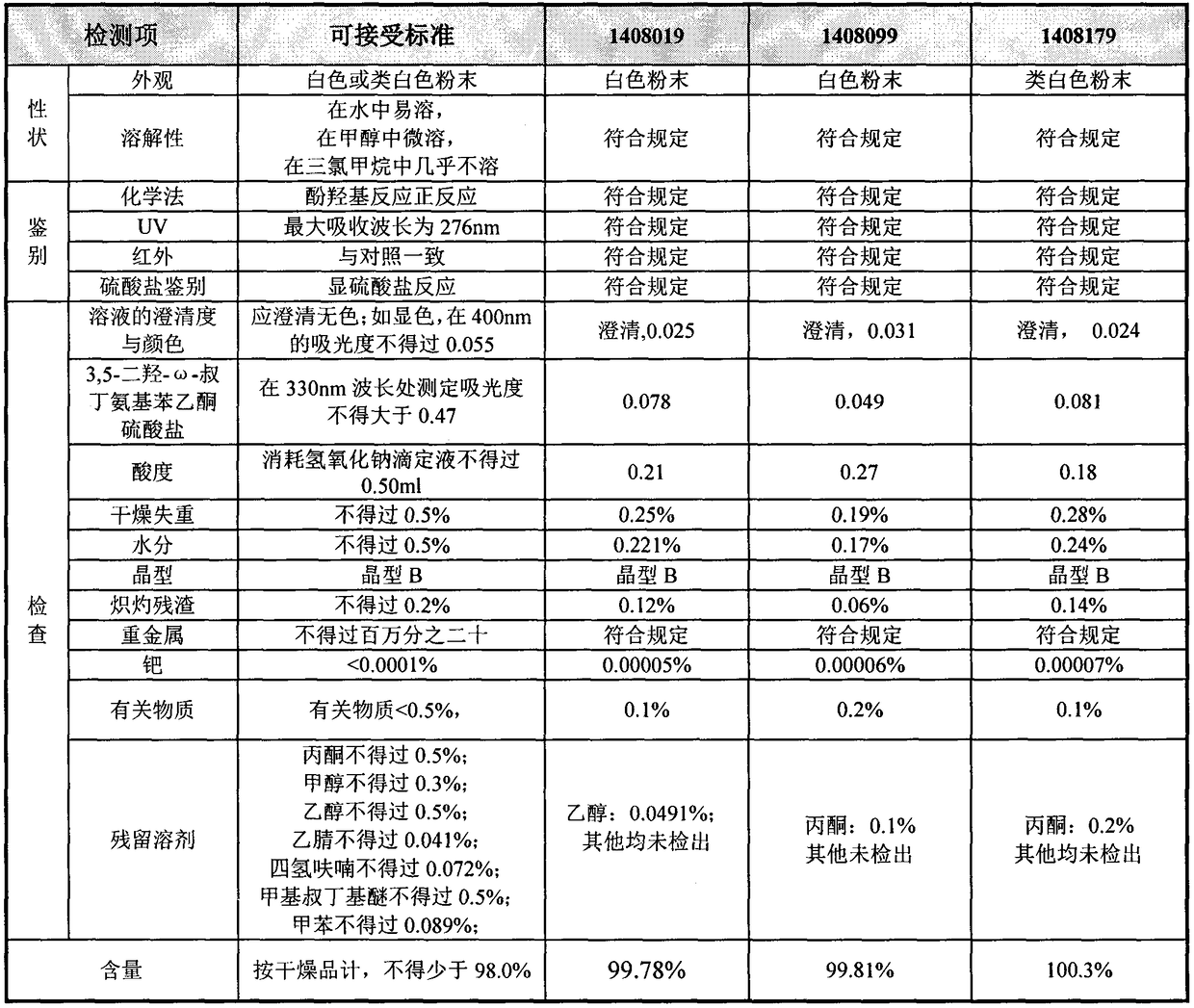
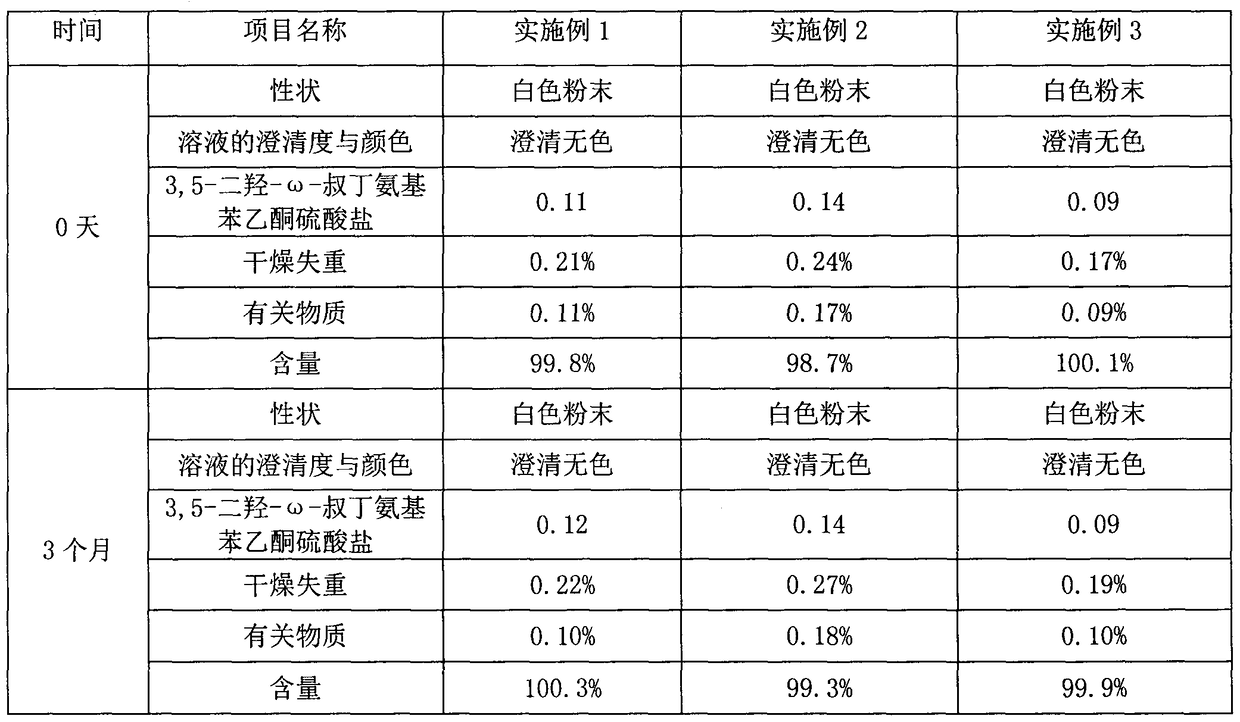
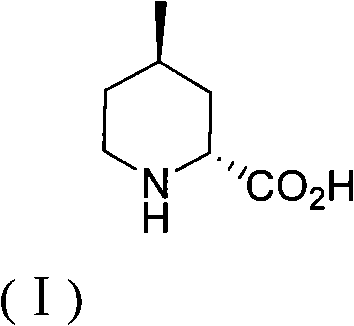Patents
Literature
175 results about "Carbonyl reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent. Typical carbonyl compounds are ketones, aldehydes, carboxylic acids, esters, and acid halides. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent; aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol can be further reduced and removed altogether.
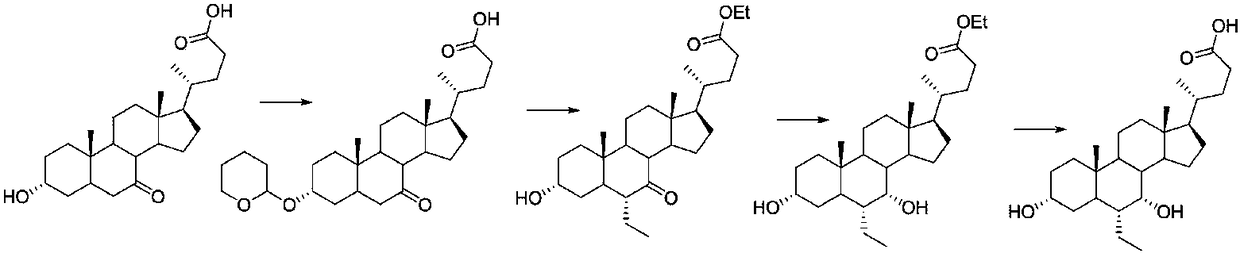
Preparation method of ursodeoxycholic acid
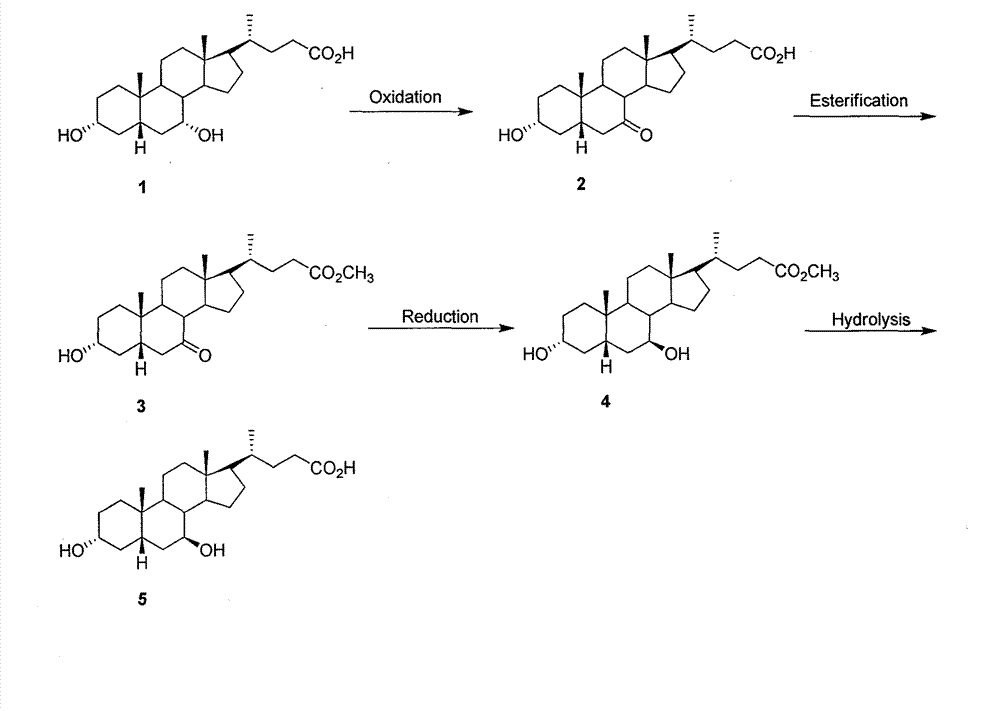
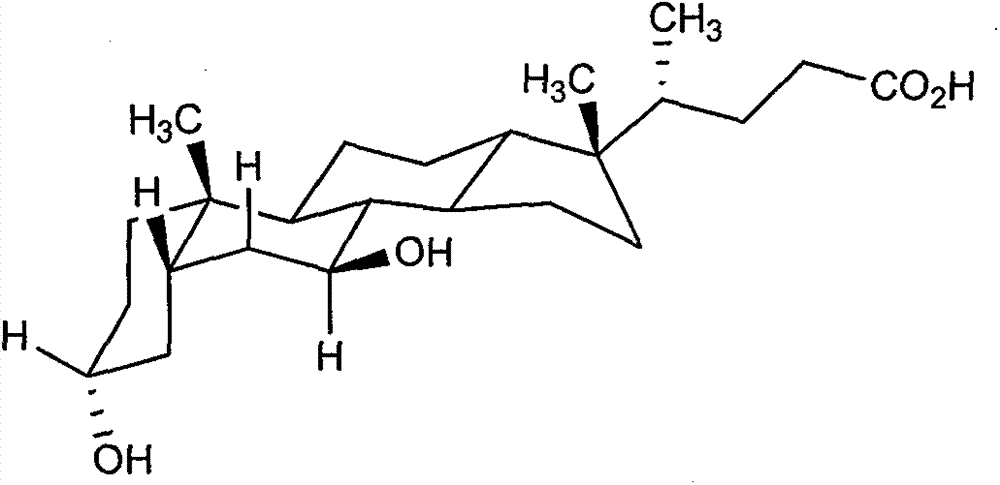
The invention provides a preparation method of ursodeoxycholic acid. Commercial available chenodeoxycholic acid is taken as raw materials, the ursodeoxycholic acid is obtained by four steps including selective oxidation, esterification, deoxidation and hydrolyzation, and the total yield is 85.7%. In a mixture of acetone and water, NBS is used for selective oxidation of hydroxy at C- 7 bit of the chenodeoxycholic acid, and the selective oxidation possesses excellent selectivity and high yield. NaBH14 / CeC13 may be used to deoxidize carbonyl at C-7 bit into hydroxy, and the ratio of alpha / beta is as high as 5 / 95.
