Patents
Literature
162 results about "Obeticholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
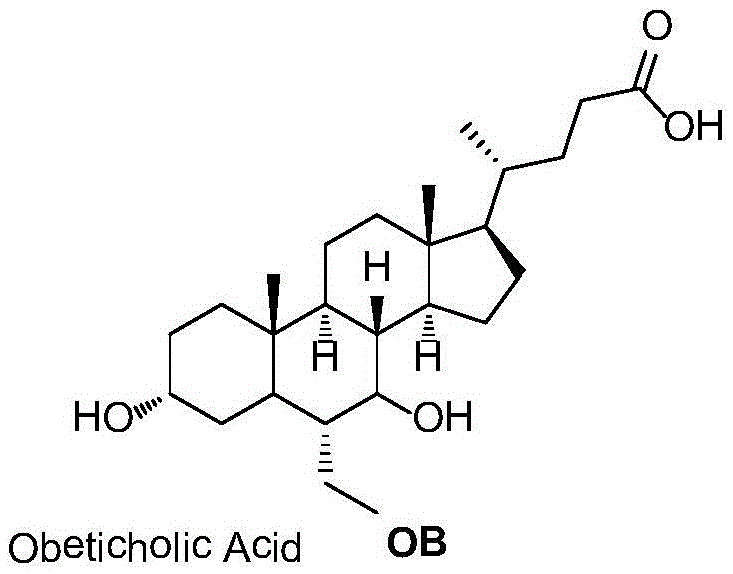
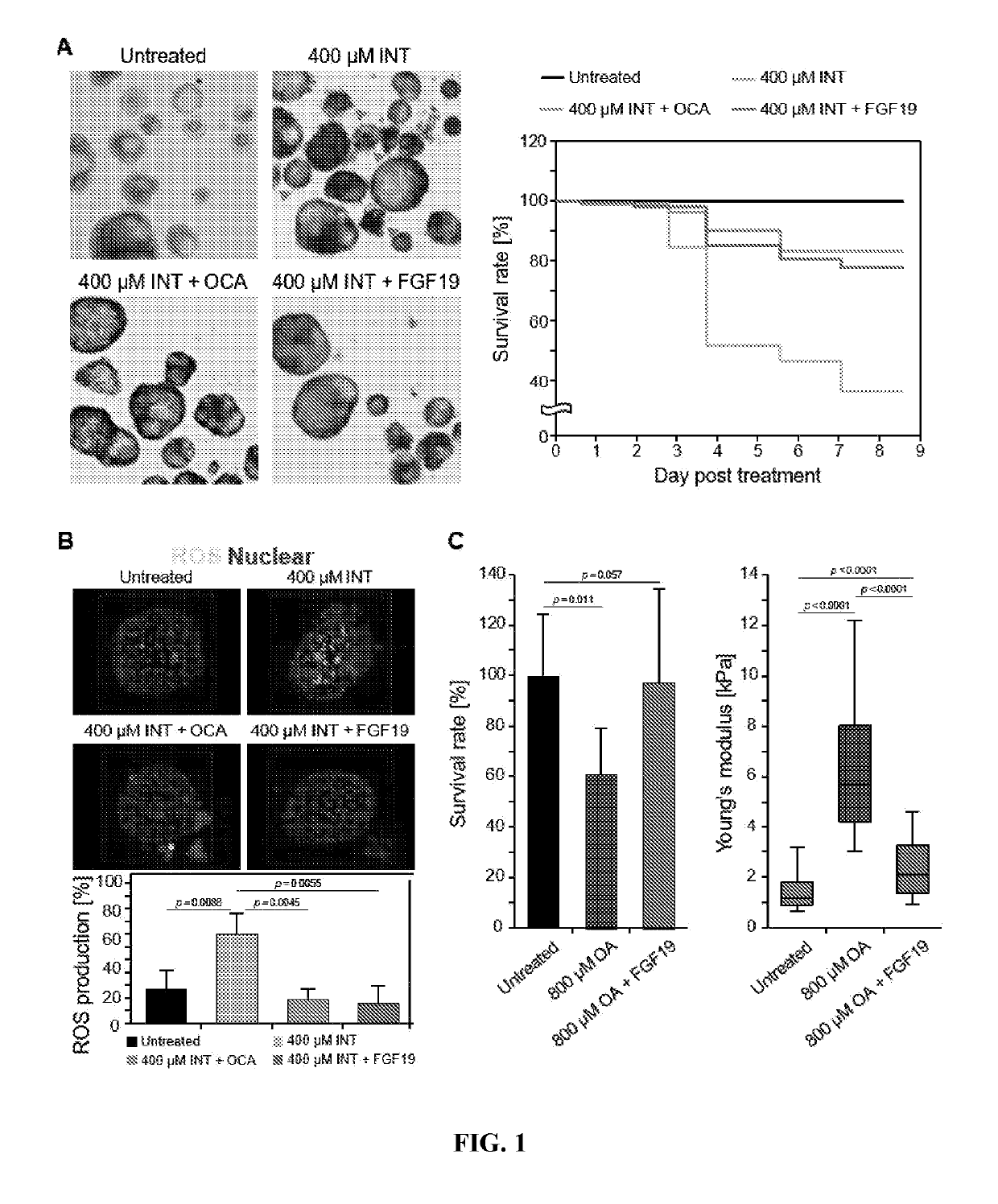
