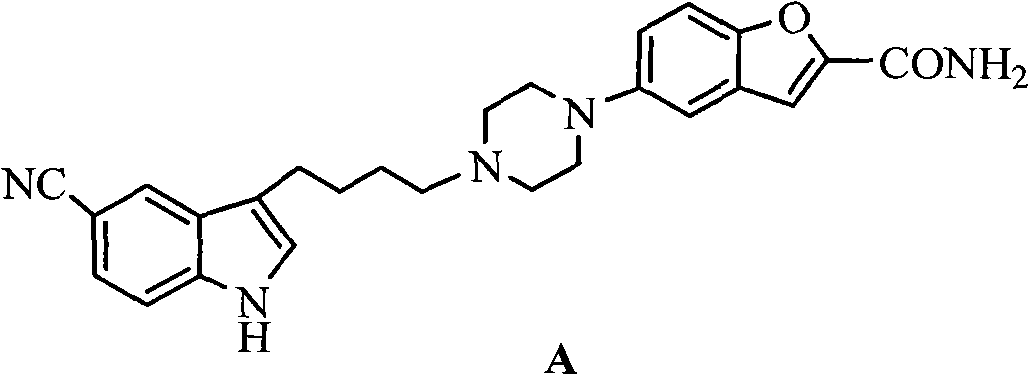
Preparation method for 3-(4- chlorobutyl)-1H-5-cyanoindole as a vilazodone intermediate
A technology of -1H-5- and vilazodone, which is applied in the field of preparation of key pharmaceutical intermediates, can solve the problems of unfavorable large-scale industrial production, difficulty in separation and purification, and low synthesis efficiency, and achieve high synthesis efficiency, cheap and easy raw materials The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
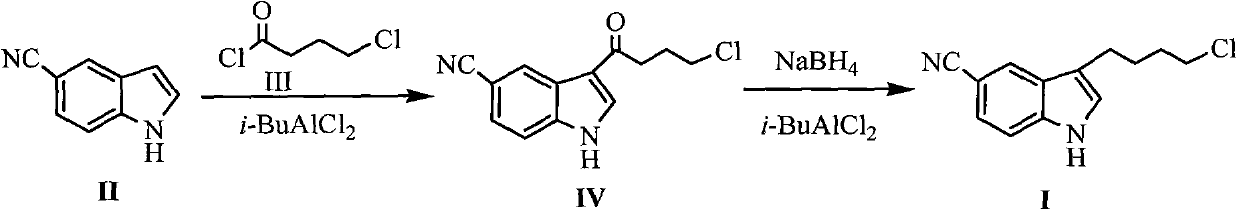
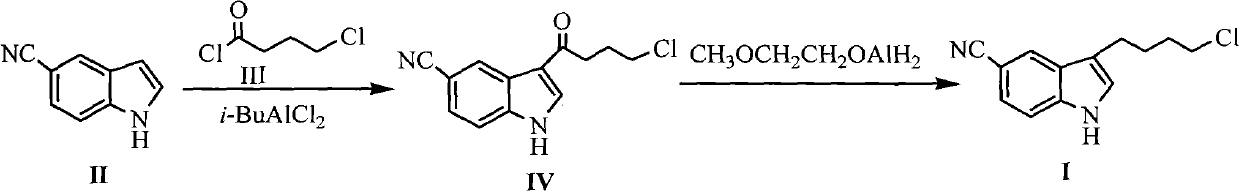
[0033] Embodiment 1 The preparation of formula (IV) compound 3-(4-chlorobutyryl)-1H-5-cyanindole
[0034] Add 16g (0.12mol) of anhydrous aluminum trichloride and 200mL of dichloromethane into the reaction flask, and cool the reaction flask to 0°C, slowly drop 28.2g (0.2mol) of compound 4-chlorobutyryl chloride into , control the reaction temperature not to exceed 5°C, and then stir at 0°C for 0.5h after dropping. Then, 14.2 g (0.1 mol) of the compound of formula (II) 5-cyanindole dissolved in 100 mL of dichloromethane was added dropwise, and the reaction was stirred at room temperature for 1.5 h after the drop was completed. 100g of ice and 100mL of concentrated hydrochloric acid were added, and the reaction was stirred at room temperature for 3h. After filtering, the filter cake was washed with dichloromethane and cold water and dried under vacuum at 50°C to obtain 19.6g of solid, melting point: 168-170°C, yield 80%.
Embodiment 2
[0035] Embodiment 2 The preparation of formula (I) compound 3-(4-chlorobutyl)-1H-5-cyanindole
[0036] Under the protection of nitrogen, 24.6g (0.1mol) of the compound of formula (IV) and 200mL of dichloromethane were added to the reaction flask, and the reaction flask was cooled to -5~-10°C, and 11.4g (0.3mol) of sodium borohydride was added, After stirring and reacting for 15 minutes, slowly add 40 g (0.3 mol) of anhydrous aluminum trichloride, control the reaction temperature not to exceed -5°C, react at -5~-10°C for 0.5h, and then stir at room temperature for 2h. Then the reaction flask was cooled to -5~-10°C, 300 mL of ice water was slowly added dropwise, and stirred at room temperature for 0.5 h after the drop was completed. The solvent was evaporated under reduced pressure, 500 mL of ethyl acetate was added to the residue, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a crude solid. ...
Embodiment 3
[0037] Embodiment 3 The preparation of formula (IV) compound 3-(4-chlorobutyryl)-1H-5-cyanindole
[0038]Add 16g (0.12mol) of anhydrous aluminum trichloride and 150mL of dichloromethane into the reaction flask, and cool the reaction flask to -5°C, slowly drop 21.2g (0.15mol) of compound 4-chlorobutyryl chloride Add, control the reaction temperature not to exceed 5°C, and stir at 0°C for 0.5h after dropping. Then, 14.2 g (0.1 mol) of the compound of formula (II) 5-cyanindole dissolved in 100 mL of dichloromethane was added dropwise, and the reaction was stirred at room temperature for 1.5 h after the drop was completed. 100g of ice and 100mL of concentrated hydrochloric acid were added, and the reaction was stirred at room temperature for 2h. After filtering, the filter cake was washed with dichloromethane and cold water and then dried in vacuum at 50°C to obtain 17.9g of solid, melting point: 168-170°C, yield 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com