Owner:EAST CHINA UNIV OF SCI & TECH
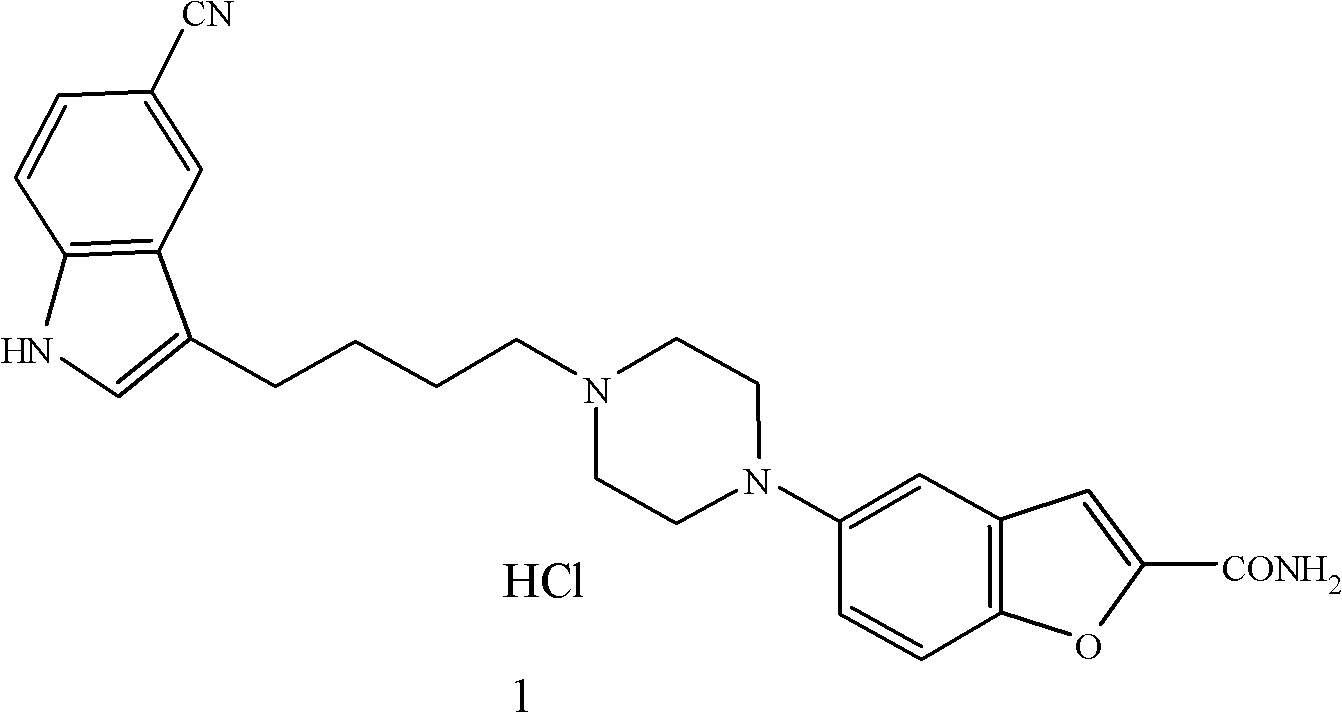
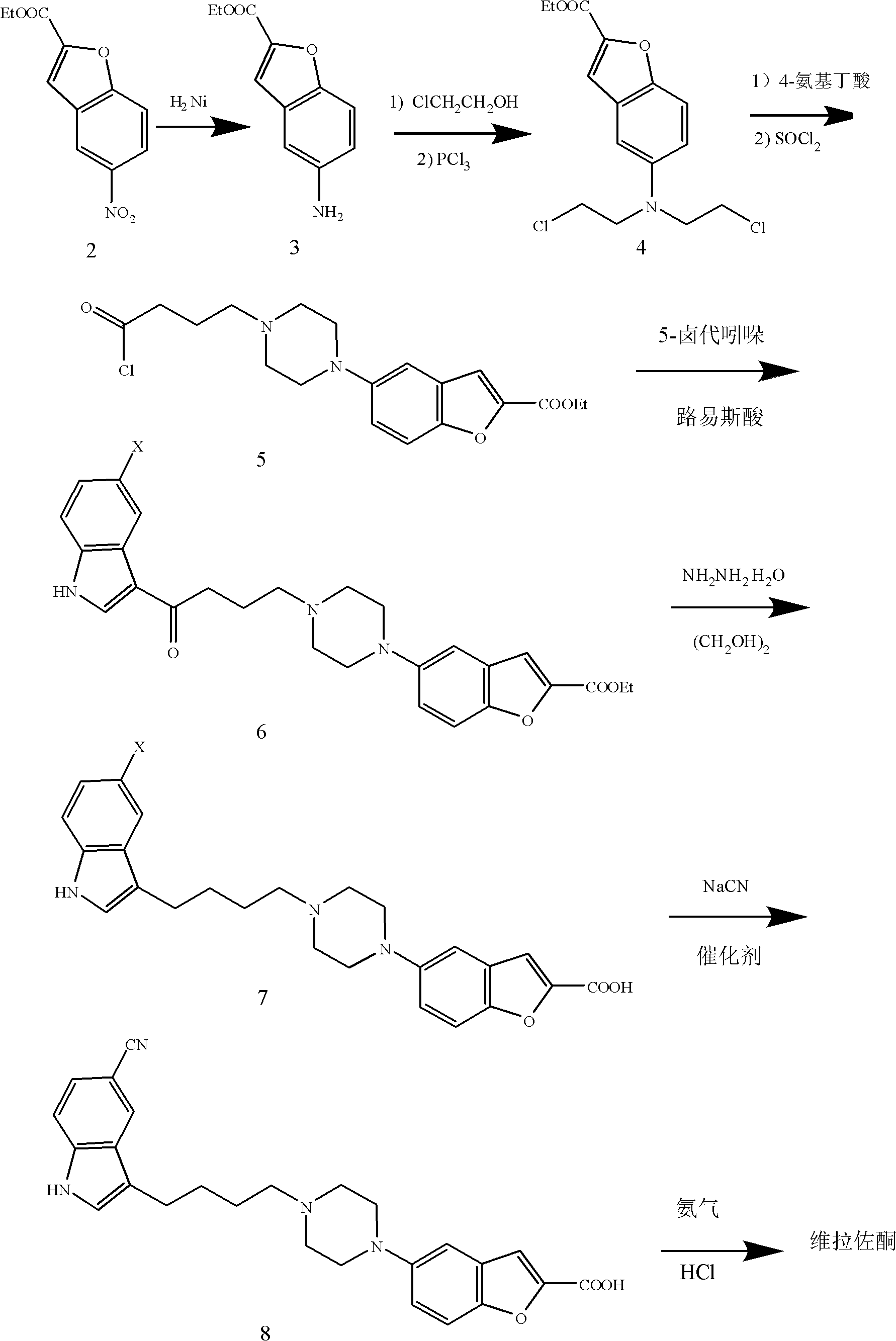
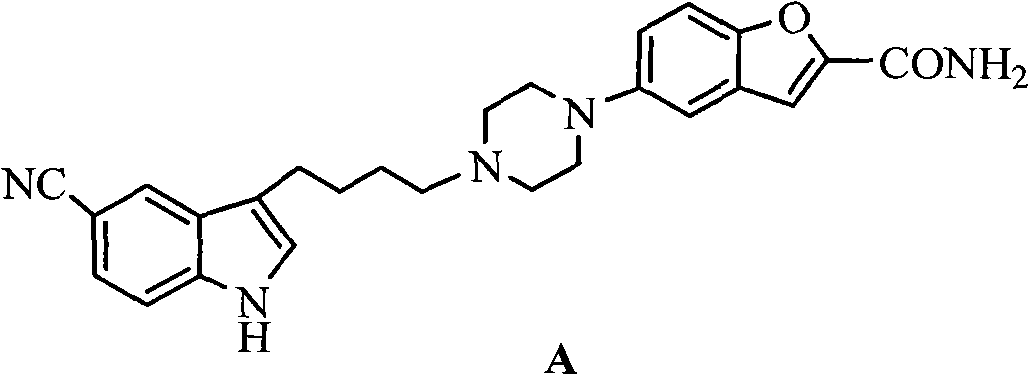
Method for preparing anti-depression medicine vilazodone
InactiveCN102180868AOvercome costsHigh reaction yieldOrganic chemistryCarboxylic acidPhosphorus trichloride
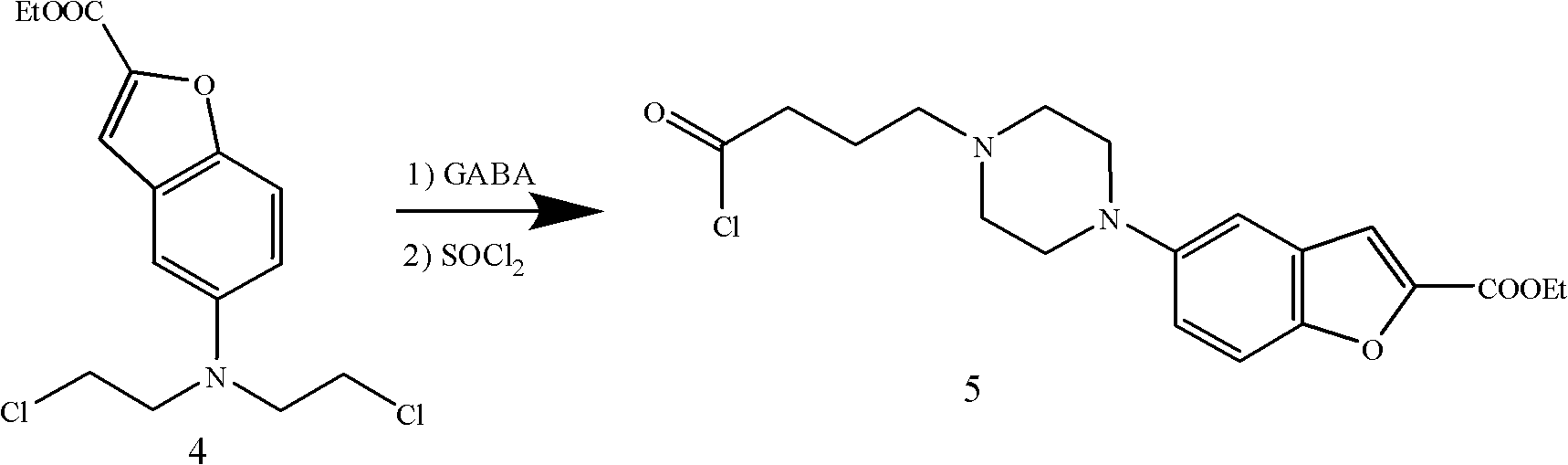
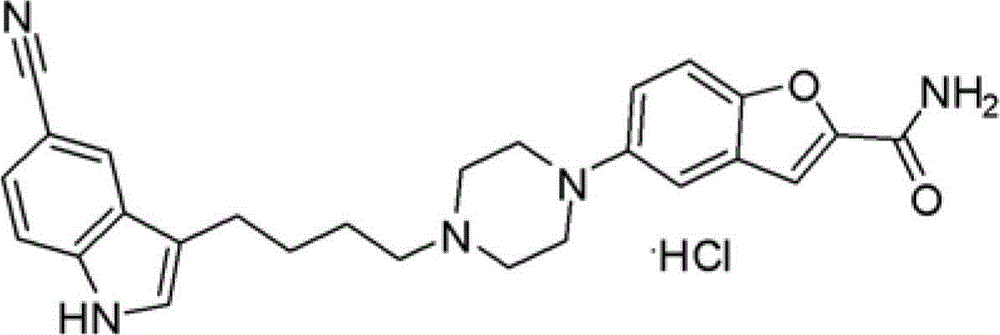
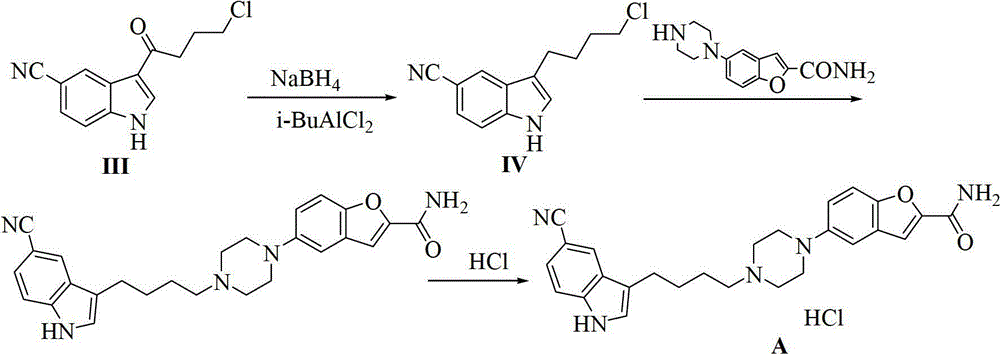
The invention provides a method for preparing an anti-depression medicine vilazodone, which comprises the following steps of: performing nitro reduction on 5-nitrobenzofuran-2-ethyl carboxylate serving as a raw material, conjugating with chlorohydrin, chlorinating by using phosphorus trichloride, cyclizing with 4-aminobutyric acid to generate a piperazine ring, performing acylchlorination, condensing with halogenated indole, performing carbonyl reduction, cyaniding, performing amidation, and the like to obtain the vilazodone. The method overcomes the defects in the prior art, is suitable for industrial production and has high application value, the used raw material is readily available, operating cost is low, and reaction yield is high.
Owner:SCI GENERAL MATERIAL & CHEM
Di-carbonyl reduction enzyme, its gene and uses thereof
InactiveCN101429514AReduction efficiently catalyzesHigh stereoselectivityOxidoreductasesGenetic engineeringBiotechnologyDICARBONYL REDUCTASE
The invention discloses a gene of dicarbonyl reductase. The nucleotide sequence of the gene has over 80 percent of homology with SEQ ID NO.1. The dicarbonyl reductase consists of an amino acid sequence having over 80 percent of homology with SEQ ID NO.2. The obtained dicarbonyl reductase can be used for a reaction of catalyzing a dicarbonyl compound to be reduced into a dicarbonyl product, and has high stereo-selectivity.