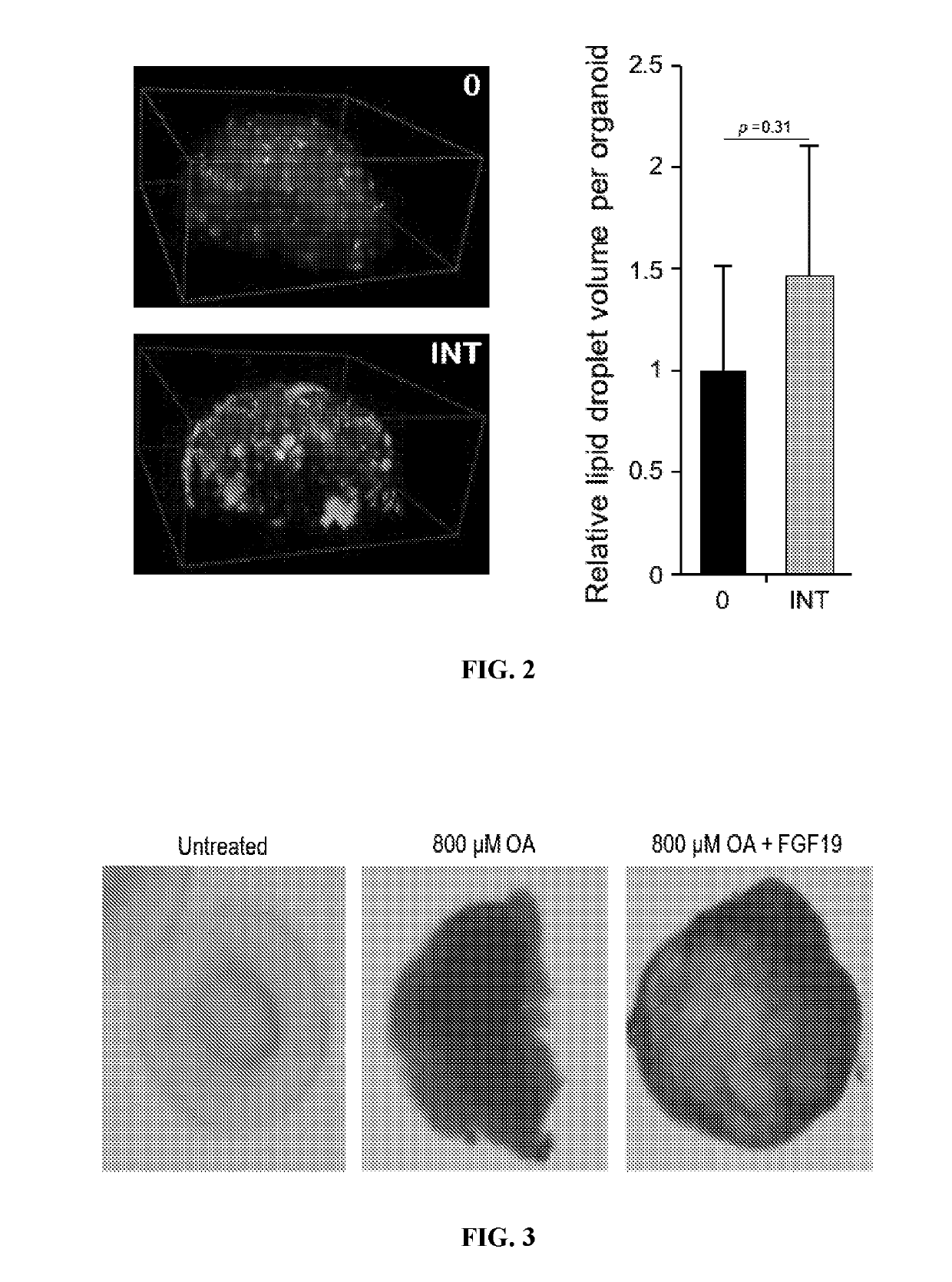
This medication is used alone or in combination treatment for a certain liver disease (primary biliary cholangitis-PBC).
