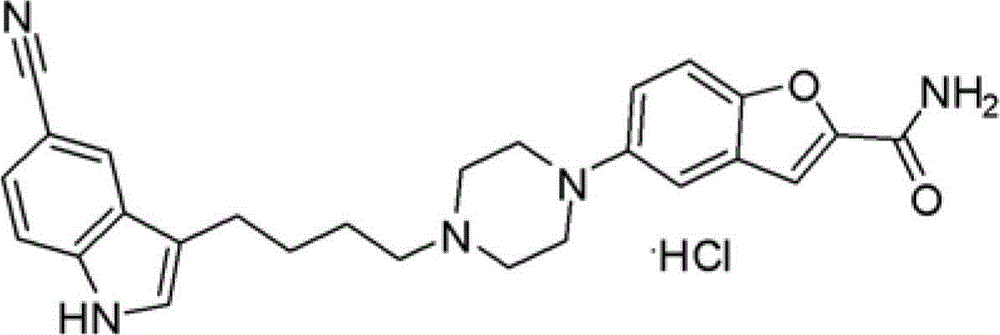
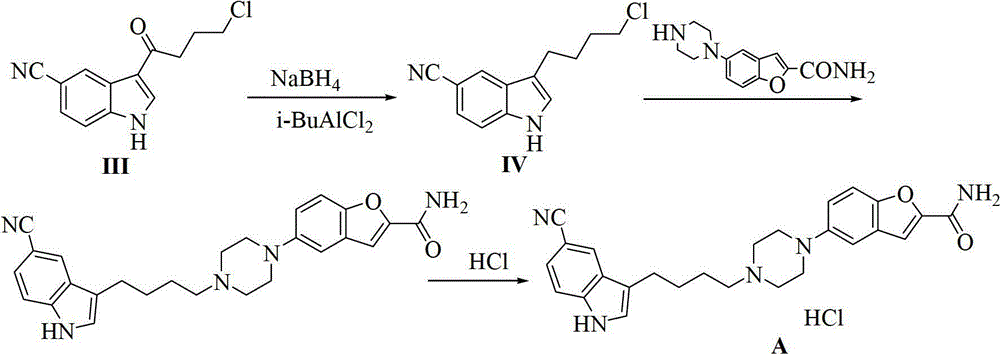
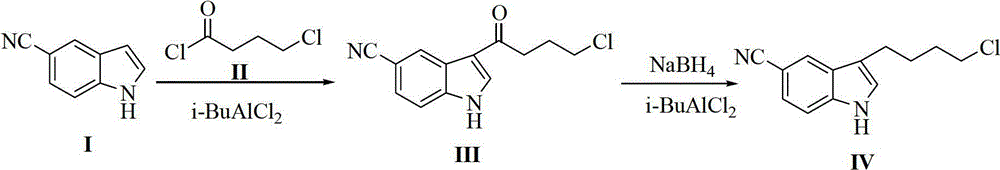
Preparation method of 3-(4-chlorobutyl)-5-cyanoindole
A cyanoindole and chlorobutyl technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of high cost, unsuitable for scale-up production, complicated post-processing operations and the like, and achieves convenient industrial production, stable and controllable quality, and high product quality. good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13-(4
[0068] The preparation of embodiment 13-(4-chlorobutyl)-5-cyanindole
[0069] Aluminum trichloride (43.2g, 0.32mol, 2.3eq) was added to a mixed solvent of nitromethane (50g) and dichloromethane (150g), and stirred. Add 4-chlorobutyryl chloride (25g, 0.177mol, 1.25eq) dropwise at 0-10°C, and then add dropwise the solution of the compound shown in formula (I) (20g (0.141mol, 1.0eq) of compound of formula I dissolved in 50g nitromethane and 150g dichloromethane). After reacting for about 2 hours, TLC showed that the reaction was complete. Add anhydrous tetrahydrofuran (400g) dropwise at 0-10°C. After dropping, add anhydrous aluminum trichloride (38g, 0.282mol, 2eq.) in batches at a temperature of -5-5°C; Add sodium borohydride (25g, 0.66mol, 4.68eq) at ~5°C. After the addition, keep stirring at -5~5°C. After the reaction is complete, add the reaction solution dropwise into ice water. The liquid was extracted and separated, and the organic phase was washed with saturated brine,...
Embodiment 23-(4
[0072] Preparation of Example 23-(4-chlorobutyl)-5-cyanindole
[0073] Aluminum trichloride (90g, 0.675mol, 2.3eq.) was added to a mixed solvent of nitromethane (100g) and dichloromethane (300g), and stirred. 4-Chlorobutyryl chloride (48.1g, 0.341mol, 1.18eq) was added dropwise at 0-10°C, and the addition was completed. Add dropwise the solution of the compound shown in formula (I) (the compound of formula I (41g, 0.288mol, 1.0eq) is dissolved in 100g nitromethane and 300g dichloromethane), dropwise at 0-10°C, after the reaction is complete, Anhydrous tetrahydrofuran (800 g) was added dropwise at 0-10° C., and the drop was completed. Add anhydrous aluminum trichloride (67.7g, 0.51mol, 1.8eq) in batches at 0-10°C; after adding, add sodium borohydride (32.7g, 0.864mol, 3eq.) at 0-10°C, Keep the temperature at 0-10°C. After the reaction was complete, the reaction solution was added dropwise to ice water. The liquid was extracted and separated, and the organic phase was washed...
Embodiment 33-(4
[0074] Preparation of Example 33-(4-chlorobutyl)-5-cyanindole
[0075] Aluminum trichloride (44g, 0.33mol, 2.3eq) was added to a mixed solvent of nitromethane (24g) and dichloromethane (96g), and stirred. 4-Chlorobutyryl chloride (23g, 0.163mol, 1.15eq) was added dropwise at 0-10°C, and the addition was completed. Add dropwise the solution of the compound shown in formula (I) (the compound of formula I (20g, 0.141mol, 1.0eq) is dissolved in 24g nitromethane and 96g dichloromethane), dropwise at 0~10°C, after the reaction is completed, Add anhydrous tetrahydrofuran (200g) dropwise to the reaction solution at 0-10°C. After the drop is complete, add anhydrous aluminum trichloride (37.5g, 0.282mol, 2.0eq) in batches at -5-5°C; after the addition, Sodium borohydride (16g, 0.423mol, 3.0eq) was added at -5~5°C, maintaining the temperature at -5~5°C. After the reaction was complete, the reaction solution was added dropwise to ice water. The liquid was extracted and separated, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com