Synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives
A technology of heterotrimeric indene and heterotrimeric indanone is applied in the field of synthesis of novel mono-substituted heterotrimeric indene derivatives, and can solve the problems of limited research and development and utilization, low yield, cumbersome and harsh conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0054] The reagents used are all commercially available products, the solvents are conventionally dried, and the synthesis and operation of sensitive compounds are performed using standard vacuum line technology. Reagent instructions: PE—petroleum ether; DCM—dichloromethane; THF—tetrahydrofuran; EtOH—ethanol; KOH—potassium hydroxide; PPA—polyphosphoric acid; EtOAc—ethyl acetate;
[0055] BINAP—1,1'-binaphthyl-2,2'-bisdiphenylphosphine; TBAB—tetra-n-butylammonium bromide
[0056] Referring to related drawings, the preparation method of related series of compounds is carried out in the following steps:
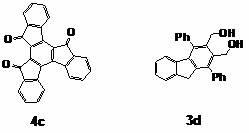
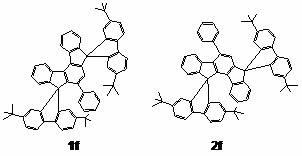
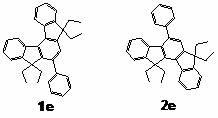
[0057] Preparation of Indenofluorene Series Derivatives
[0058] Precursor compound 1,3-diphenyl-indene[ a ]cyclopentadiene-2,8-dione was prepared by referring to literature (Ried, W.; Freitag, D. Chem. Ber. 1966, 99, 2675-2677).
[0059] Dissolve ninhydrin (4.86 g, 27.3 mmol) and 1,3-diphenylacetone (5.73 g, 27.3 mmol) in 40 mL of hot ethanol, slowly add 3.5 mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com