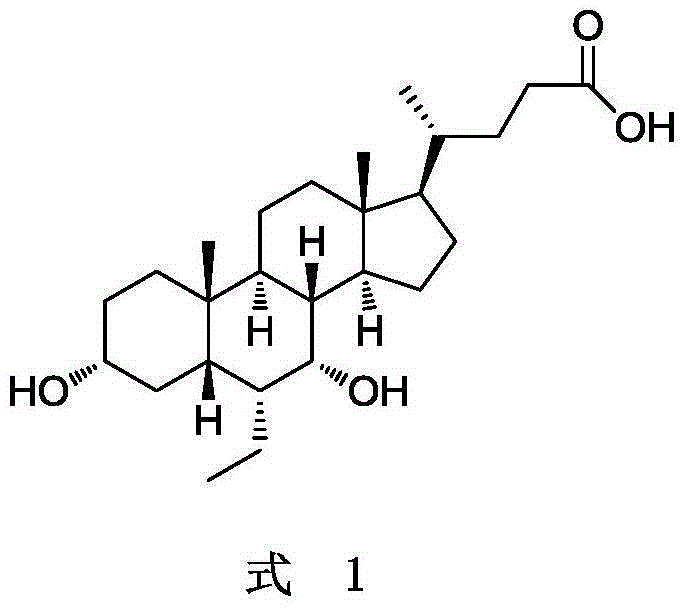
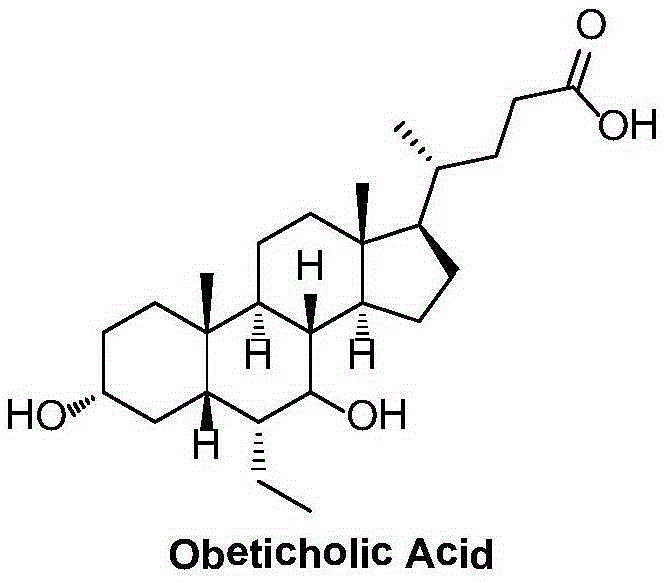
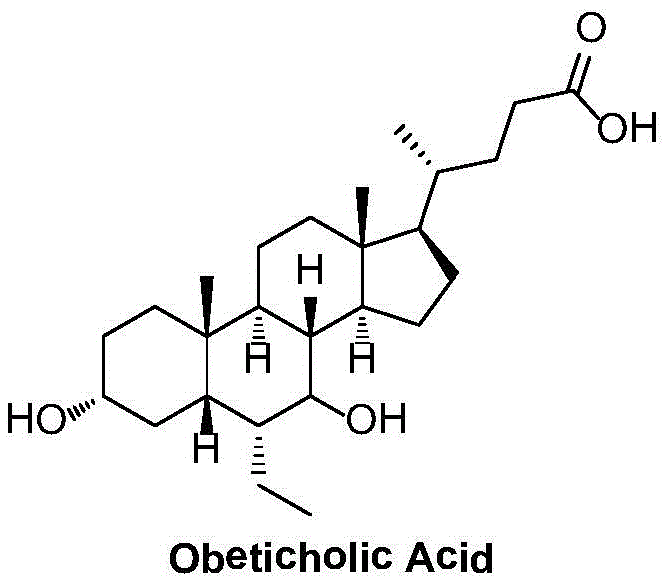
Obeticholic acid crystal form I, preparation method, pharmaceutical composition, and application thereof
ActiveCN105175473AImprove stabilitySimple processOrganic active ingredientsMetabolism disorderFiberAlkane