Owner:常州金隆生物医药有限公司
Method for catalytically synthesizing atazanavir intermediate
ActiveCN104911224AImprove recycling ratesImprove regeneration efficiencyChemical industryFermentationTert-Butyloxycarbonyl protecting groupButanone
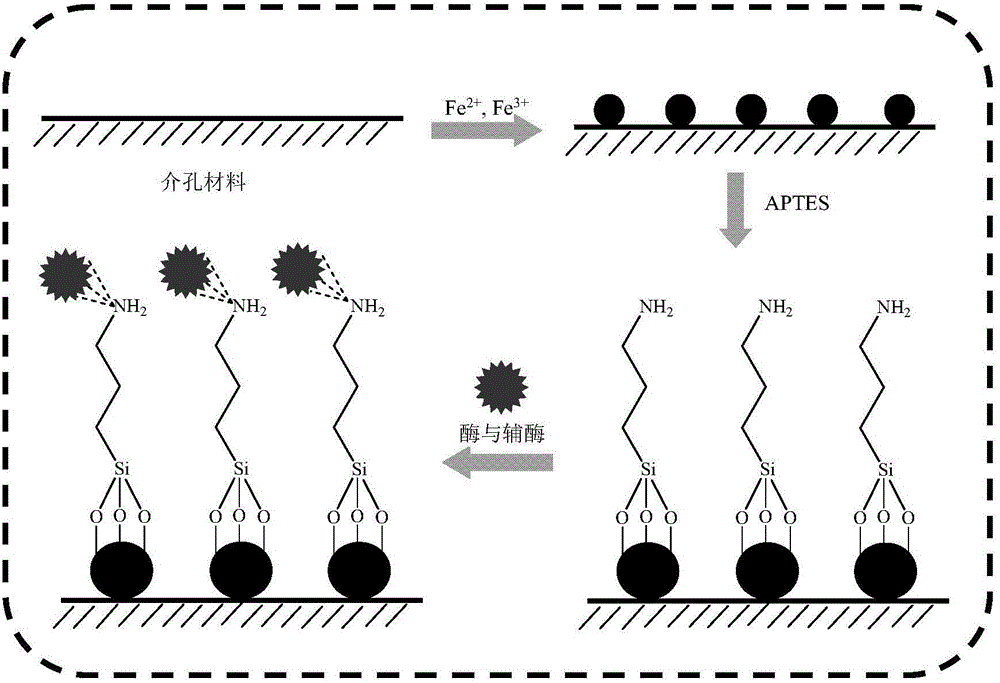
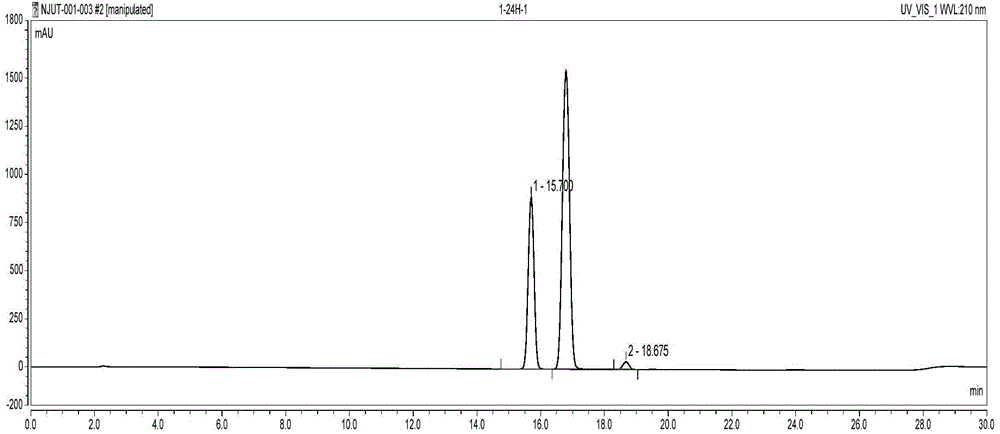
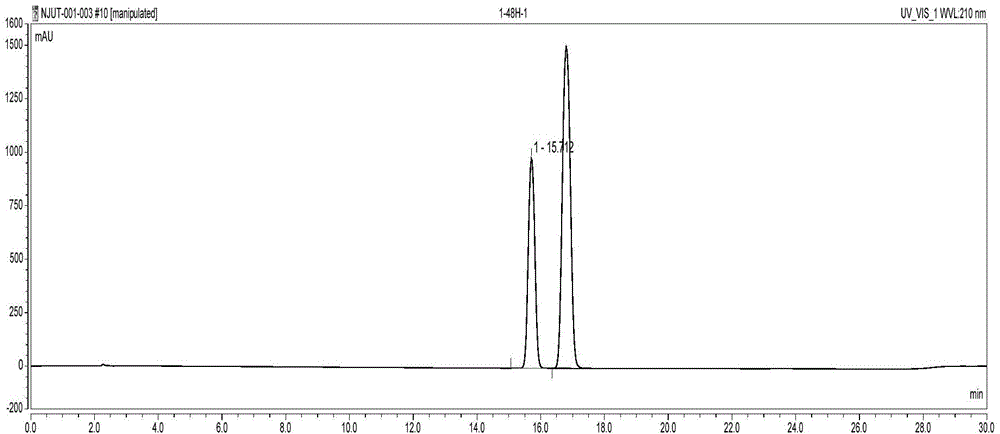
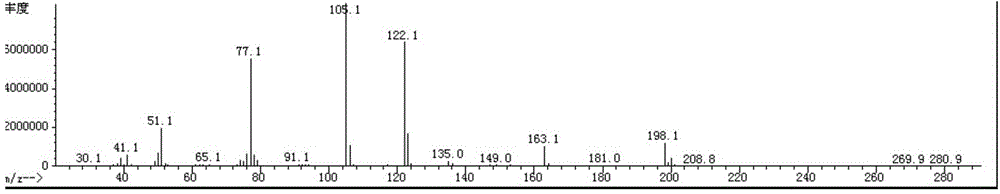
The invention discloses a method for catalytically synthesizing an atazanavir intermediate, which comprises the following steps: preparing a magnetic modified mesoporous material, co-immobilizing a carbonyl reduction enzyme and a coenzyme in the magnetic mesoporous material, and catalyzing (3S)-3-(tert-butyloxycarbonyl)amino-1-chloro-4-phenyl-(2R)-butanone by using the immobilized enzyme to generate the (3S)-3-(tert-butyloxycarbonyl)amino-1-chloro-4-phenyl-(2R)-butanol intermediate. The method has the advantages of high recovery rate of the carbonyl reduction enzyme and coenzyme, high regeneration efficiency of the coenzyme, low cost, wide market prospects and the like.
Owner:NANJING UNIV OF TECH
Preparation method of silodosin intermediate
ActiveCN104974072ALower purchase costReduce manufacturing costCarboxylic acid salt preparationIndolineCarbonyl reduction
The invention discloses a preparation method of a silodosin intermediate, wherein the structure of the silodosin intermediate is represented as the formula A. The preparation method, wherein indoline is employed as a start raw material, includes the reactions of Friedel-Crafts acylation, carbonyl reduction, Gabriel reaction and chiral resolution and the like. The preparation method is simple in operation, is low in cost, is high in yield, allows the product to purify easily and is stable in processes, and is suitable for industrial production. The invention also discloses a new intermediate compound which is related in the method.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
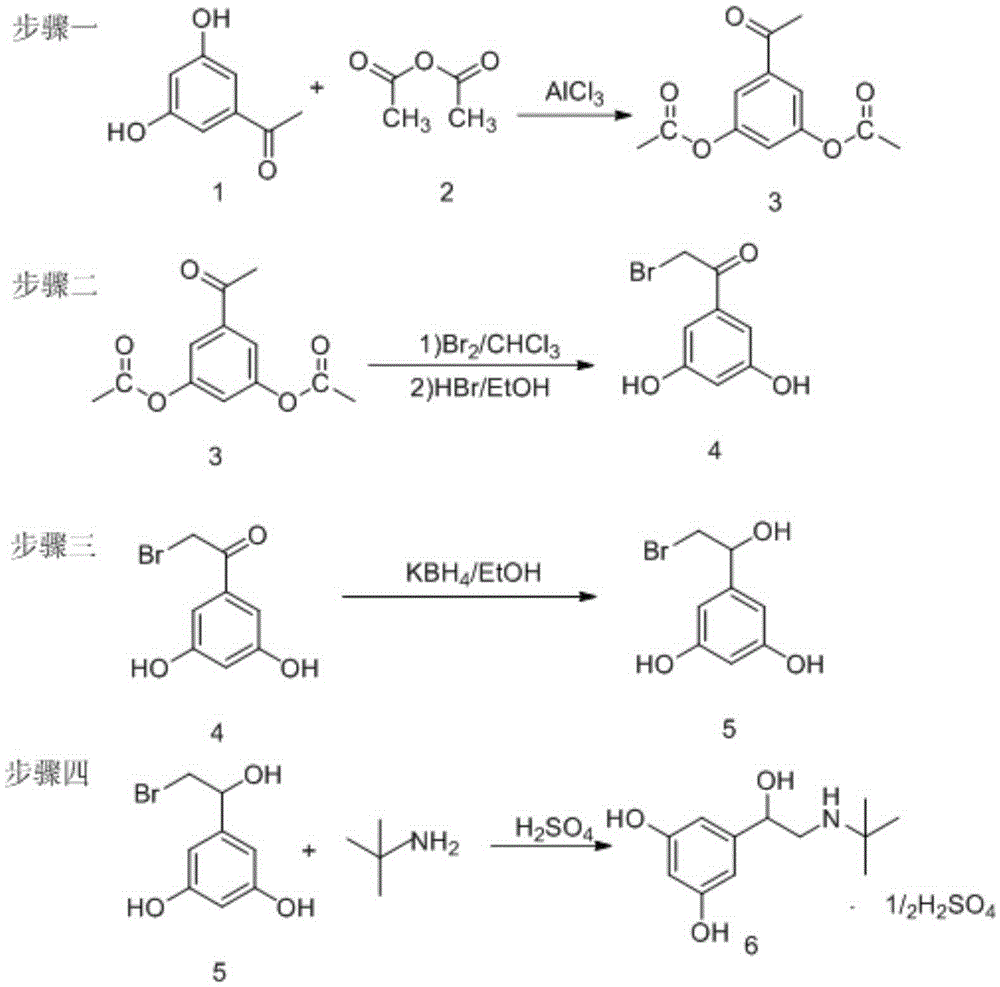
Preparation method of terbutaline sulphate
ActiveCN105254512AMild responseImprove securityAmino preparation from aminesBulk chemical productionLithiumHigh pressure
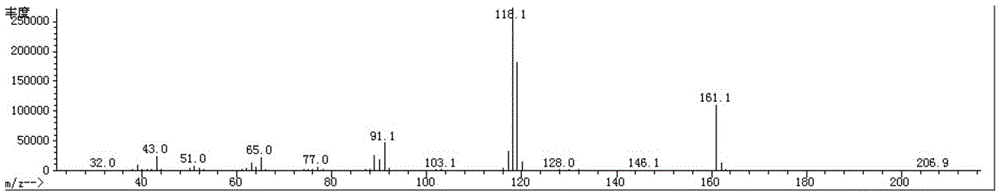
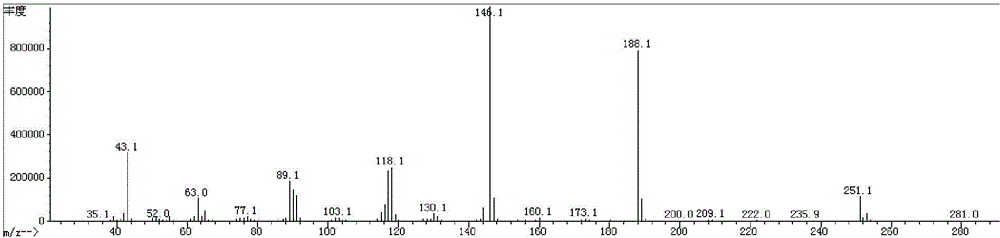
The invention relates to a preparation method of terbutaline sulphate. The technical problems that according to an existing preparation method of terbutaline sulphate, high-pressure hydrogenation and other high-risk operations, and lithium methide, azomethane and other high-risk reagents exist, and cost is high are solved through the preparation method. 3,5-resacetophenone serves as a raw material, and terbutaline sulphate is obtained through hydroxyl protection, the bromination reaction, carbonyl reduction, the condensation reaction and sulphating. The preparation method can be suitable for industrially-produced terbutaline sulphate.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
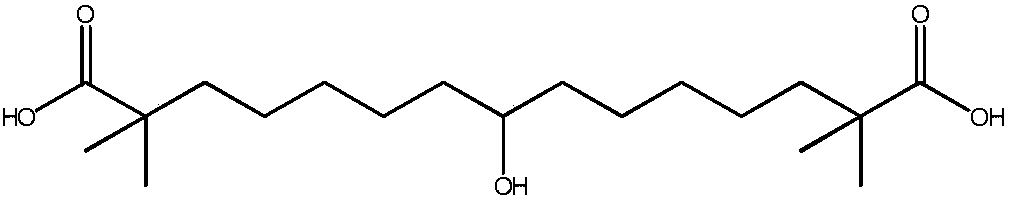
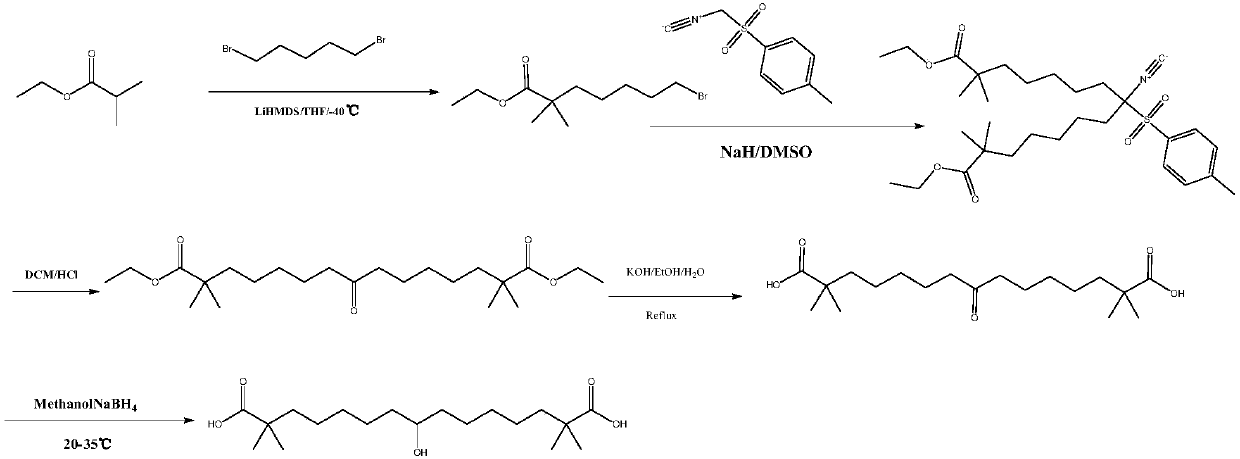
Synthetic method for 8-hydroxyl-2,2,14,14-tetramethyl-pentadecanedioic acid
PendingCN109721486AReduce cost inputReduce dosagePreparation from carboxylic acid saltsOrganic compound preparationSolventMethyl group
The invention belongs to the technical field of medicines, and specifically provides a synthetic method for 8-hydroxyl-2,2,14,14-tetramethyl-pentadecanedioic acid. The synthetic method comprises the following steps: (1) pouring 8-keto-2,2,14,14-tetramethyl-pentadecanedioic acid into purified water, and adding a proper amount of alkali until the 8-keto-2,2,14,14-tetramethyl-pentadecanedioic acid iscompletely dissolved so as to obtain 8-keto-2,2,14,14-tetramethyl-pentadecanediate; (2) adding sodium borohydride into an above-mentioned reaction solution, and carrying out a carbonyl reduction reaction under the condition of 20 to 25 DEG C so as to obtain 8-hydroxyl-2,2,14,14-tetramethyl-pentadecanediate; and (3) adding concentrated hydrochloric acid into an above-mentioned reaction solution until the pH value of the reaction solution is adjusted to 1 to 3 so as to generate the 8-hydroxyl-2,2,14,14-tetramethyl-pentadecanedioic acid. The synthetic method provided by the invention reduces theusage amount of the sodium borohydride, decreases the dangerousness of a reaction, simplifies operation, reduces the cost input amounts of a solvent and raw materials, and is applicable to industrialproduction.