The invention relates to obeticholic acid crystal form I, which is represented in the description, and a preparation method. The preparation method comprises the following steps: dissolving obeticholic acid in an organic solvent to form a solution, wherein the organic solvent is halogenated alkane or ester, and volatilizing the solution until all solvent is completely volatilized so as to obtain the obeticholic acid crystal form I. Compared with the known solid forms of obeticholic acid, the novel crystal form is more stable and is more suitable for being applied to industrial production. The invention also relates to a pharmaceutical composition of the novel crystal form and an application thereof in the preparation of drugs for treating and / or preventing FXR mediated impaired adjustment, cardiovascular disease, cholestatic liver disease, high HDL cholesterol, high triglyceride, and fibrosis diseases.
Owner:LIVZON PHARM GRP INC
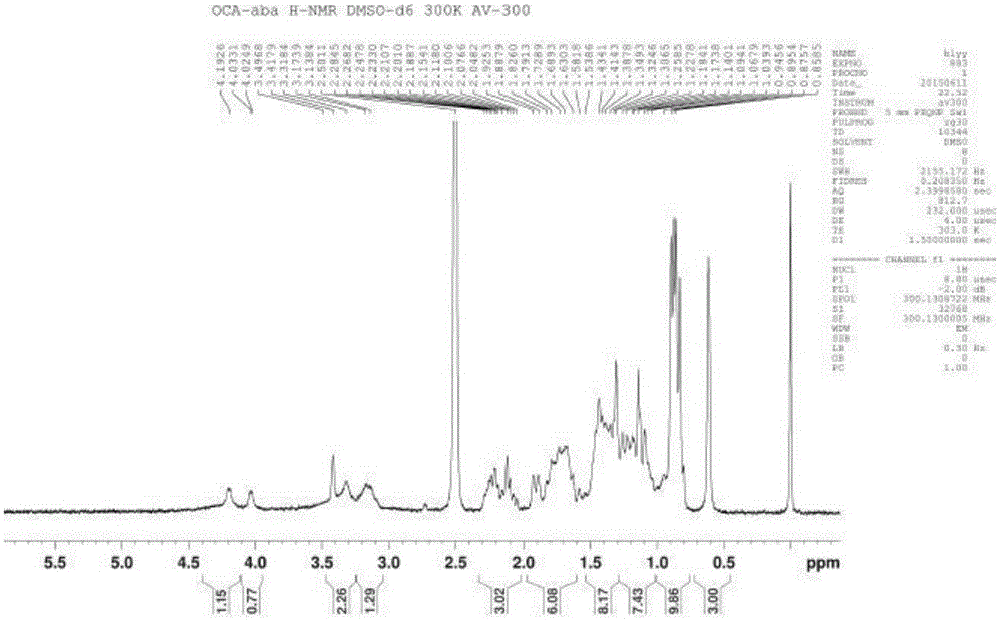
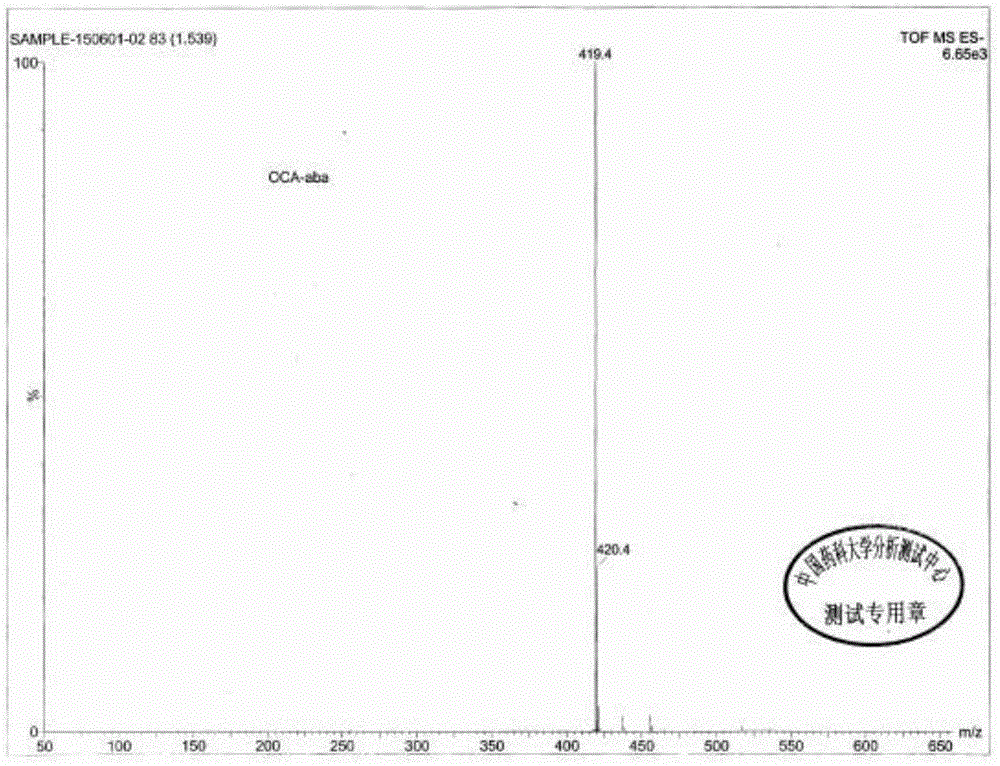