Owner:LUNAN PHARMA GROUP CORPORATION
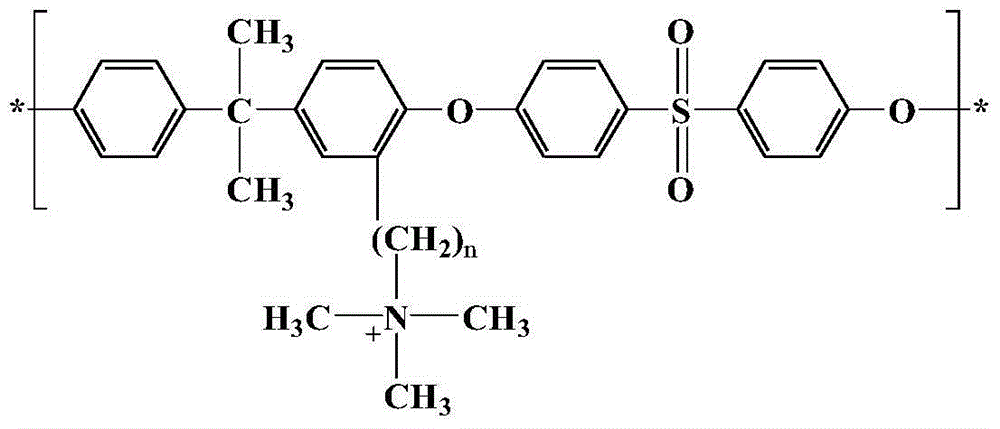
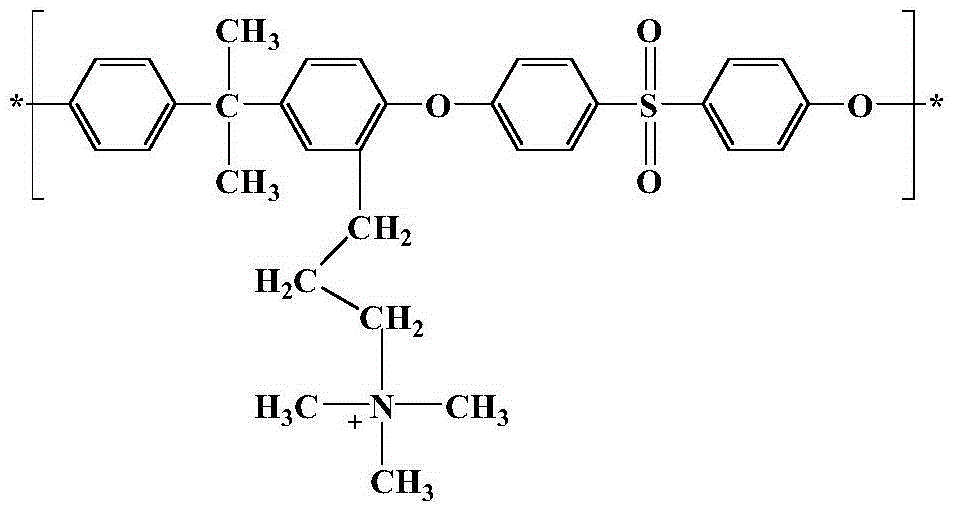
Long-branched-chain polysulfone anionic membrane and preparation method thereof
ActiveCN104877136AImprove hydroxide ion conductivityImprove connectivityCell component detailsFuel cell detailsHydrophobic polymerPhysical chemistry
The invention discloses a long-branched-chain polysulfone anionic membrane and a preparation method thereof. The structure of the membrane material is disclosed in the specification. The preparation method comprises the following steps: polysulfone acylation, carbonyl reduction, quaternization, film formation, alkalification and the like. Compared with the traditional anion-exchange membrane sequentially subjected to quaternization and functionalization, the polysulfone anion-exchange membrane contains the long branched chain on the side chain, so that the hydrophilic group is far away from the hydrophobic polymer main chain, thereby enhancing the phase separation driving force in the film formation process, improving the connectivity of the ion channels in the membrane and further enhancing the hydroxide ion conductivity of the membrane. The long alkyl side chain can obviously reduce the degree of swelling of the quaternary ammonium salt membrane and reduce the nucleophilic attack of water or OH-. The long-branched-chain polysulfone anionic membrane can obtain higher ion conductivity on the premise of relatively lower water absorptivity, thereby solving the contradiction in the anionic membrane for a long time.
Owner:DALIAN UNIV OF TECH
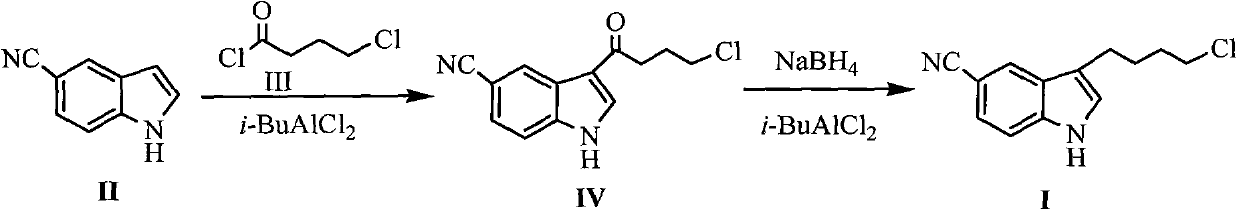
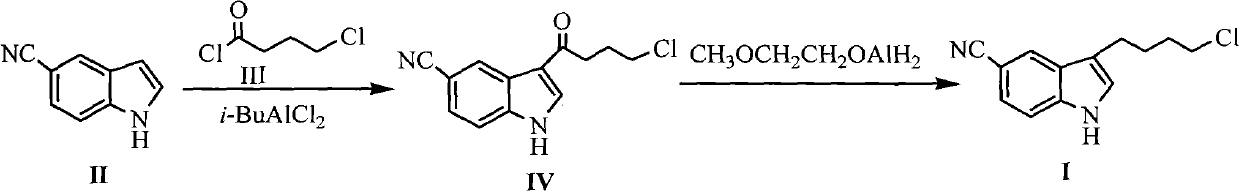
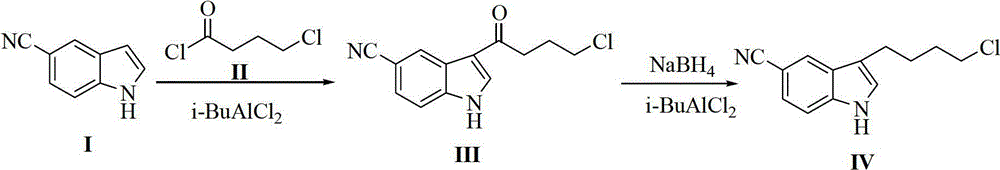
Preparation method for 3-(4- chlorobutyl)-1H-5-cyanoindole as a vilazodone intermediate
InactiveCN102690224ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryOrganic solventCarbonyl reduction
The invention discloses a new method for preparing 3-(4- chlorobutyl)-1H-5-cyanoindole as a vilazodone intermediate, comprising the following steps: step (1), a compound of formula II is reacted with a compound of formula III in the presence of an acylation catalyst in an organic solvent to form a compound of formula IV, step (2), the compound of formula IV formed in step (1) is subjected to carbonyl reduction reaction in the presence of a reducing catalyst in the organic solvent. The preparation method for 3-(4- chlorobutyl)-1H-5-cyanoindole as the vilazodone intermediate has cheap and easily-available raw materials, mild reaction condition, simple operation, high synthesis efficiency and is suitable for industrial production, thereby providing a new way for preparing vilazodone.
Owner:HUAIHAI INST OF TECH
New process for synthesis of asenapine
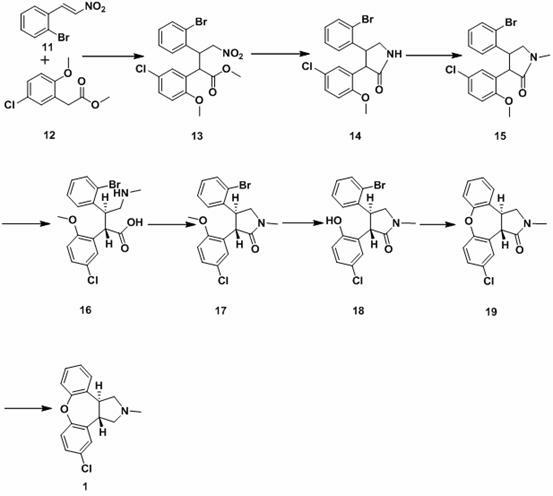
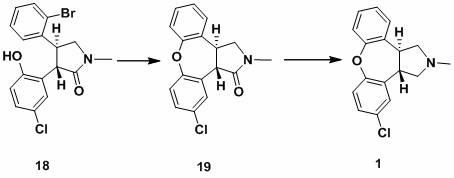
ActiveCN102229613AReaction transposition effect is goodHigh yieldOrganic chemistryLoop closingMethyl group
The invention discloses a process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to acondensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:安庆润科生物医药科技有限公司
Preparation method of 3-(4-chlorobutyl)-5-cyanoindole
ActiveCN102875440AReduce usageOperational securityOrganic chemistryPotassium borohydrideLewis acid catalysis
The invention discloses a preparation method of 3-(4-chlorobutyl)-5-cyanoindole which is shown as the formula IV. The preparation method comprises the steps as follows: carrying out following carbonyl reduction reaction on a compound III and a hydroboron reducing agent in the solvent under the catalyzing of Lewis acid to obtain the product, wherein Lewis acid is one or more of aluminium trichloride, magnesium chloride, zinc chloride and ferric chloride; and the hydroboron reducing agent is one or more of sodium borohydride, potassium borohydride, lithium borohydride and borane. The preparation method disclosed by the invention is safe in operation, low in requirement on equipment, low in cost, simple in post-processing steps, high in yield of product, high in purity, and is suitable for industrialization.
Owner:CHIRAL QUEST (SUZHOU) CO LTD +1
Preparation method and application of glycopyrronium bromide chiral antipode
The invention belongs to the technical field of medicine, and discloses a preparation method of (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) four type chiral monomers of muscarine receptor antagonist racemic medicine glycopyrronium bromide. The method comprises the following steps: resolving racemic alpha-cyclopentylmandelic acid by a chemical resolution method by using L-Tyrosine methyl ester and (R)-alpha-phenylethylamine as resolution reagents to respectively prepare (S)-alpha-cyclopentylmandelic acid and (R)-alpha-cyclopentylmandelic acid; and carrying out esterification reaction to respectively obtain chiral intermediates (S) / (R)-alpha-cyclopentylmethyl mandelate. L / D-malic acid used as the raw material is subjected to four reaction steps, including condensation, carbonyl reduction, catalytic hydrogenation or transfer hydrogenation reduction debenzylation, and reduction alkylation or alkylogen alkylation, in a chiral synthesis mode to obtain another important chiral intermediate (S) / (R)-N-methyl-3-hydroxypyrrolidine. The chiral intermediate is subjected to ester exchange and quaterisation to respectively obtain the four (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) type glycopyrronium bromide chiral monomers. The result indicates that the (3R,2'S)-glycopyrronium bromide has the strongest cholinergic antagonistic action.
Owner:SHENYANG PHARMA UNIVERSITY +1
Synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives
InactiveCN102627522AOrganic compound preparationHydrocarbon from oxygen organic compoundsEthyl groupKetone
The invention discloses a synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives. The synthesis method of indenofluorene derivatives is characterized in that ester-group-containing compounds are formed through Diels-Alder reaction of methyl propiolate and indenocyclopentadienone, ethyl substituted indenofluorene derivatives are formed through hydrolysis, acid catalytic ring closing, carbonyl reduction and introduction of ethyl onto methylene, diketone compounds produced after ring closing react with lithium salt of 4, 4'-di-tert-butyl-2-brominated biphenyl, and then acidification and ring closing are conducted to obtain indenofluorene derivatives with spirofluorene. The synthesis method of isotruxene is characterized in that 1, 4-diphenyl-2, 3-di (carbalkoxy) fluorenone products are formed through the Diels-Alder reaction of indenocyclopentadienone and dimethyl acetylenedicarboxylate, isotruxene ketone is obtained through hydrolysis and acid catalytic ring closing and finally isotruxene is obtained; dibenzyl alcohol products are formed through the Diels-Alder reaction of 1, 4-butynediol and indenocyclopentadienone, and isotruxene is obtained through acetone protection, carbonyl reduction, acetone removal and polyphosphoric acid (PPA) ring closing; and oriented oxy substituted products, i.e. isotruxene ketone which is derived from isotruxene with methylene at a No.5 position being substituted, and corresponding diethyl substituted products are additionally obtained. The indenofluorene derivatives, the isotruxene and the mono-substituted isotruxene derivatives disclosed by the invention can be used in the field of organic electroluminescence and organic micro-molecule solar cells.
Owner:EAST CHINA NORMAL UNIV
Candida parapsilosis CCTCC M203011 (S)- carbonyl reduction enzyme gene sequence and amino acid composition
The invention relates to gene order and amino acid component of (S)-carbonyl reductase of Candida parapsilosis CCTCC M203011, and the (S)-special carbonyl reductase gene can be used in separating recemic compound with biological method, which belongs to biological engineering sphere. The invention is to confirm (S)-special carbonyl reductase of Candida parapsilosis CCTCC M203011, its gene nucleotide sequence is SEQ ID NO:1, and its amino acid component is SEQ ID NO:2. By this gene, the recombination strain can be used in racemic chirality resolution and can arrange conditions for the heterogeneity, conversion approach and reaction mechanism of the microbial stereoselectivity oxidoreductase used in chirality resolution.
Owner:JIANGNAN UNIV
Triadimefon and triadimenol compounds having antimicrobial activity, salts, synthetic methods and uses thereof
The invention relates to a method for synthesizing a novel triazolone and a triadimenol compound, namely, benzene or substituted benzene is used as a raw material and subjected to Friedel-Crafts acylation, Tolyltriazole alkylation and halogenous-benzylation to obtain the novel triazolone compound; then carbonyl reduction is carried out to obtain the novel triadimenol compound. The invention also relates to medical and pesticide applications of the novel triazolone and triadimenol compounds.