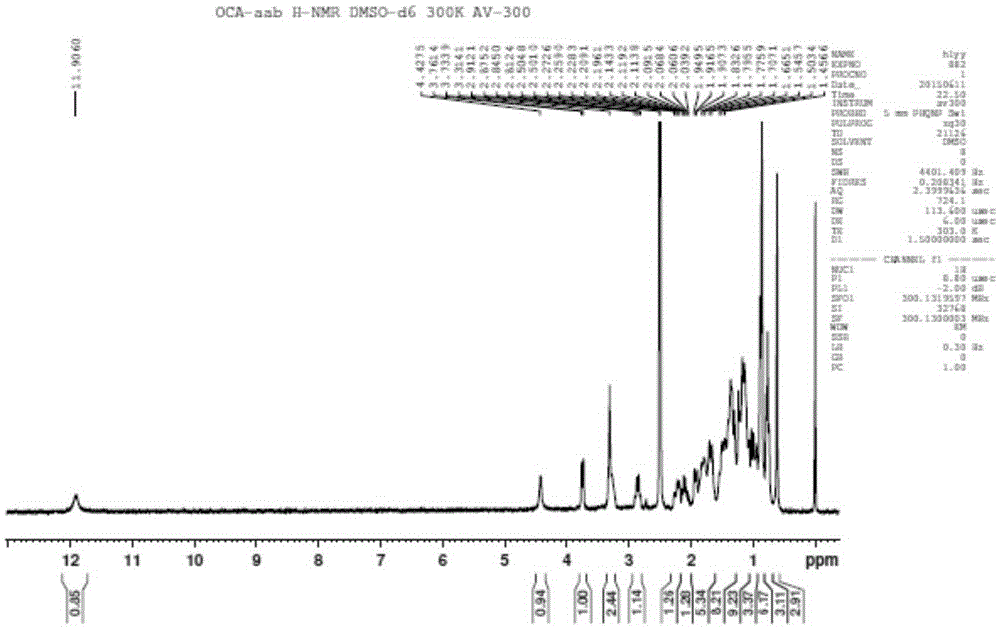
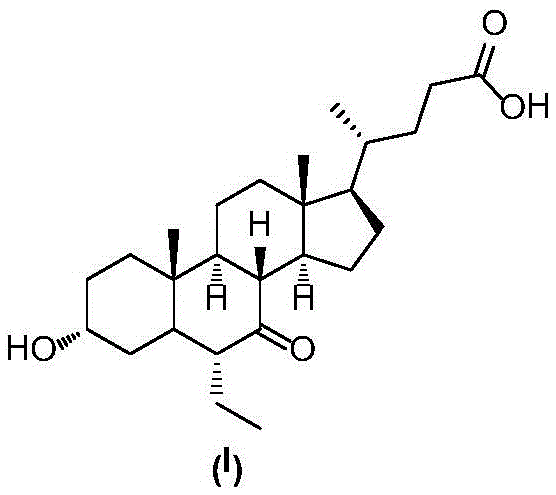
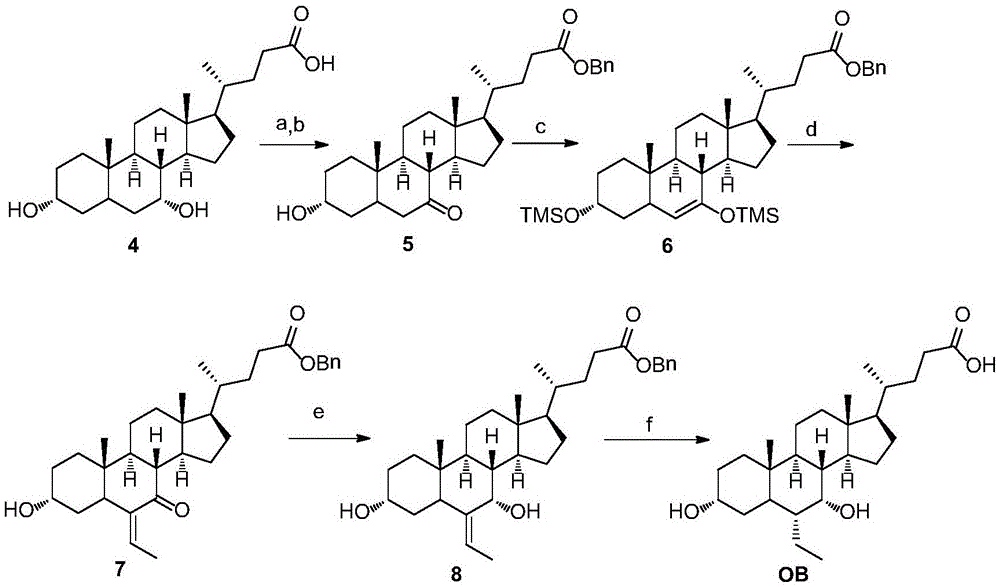
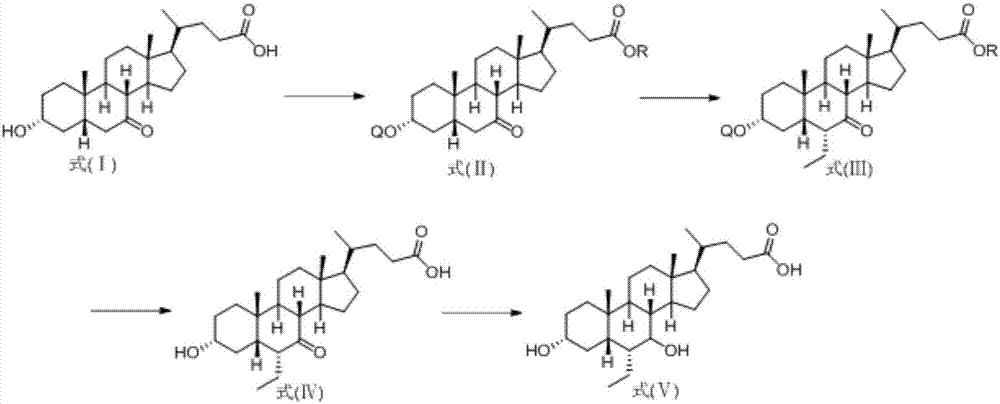
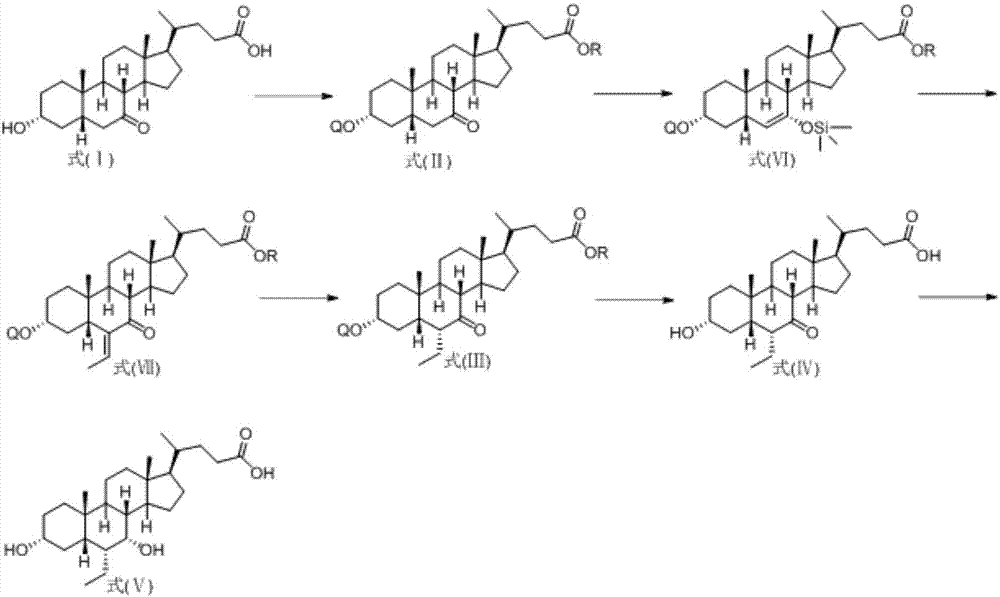
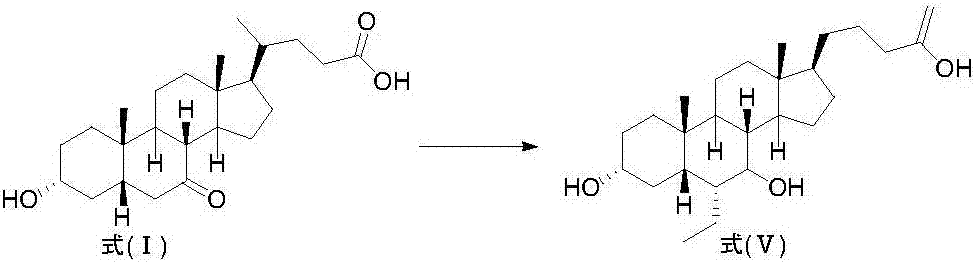
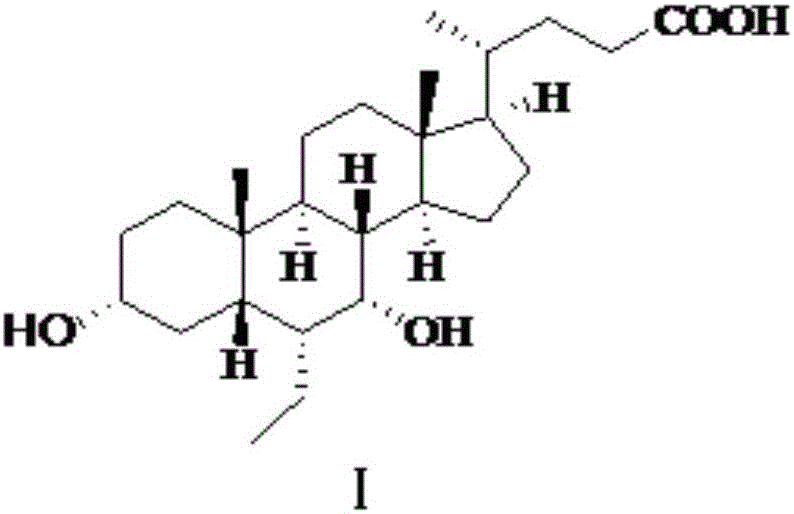
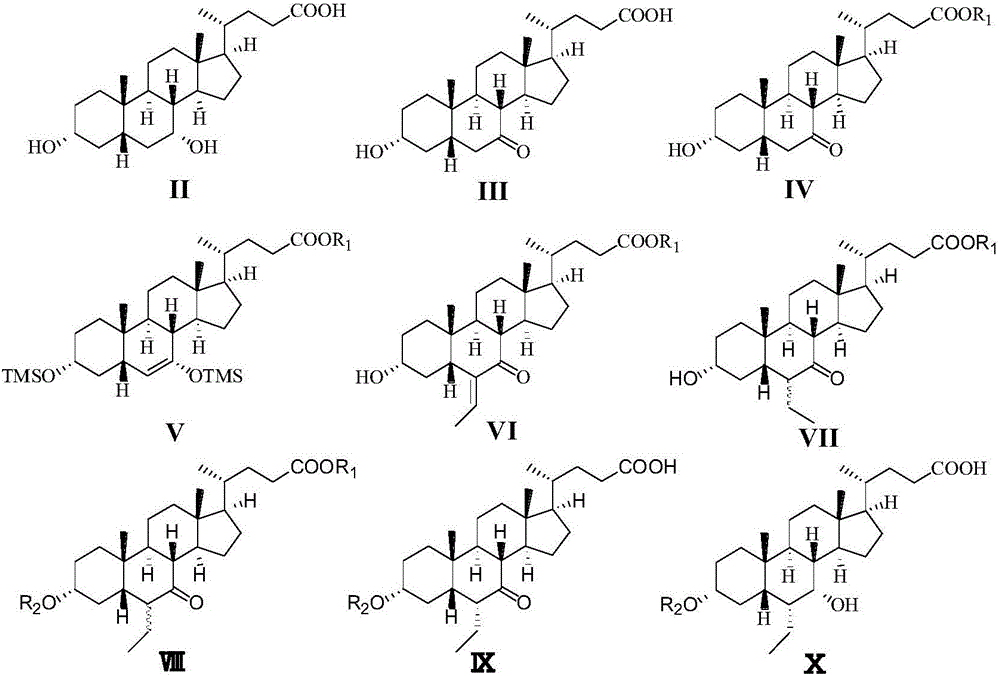