Owner:SOUTHWEST UNIVERSITY
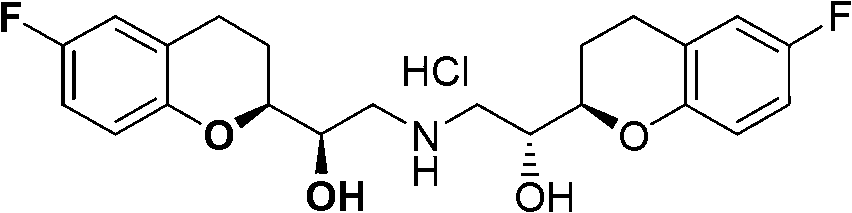
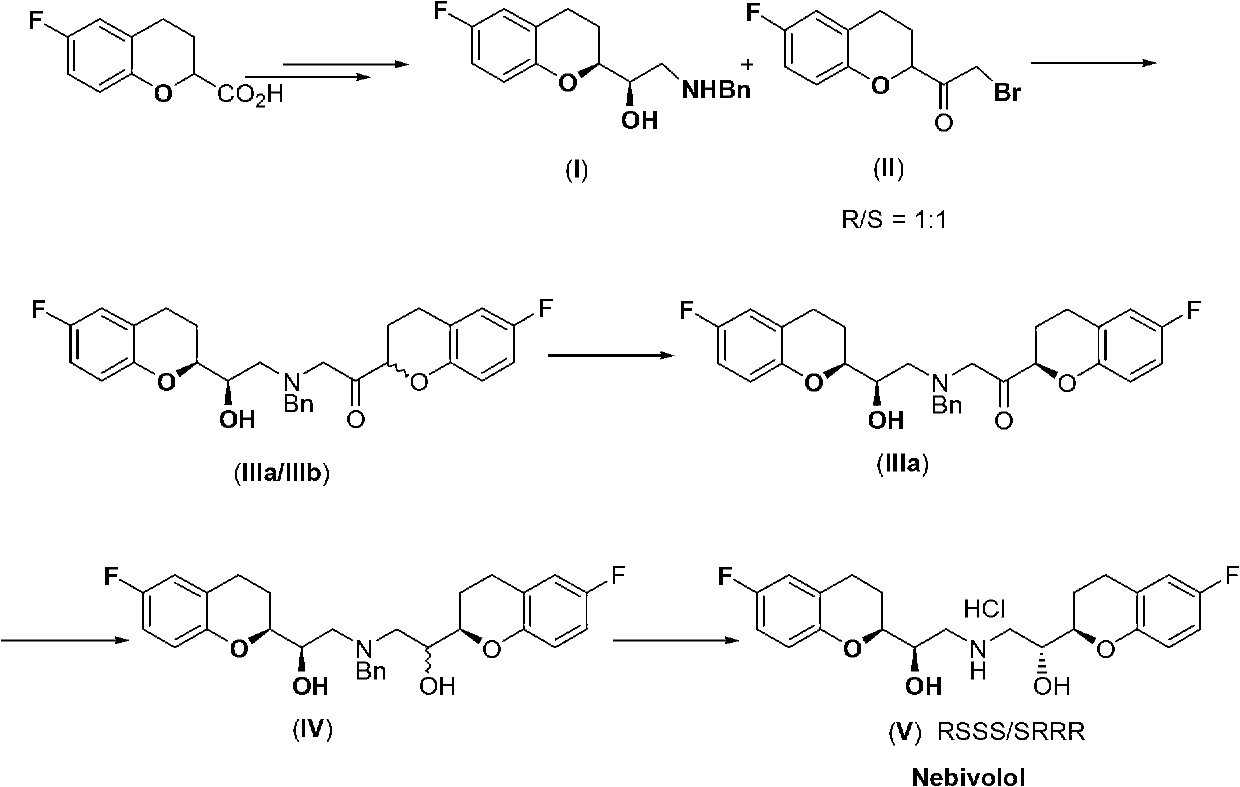
Method for preparing nebivolol racemate hydrochloride
InactiveCN102816141AHigh chemical purityReduce the discharge of three wastesOrganic chemistryBulk chemical productionIsomerizationAcyloin condensation
The invention discloses a method for preparing nebivolol racemate hydrochloride and belongs to the technical field of pharmaceutical synthesis. The method includes that a formula I compound and a formula II compound are used as raw materials to be subjected to an acyloin condensation to prepare a formula III compound, the formula III compound is subjected to an epimerization reaction to increase the ratio between III a and III b from 1:1 to 9:1, the formula III compound is subjected to a recrystallization purification to obtain a high purity III a, the III a is subjected to a carbonyl reduction to generate a formula IV compound, the formula IV compound is directly subjected to a catalytic hydrogenation to remove a benzyl protecting group to obtain a nebivolol racemate crude product, the nebivolol racemate crude product is directly salified by hydrogen chloride, and the recrystallization is performed to obtain the high purity nebivolol racemate hydrochloride. According to the method, the reaction is easy to control, the postprocessing is easy, and the production efficiency is high.
Owner:JINAN ASIA PHARMA TECH
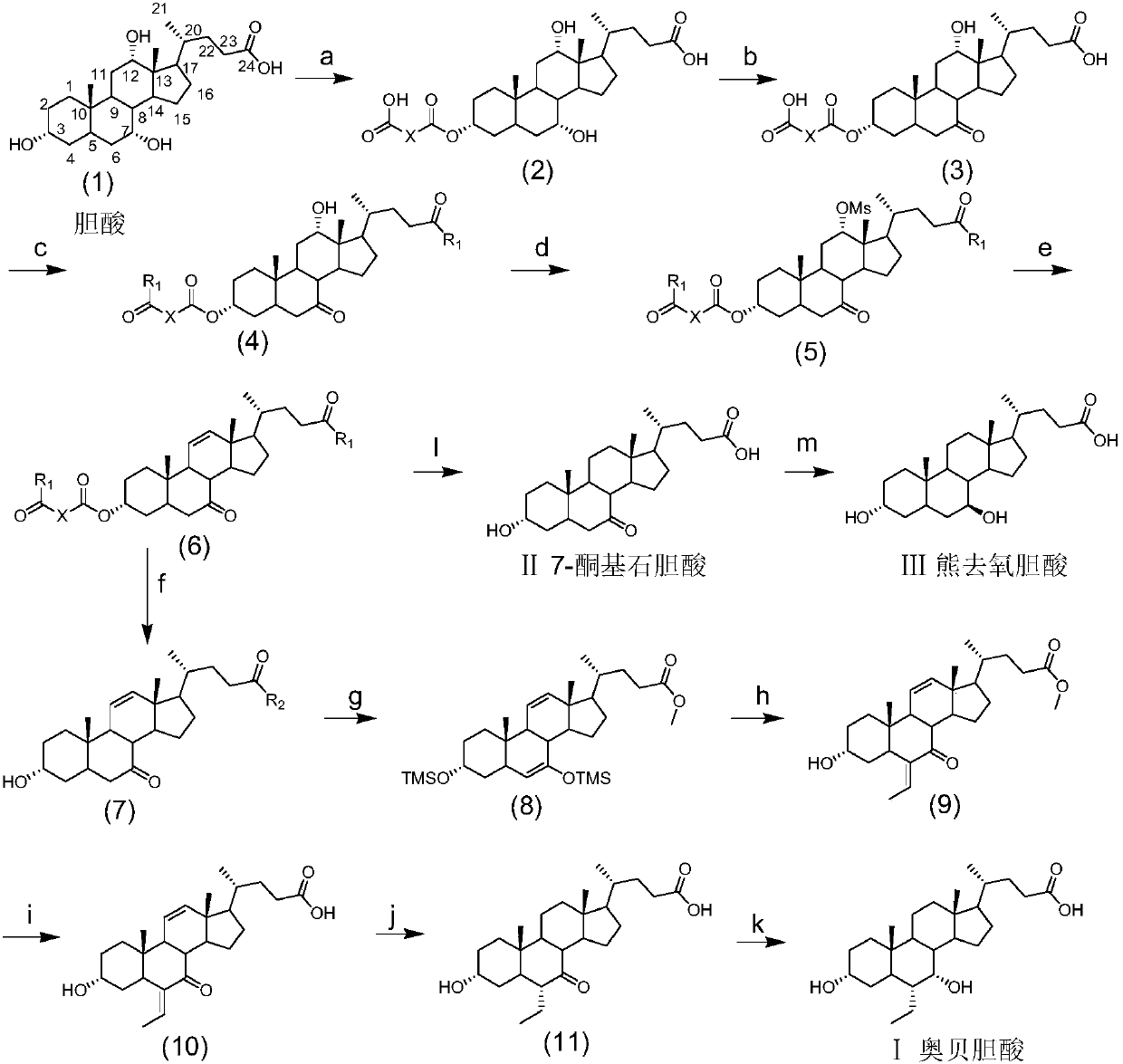
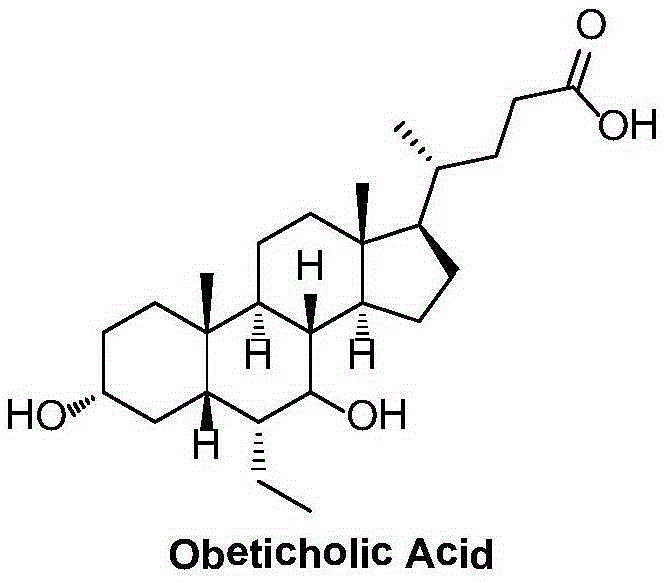
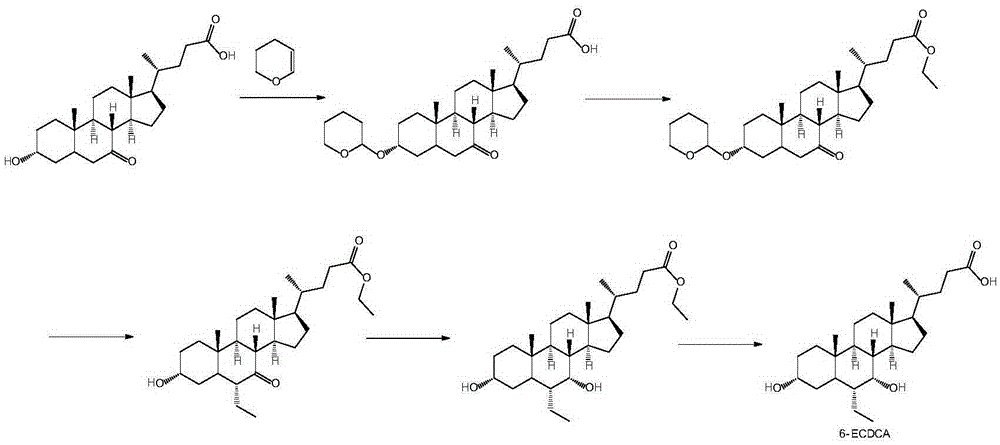
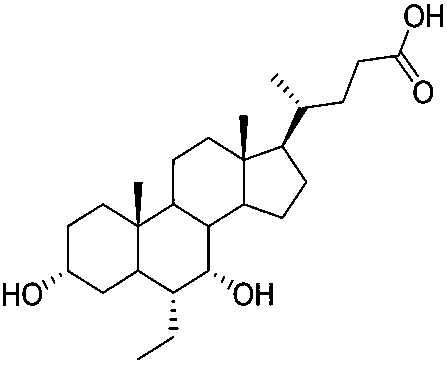
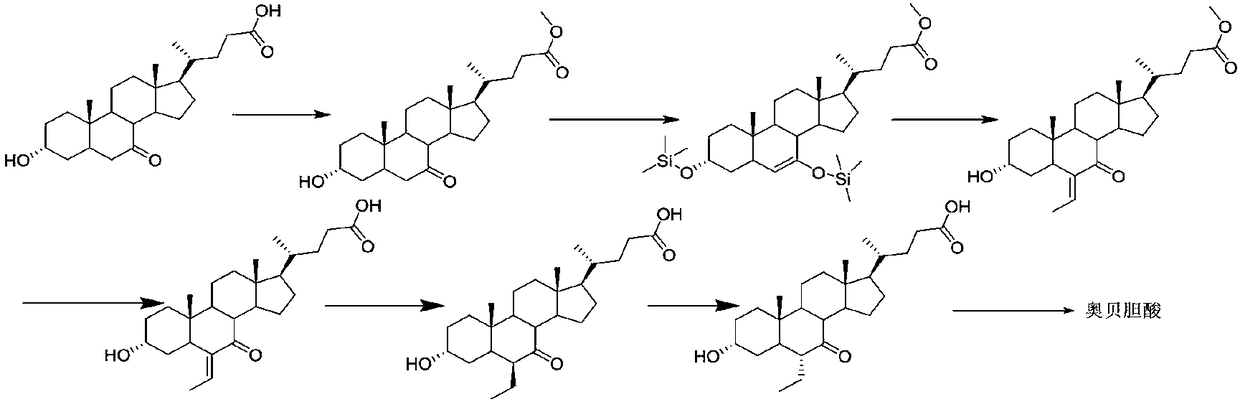
Method for preparing obeticholic acid, ursodeoxycholic acid and 7-ketolithocholicacid
InactiveCN108676049AEasy to manufactureHigh yieldSteroidsBulk chemical productionCholic acidSynthesis methods
The invention discloses a method for preparing obeticholic acid, 7-ketolithocholicacid and ursodeoxycholic acid. Cholic acid is used as a raw material for preparing obeticholic acid through selectiveprotection by a 3-alpha-hydroxyl group, selective oxidation of a 7-alpha-hydroxyl group, esterification of a 24th carboxyl group, methanesulfonation of a 12-alpha-hydroxyl group, elimination, hydrolysis, silylation, condensation, hydrolysis, catalytic hydrogenation, carbonyl reduction and other reactions; an intermediate is subjected to catalytic hydrogenation to prepare the 7-ketolithocholicacidand then is reduced to prepare the ursodeoxycholic acid. The method provided by the invention uses cheap cholic acid as the raw material, and has advantages of novel synthesis method, low cost, high yield, mild reaction condition, high simplicity in operation, environmental friendliness and high convenience in industrial production.
Owner:EAST CHINA NORMAL UNIV
Method for preparing gemcitabine hydrochloride
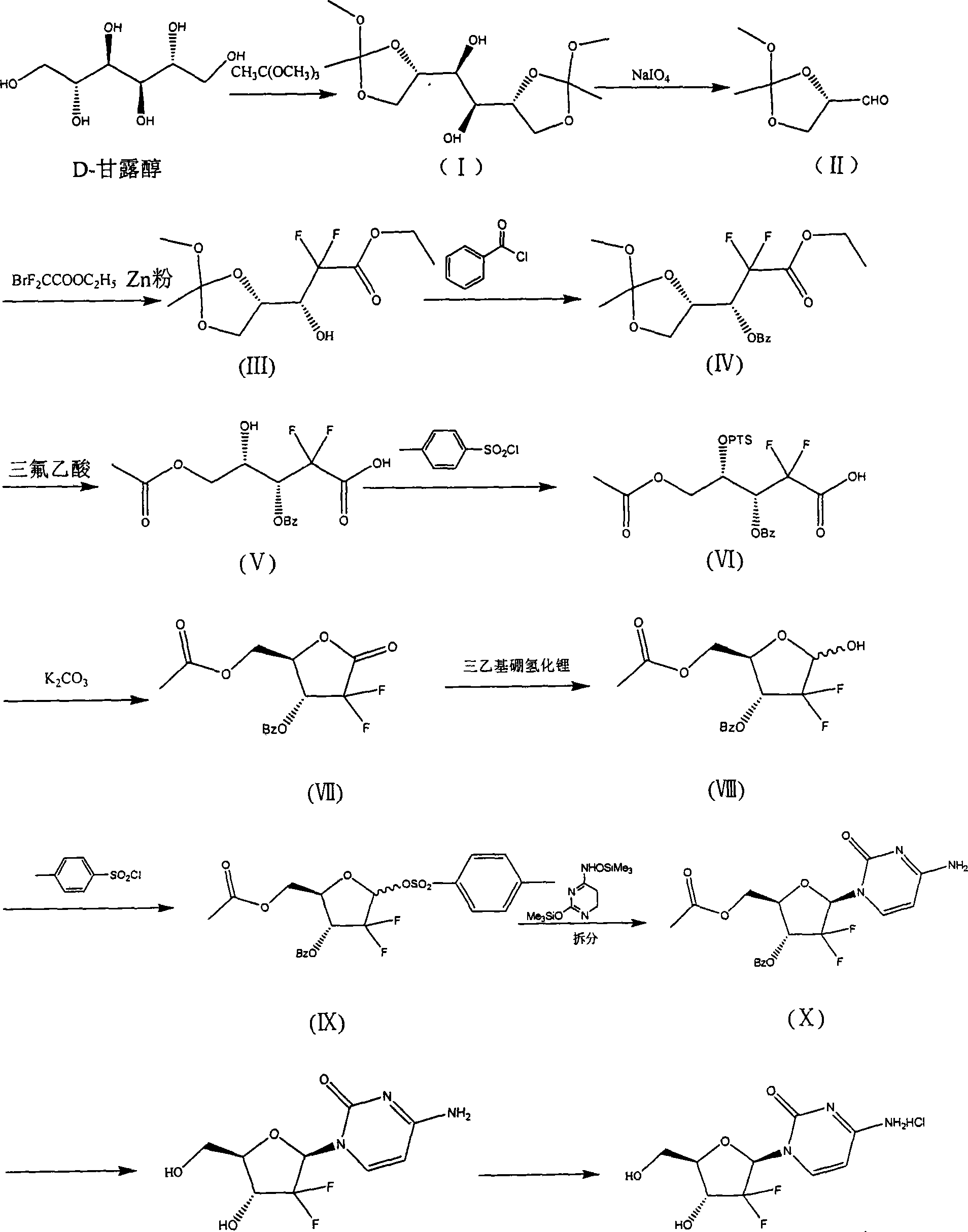
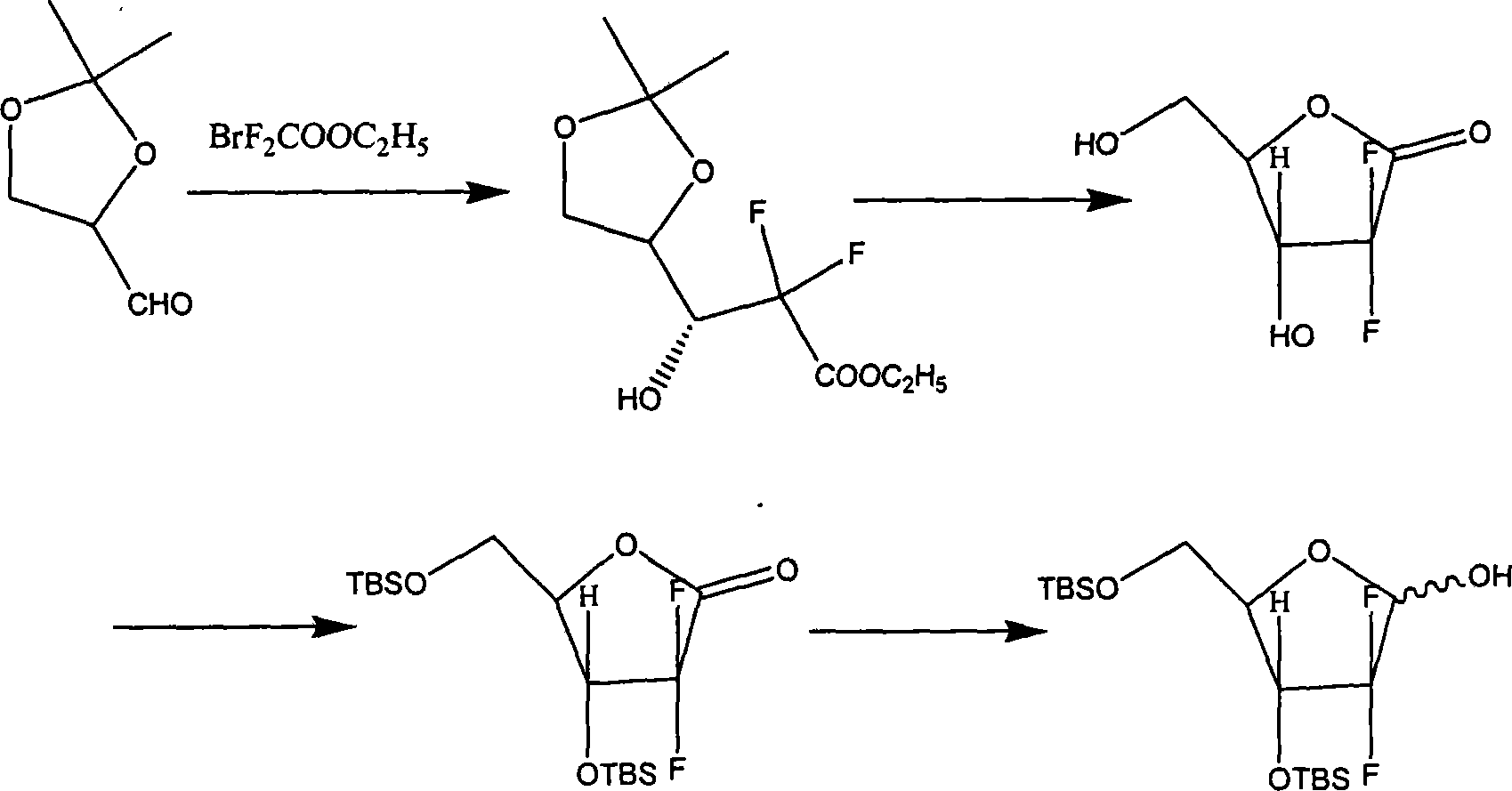
The invention discloses the compounding method for hydrochloric acid Gemcitabine. It uses D-mannitol as raw material and takes the processes of hydroxyl protection, oxidation, and addition of reformatsky, hydroxy benzoylation, hydrolysis, hydroxyl sulfonylation, ring closure, carbonyl reduction, hydroxyl sulfonylation, condensation, hydrolysis and crystallization to gain hydrochloric acid Gemcitabine. It has the advantages of high yield, simple operation, and is suitable to industrial producing.
Owner:HUBEI YITAI PHARMA
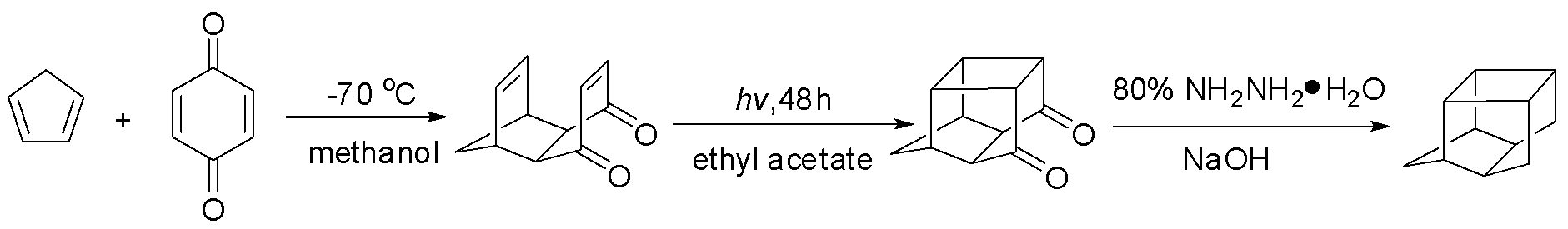
Synthetic method of pentacycloundecane
ActiveCN103204760AImprove efficiencyShort cycleHydrocarbonsHydrocarbon preparationRamjetSynthesis methods
The invention provides a synthetic method of pentacycloundecane (PCUD). The method uses cyclopentadiene and benzoquinone as initial raw materials, and a high boiling solvent; and the pentacycloundecane is synthesized through the following three reactions: Diels-Alder addition, ultraviolet cyclization and carbonyl reduction. According to the invention, the Diels-Alder addition, the ultraviolet cyclization and the carbonyl reduction can be continuously carried out without post-treatment, and unnecessary processes in the reactions is simplified, thereby achieving purposes of improving efficiency and reducing cost. The method has advantages of short period, low cost and easily expanded technology; and the synthesized target compound PCUD can be used as a high-energy additive for fuel of solid turbine stamping engines or liquid rocket fuel.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
Preparation method for hypoglycemic drugdapagliflozin
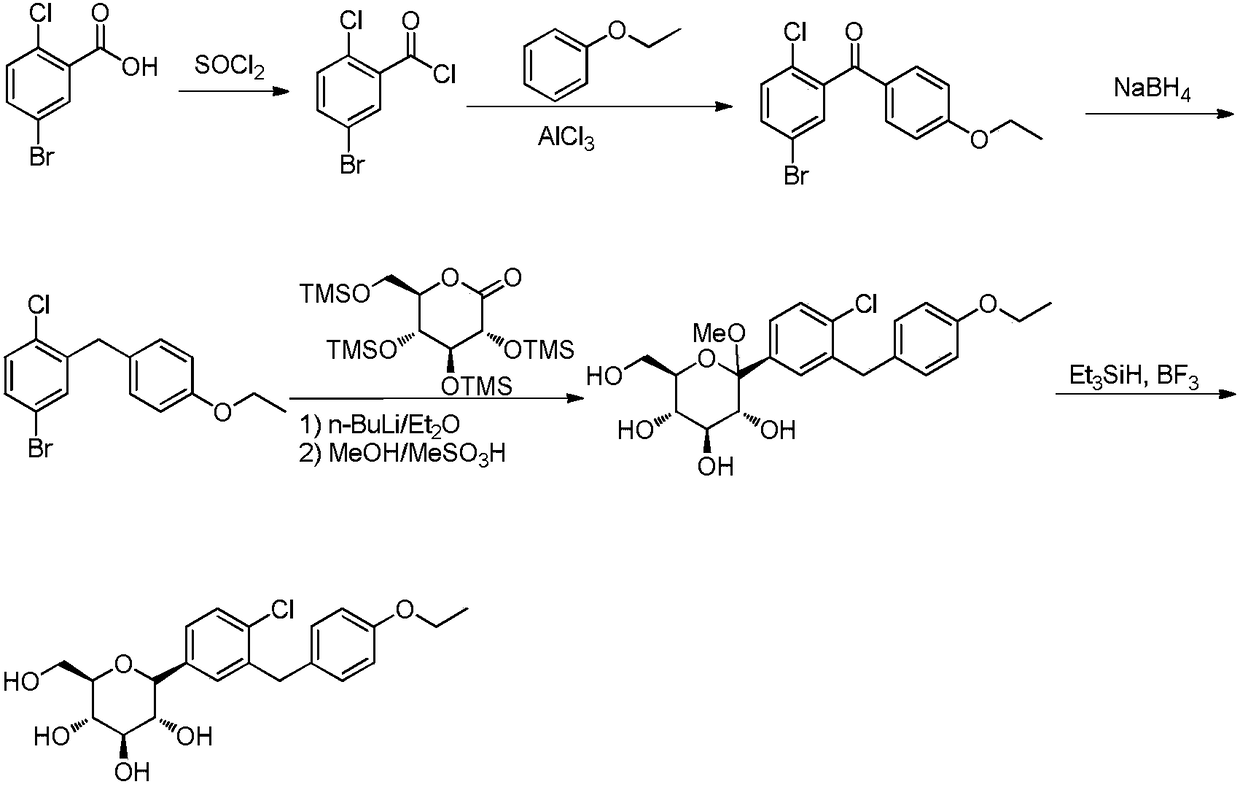
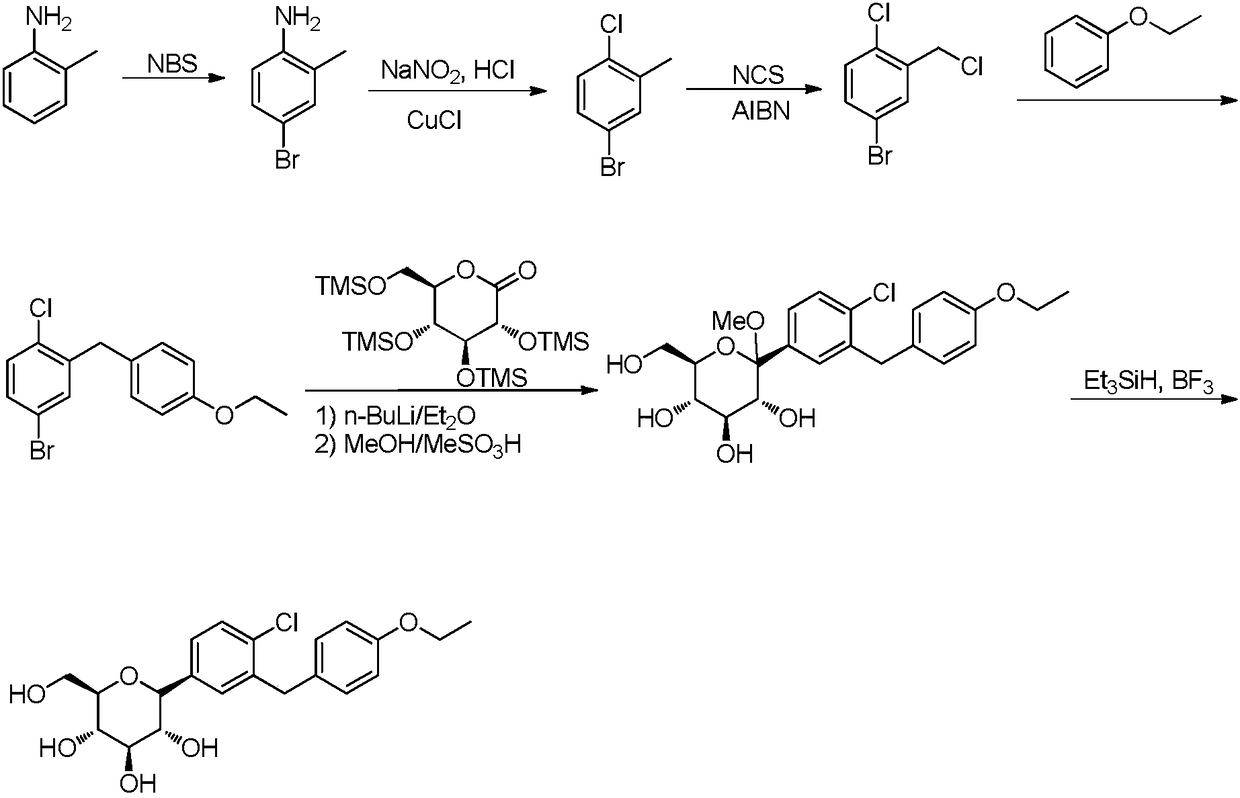
ActiveCN108084130ACheap and easy to getHigh purityOrganic chemistryMetabolism disorderDiphenylmethaneAlkyl transfer
The invention discloses a preparation method for hypoglycemic drugdapagliflozin. The method comprises the steps that 4-hydroxybenzaldehyde is adopted as a starting raw material, alkylation, carbonyl reduction, chlorination and alkylation reaction with asepsin, diazotization and chlorination are performed to obtain a dapagliflozinmidbody 5-bromo-2-chloro-4'-ethyoxyl diphenylmethane, then, the midbody and 2,3,4,6-tetra-O-trimethyl silicone-D-glucolactone are subjected to condensation, etherification and methoxyl removal to obtain the hypoglycemic drugdapagliflozin. The raw materials adopted by the technological path are low in price and easy to obtain, the technology can achieve industrialization easily, and the final product is high in purity; the technological path is novel, the syntheticroute is short, no risky or complex technology exists in the reactions, equipment is simple, operation is easy and convenient, and the method is suitable for industrial production.
Owner:SOUTHEAST UNIV
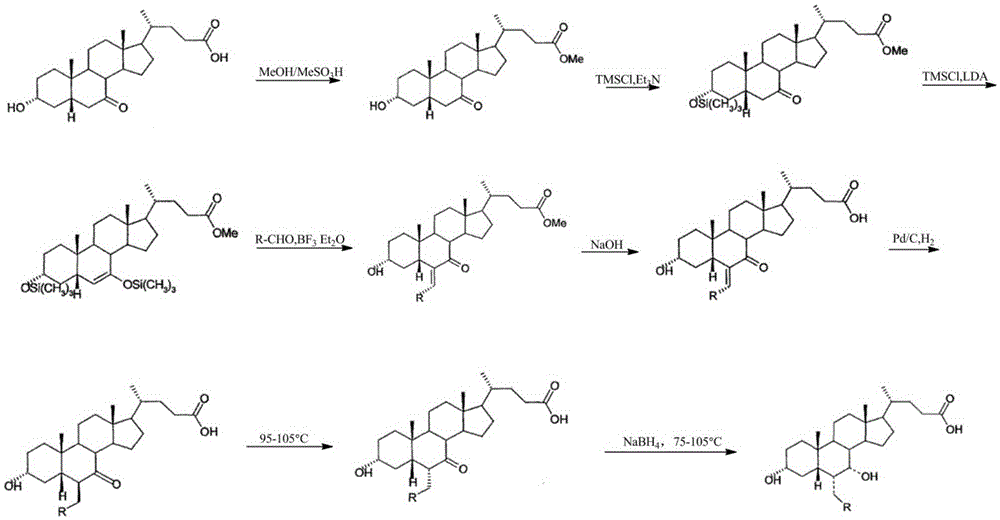
Preparation method of high-purity obeticholic acid
ActiveCN106749466AImprove protectionRaw materials are easy to getSteroidsChenodeoxycholic acidCarbonyl reduction
The invention relates to a preparation method of high-purity obeticholic acid. A compound chenodeoxycholic acid (CDCA) shown as a formula II is used as a starting raw material and subjected to oxidation, esterification, hydroxy protection, ethylidene formation, catalytic hydrogenation, carbonyl reduction and esterolysis reaction to obtain the high-purity obeticholic acid. The preparation method of the high-purity obeticholic acid, provided by the invention, has the advantages of low toxicity, low pollution, high purity, good stereoselectivity, low content of impurities, mild reaction conditions, high safety, simplicity and convenience in production operation and the like, and is suitable for industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
Bithienofluorene, its derivative and preparation method
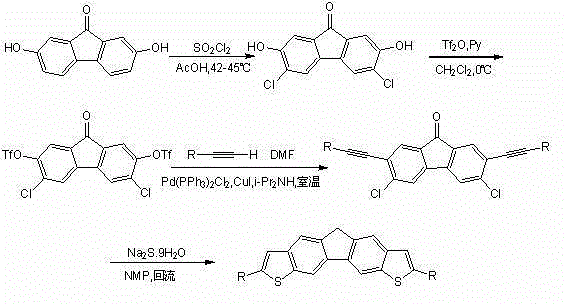
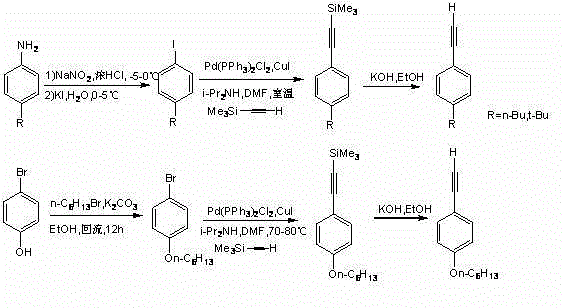
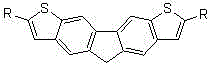
The invention discloses bithienofluorene, its derivative and a preparation method. The compounds are characterized in that: a fluorene ring is in connection with thieno rings, and the 2- position of thiophene has a substituent group, which can be hydrogen, alkyl or aryl. The preparation method consists of: taking 2, 7-dyhydroxyl-9-fluorenone as a raw material, employing sulfonyl chloride to conduct chlorination so as to synthesize 3, 6-dichloro-2, 7-dyhydroxyl-9-fluorenone; and then adopting trifluoromethanesulfonic anhydride to carry out esterification, and performing a Sonogashira coupling reaction to obtain series of 2, 7-disubstituted ethynyl-3, 6-dichloro-9-fluorenone, and finally conducting ring closing by sodium sulfide and reduction to obtain bithienofluorene and its derivative. The series of compounds involved in the invention provides a new option for organic field effect transistor materials, and the preparation method provides a new method for aromatic ketone carbonyl reduction.
Owner:EAST CHINA NORMAL UNIV
Preparation method of high-purity injection-grade terbutaline sulfate
InactiveCN109305920AAddressing risks with a high risk of contaminationLow costOrganic compound preparationCarbonyl compound preparationSubstitution reactionCarbonyl reduction
The invention provides a preparation method of high-purity injection-grade terbutaline sulfate. The preparation method is characterized in that 3,5-dibenzyloxyacetophenone is adopted as a raw material, a bromination reaction, a substitution reaction, carbonyl reduction and hydrogenation reduction are conducted, salifying with sulfuric acid is conducted, and the terbutaline sulfate is obtained. A synthetic route is shown in the description.
Owner:HAINAN LEVTEC PHARMA
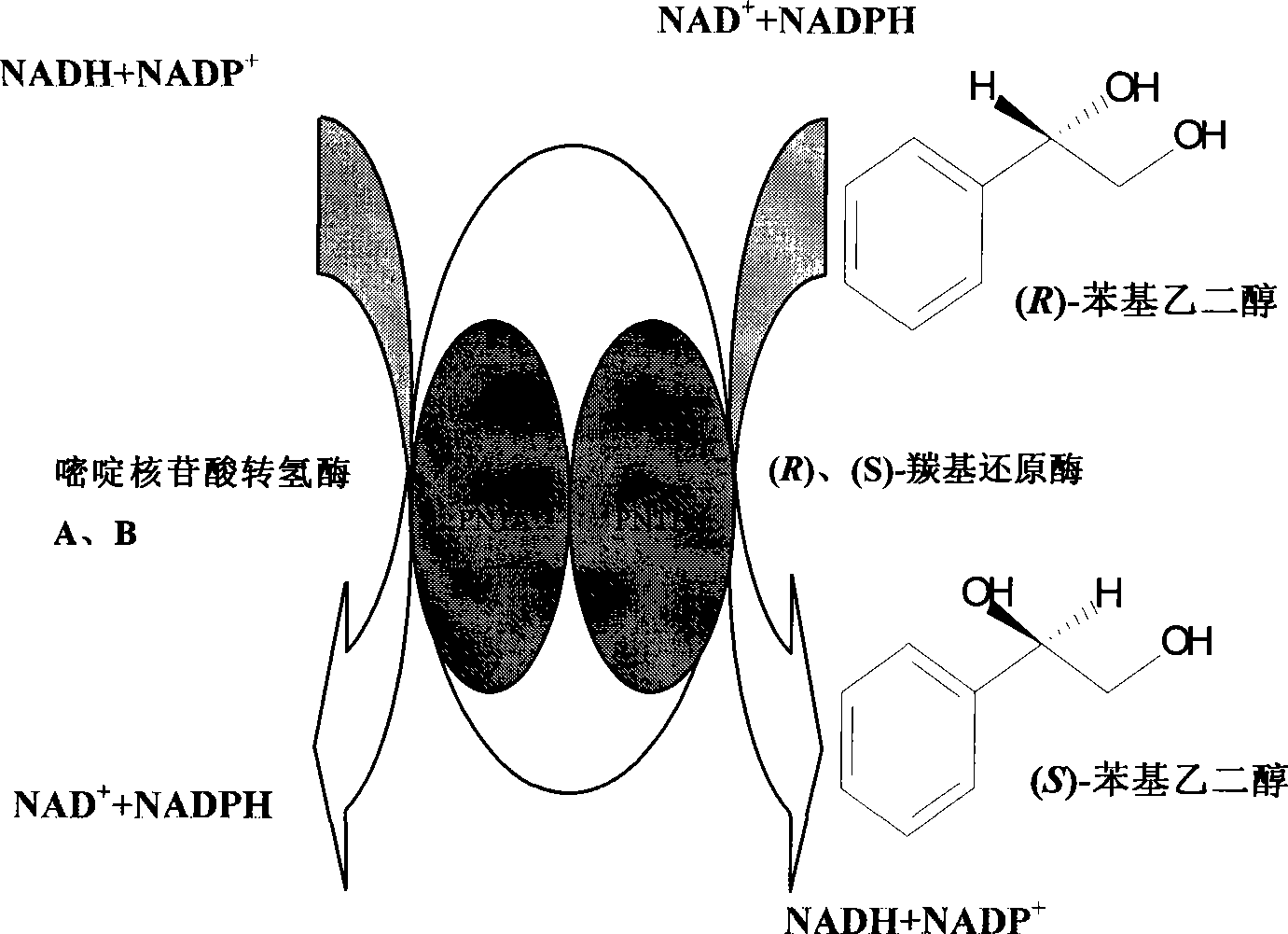
Synthesis of (R)-styrene glycol by coupling acceleration of (R)-carbonyl reduction enzyme and formic dehydrogenase
ActiveCN101469318AHigh optical purityHigh yieldBacteriaMicroorganism based processesEscherichia coliHigh concentration
The invention relates to promotion of synthesis of (R)-styrene glycol by coupling (R)-carbonyl reductase and formic dehydrogenase, which belongs to the technical field of asymmetrical transformation of biological catalysis. The invention provides a recombinant Escherichia coli, namely E.coli Rosetta / pETDuet-rcr-fdh, with a preservation number of CCTCC NO: M208123, wherein the (R)-special carbonyl reductasei and the formic dehydrogenase are coupled by a co-expression mode; asymmetrical transformation reaction is catalyzed by utilizing a cultured recombinant strain and taking 2-hydroxyacetophenone as a substrate; and the optical purity of the (R)-styrene glycol reaches 100 percent e.e., and the yield of the (R)-styrene glycol reaches 85.9 percent by optimizing the biotransformation reaction conditions. The invention utilizes double-enzyme coupling technology to solve the problem of limitation of regeneration cycle of coenzyme under the condition of high-concentration substrate, provides an effective path for high-efficiency and low-cost preparation of the (R)-styrene glycol, and lays a foundation for industrial application of the biosynthesized (R)-styrene glycol.
Owner:JIANGNAN UNIV
Method for preparing (S)-styrene glycol with carbonyl reduction enzyme and pyrimidine nucleoside acid transhydrogenase couplet
ActiveCN101368168AAchieve couplingSimplified chiral conversion pathwaysBacteriaMicroorganism based processesEscherichia coliAlcohol
A method for preparing (S)-phenylethylene glycol by coupling of carbonyl reductase and pyrimidine nucleotide transhydrogenase, which belongs to the technical field of biocatalysis asymmetric transformation. In the invention, (R)-carbonyl reductase, (S)-carbonyl reductase and pyrimidine nucleotide transhydrogenaseA, B are respectively constructed to two co-expression vectors, and the constructed recombinant plasmid pETDuet-rcr-scr and pACYCDuet-pnta-pntb are co-transformed into Escherichia coli to obtain recombinant strain E. Coli BL21(pETDuet-rcr-scr / pACYCDuet-pnta-pntb). The accession number is CCTCC NO: M208126. The recombinant strain not only realizes one-step transformation from substrate (R)-phenylethylene glycol to product (S)-phenylethylene glycol, but also removes a restriction of coenzyme regeneration cycle in a transformation reaction. The method provides an effective way for producing chiral alcohol through catalytic reaction of enzyme and regeneration of in-situ coenzyme under high substrate concentration.
Owner:JIANGNAN UNIV
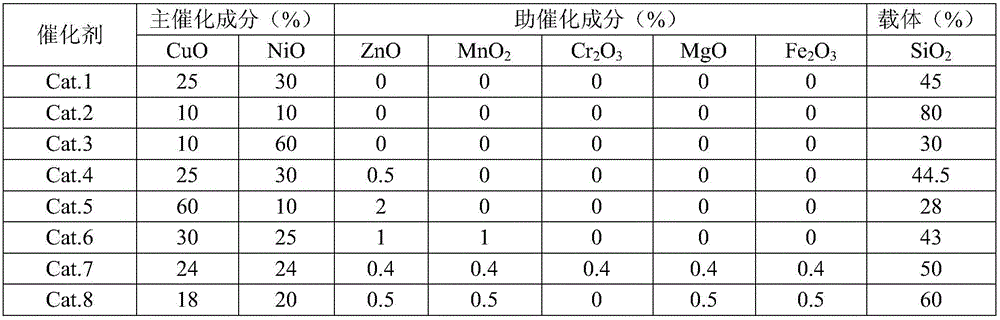
Dual-function catalyst for hydrogenating benzene rings and reducing carbonyl, preparation of dual-function catalyst and application thereof
ActiveCN105688915AAvoid replacementMild reaction conditionsOrganic compound preparationHydrocarbon by hydrogenationBenzeneCopper oxide
The invention discloses a dual-function catalyst for hydrogenating benzene rings and reducing carbonyl. The dual-function catalyst comprises, by weight, 10-60% of copper oxide, 10-60% of nickel oxide, 28-80% of carriers and 0-2% of catalysis assisting components. The catalysis assisting components include at least one type of ZnO, MnO2, Cr2O3, MgO and Fe2O3. The invention further discloses a method for preparing the dual-function catalyst and application of the dual-function catalyst to catalytically hydrogenating the benzene rings, hydrogenating and reducing the carbonyl and preparing 1, 4-cyclohexyl dimethyl carbinol.
Owner:KAILING CHEM ZHANGJIAGANG CO LTD
3,5-dihydroxyamylbenzene synthesis method
ActiveCN109928867ACheap and easy to getMild reaction conditionsOrganic chemistryOrganic compound preparationChemical industrySynthesis methods
The present invention provides a 3,5-dihydroxyamylbenzene synthesis method, which comprises: carrying out a Friedel-crafts acylation reaction on benzene under the catalysis of an acid to obtain valerophenone, wherein the acid is a Lewis acid; carrying out a nitrification reaction on the obtained valerophenone to obtain 3,5-dinitrovalerophenone; reducing the carbonyl into methylene, and reducing the nitro group to obtain 3,5-diaminoamylbenzene; and diazotizing the 3,5-diaminoamylbenzene to change into the hydroxy group so as to obtain the 3,5-dihydroxyamylbenzene. According to the present invention, the used raw materials are bulk chemical industry raw materials with low price and easy availability, the conditions of each reaction are relatively mild, the reactions belong to a large numberof the conventional reactions used in the chemical industry, and the final product 3,5-dihydroxyamylbenzene (Olivetol) obtained according to the process has high purity, wherein the purity can achievemore than 98.5%, and the single impurity is within 0.3%.
Owner:JIANGSU JIMING PHARMA TECH
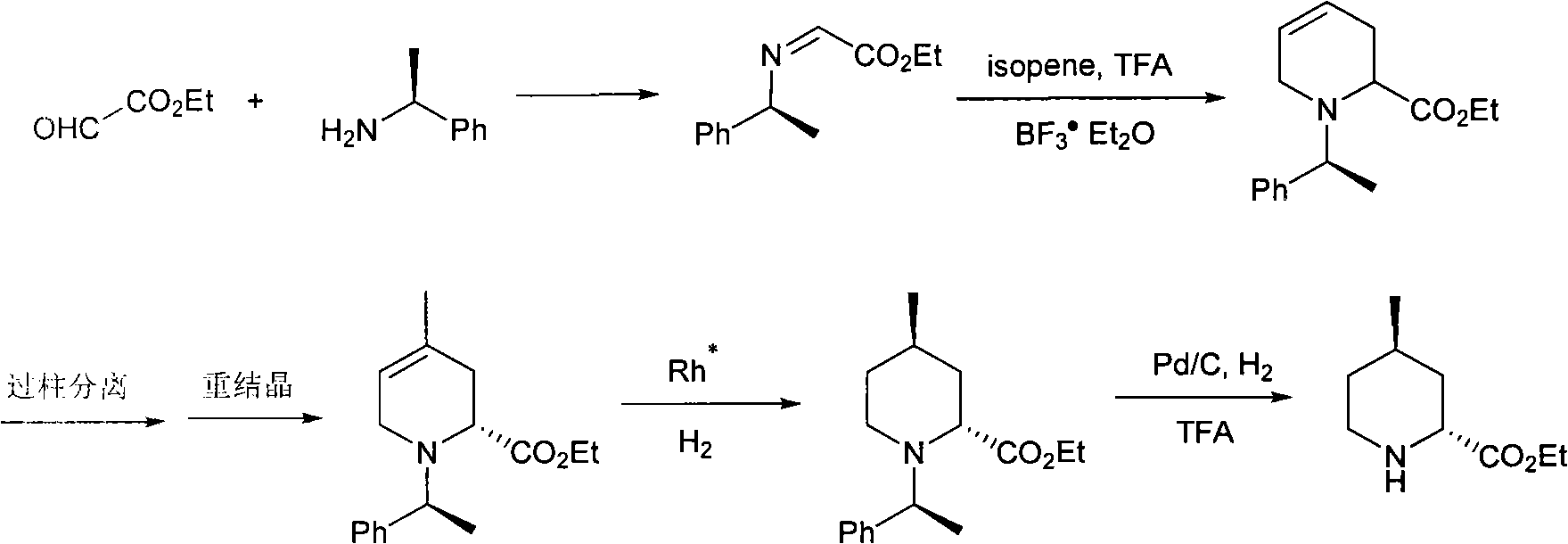
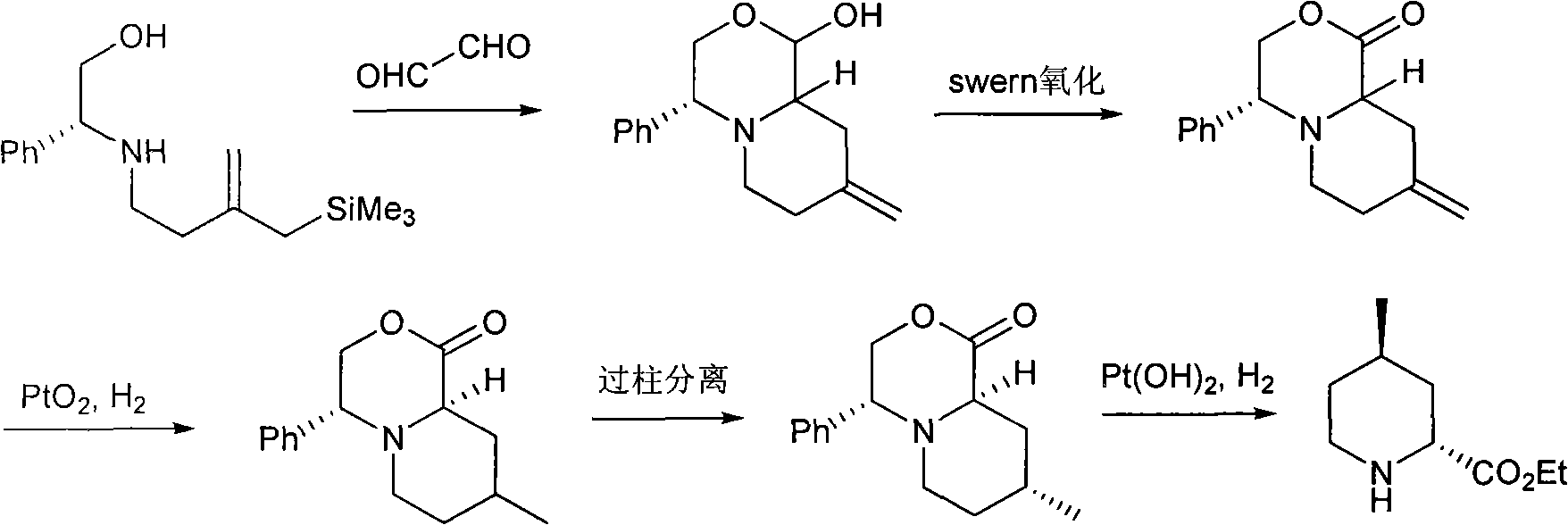
Method for preparing (2R,4R)-4-methyl-2-pipecolic acid
InactiveCN102807523AEasy to manufactureAvoid splittingOrganic chemistryBulk chemical productionL-AspartateFormate
The invention discloses a method for preparing (2R,4R)-4-methyl-2-pipecolic acid (I). The method comprises the following steps that: L-aspartic acid is subjected to selective esterification to obtain L-aspartic acid-4-alkyl ester hydrochlorate (II); the resulting (II) and acrylate are subjected to a reaction and an amino protection reaction to generate N-(2-carboalkoxy(aryloxy))ethyl-N-protective group-L-aspartic acid-4-alkyl ester (III); the resulting (III) is subjected to intramolecular ring closing and decarboxylation under an alkaline environment to generate (R)-N-protective group-4-oxopiperidine-2-formic acid (IV); the carboxyl of the resulting (IV) is subjected to esterification to generate (R)-N-protective group-4-oxopiperidine-2-formate (V); the resulting (V) is subjected to selective carbonyl reduction under an effect of a reducing agent to generate cis (2R,4S)-N-protective group-4-hydroxypiperidine-2-formate (VI); the hydroxyl of the resulting (VI) is activated to generate cis (2R,4S)-N-protective group-4-oxysulfonyl piperidine-2-formate (VII); the resulting (VII) and a nucleophilic methyl metal reagent SN2 are subjected to a substituted reaction to generate trans (2R,4R)-N-protective group-4-methyl piperidine-2-formte (VIII); and amino in the compound (VIII) and the protective group on the acid are removed to generate a target compound (2R,4R)-4-methyl-2-pipecolic acid (I). The method of the present invention has advantages of low cost, environmental friendliness, high selectivity, high yield, and the like, and is suitable for industrial production.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
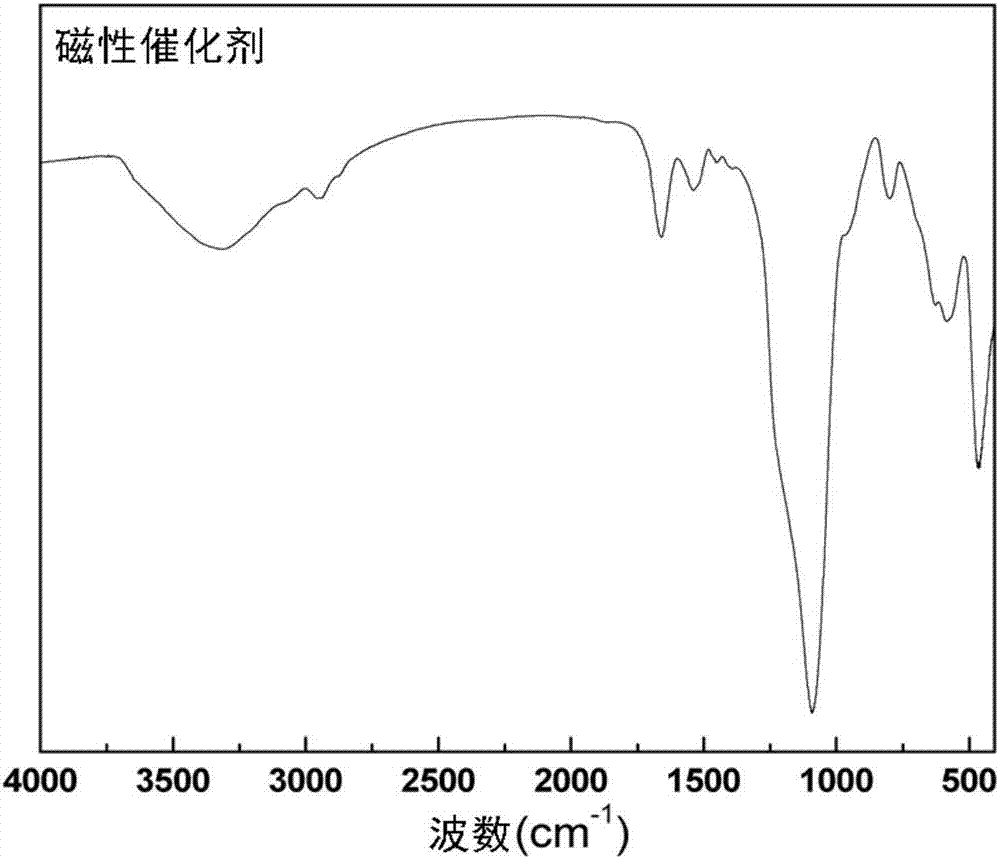
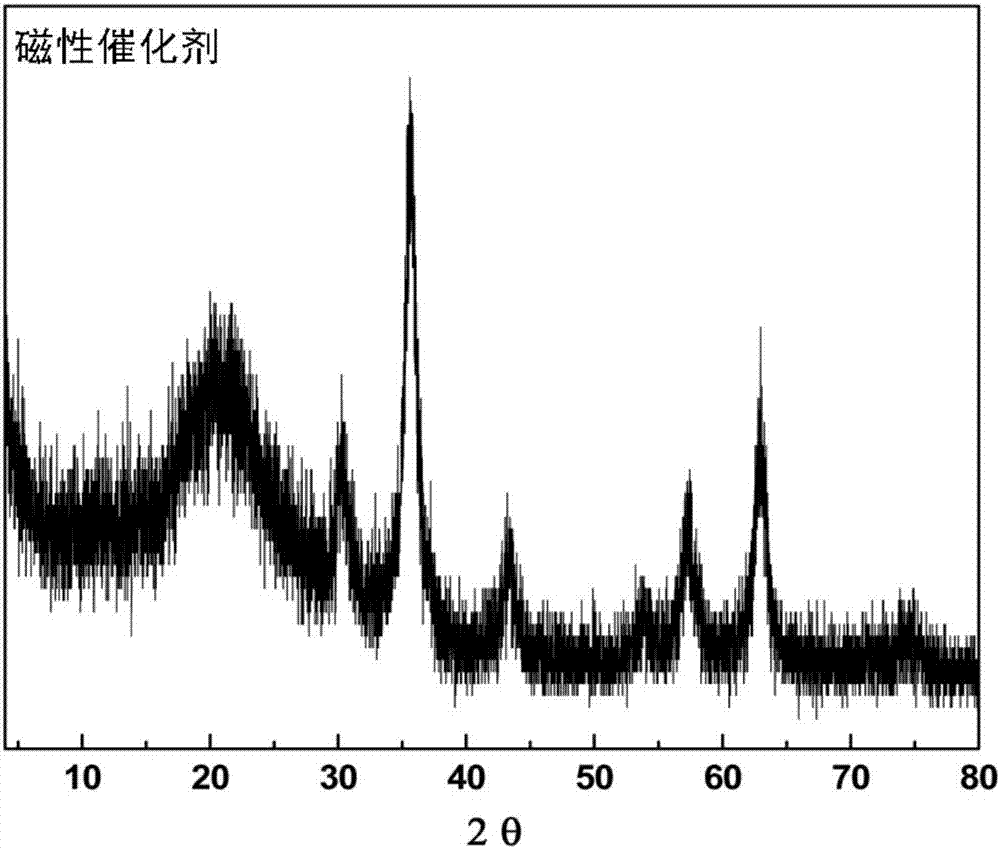
Magnetic combined crosslinked enzyme aggregate biocatalyst and preparation method and application thereof
InactiveCN107227301AIncreased Enzyme LoadingIncrease loadOxidoreductasesFermentationCross-linked enzyme aggregateEnantioselective synthesis
The invention provides a magnetic combined crosslinked enzyme aggregate biocatalyst. The biocatalyst can produce diffraction peaks at diffraction angles 2 theta of 30.5+ / -0.2 degrees, 35.4+ / -0.2 degrees, 44.7+ / -0.2 degrees, 57.3+ / -0.2 degrees and 63.4+ / -0.2 degrees. A preparation method of the biocatalyst is simple, does not need special equipment, needs mild process conditions, is easy to operate, realizes a low cost and is suitable for industrialization. The biocatalyst is used for asymmetric synthesis of (R)-3-quinuclidinol, realizes synchronous carbonyl reduction and coenzyme NADH / NAD<+> in-situ regeneration, prevents diffusional limitation to a substrate , a coenzyme and a product, has good catalytic activity and high catalytic efficiency, greatly shortens reaction time and has obvious economic values.
Owner:CHONGQING MEDICAL UNIVERSITY
Obeticholic acid derivative and obeticholic acid preparation method
InactiveCN108456238AHigh purityLow efficiencyOrganic chemistry methodsSteroidsHydrolysisCarbonyl reduction
The present invention provides an obeticholic acid derivative and an obeticholic acid preparation method, wherein configuration transformation of a compound represented by a formula III, carbonyl reduction and amide hydrolysis are performed to obtain obeticholic acid. According to the present invention, the route has characteristics of simple operation, convenient post-treatment, and high yields and high purities of intermediates in each step.
Owner:ZHEJIANG JINGXIN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com