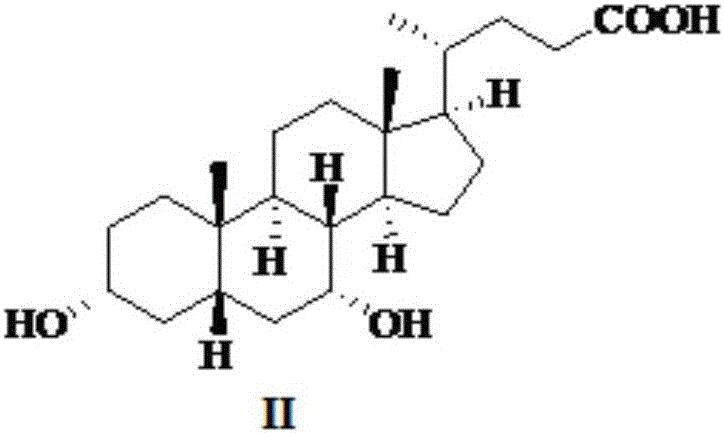
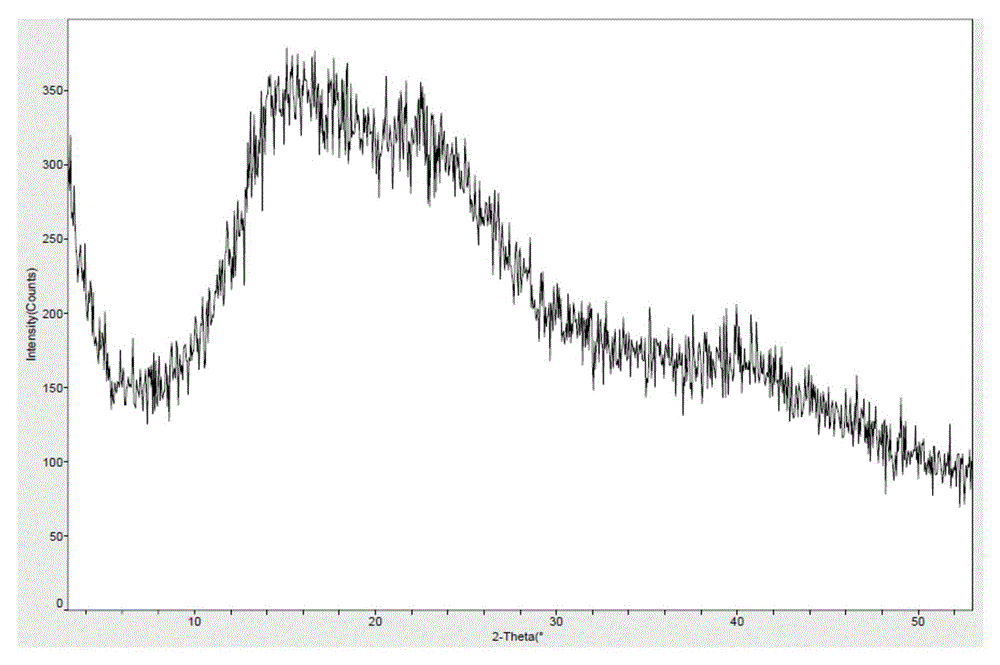
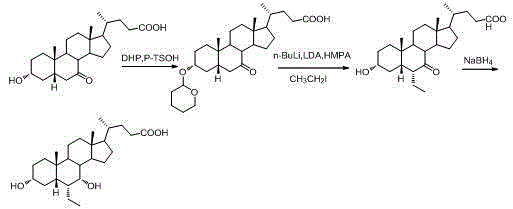
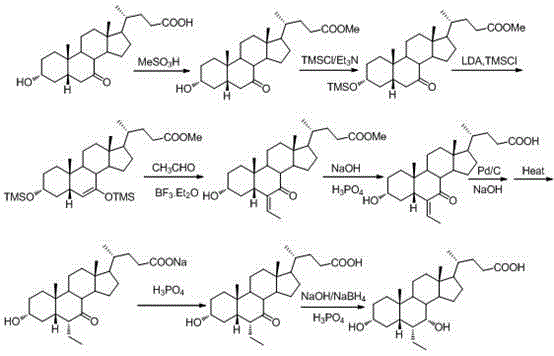
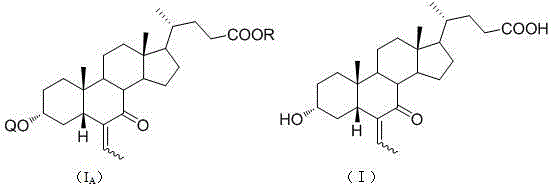
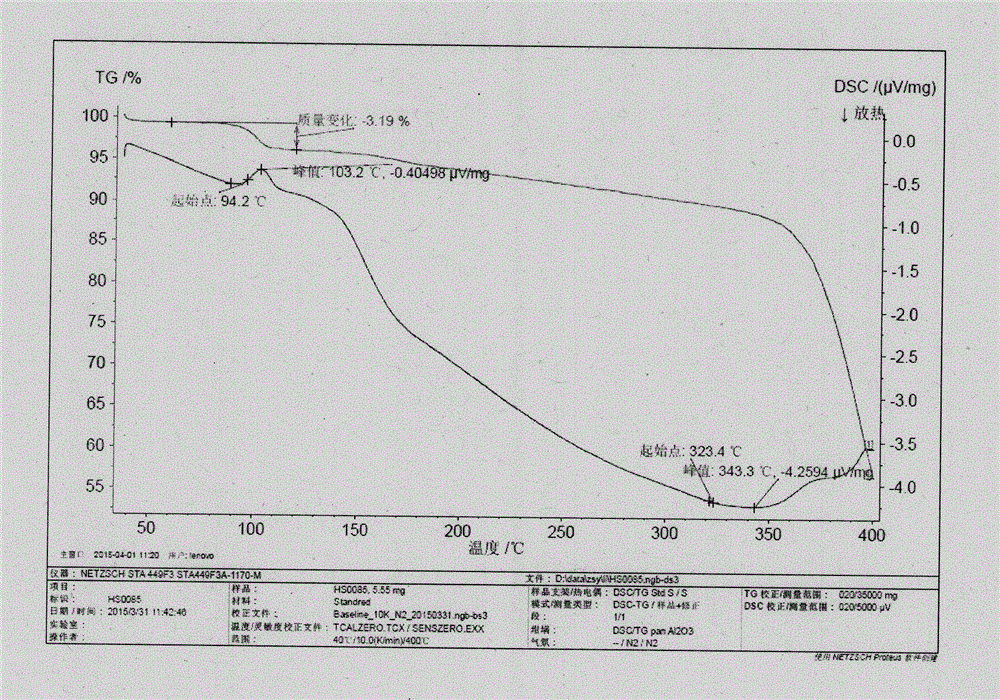
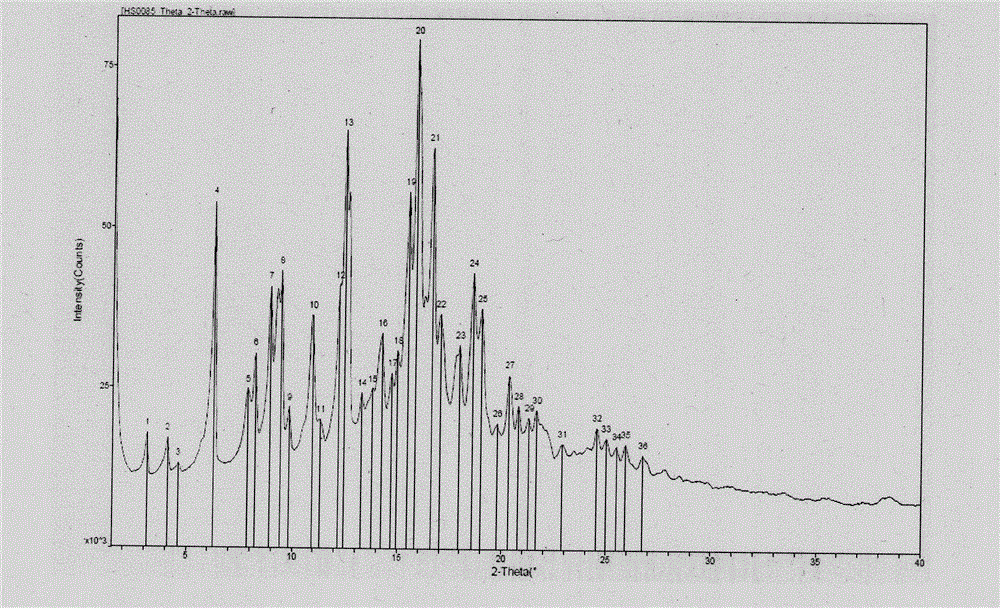
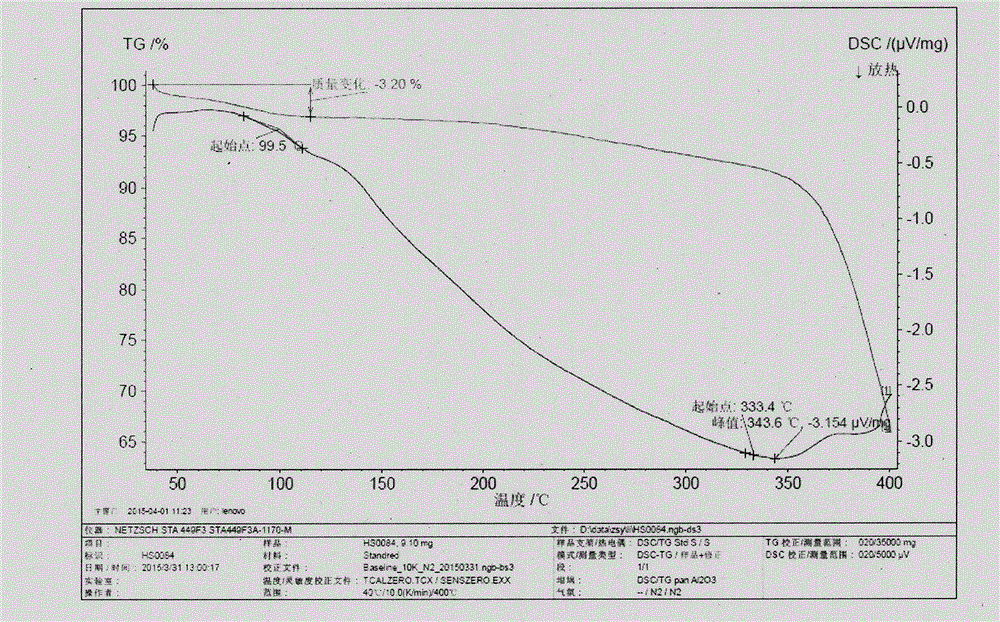
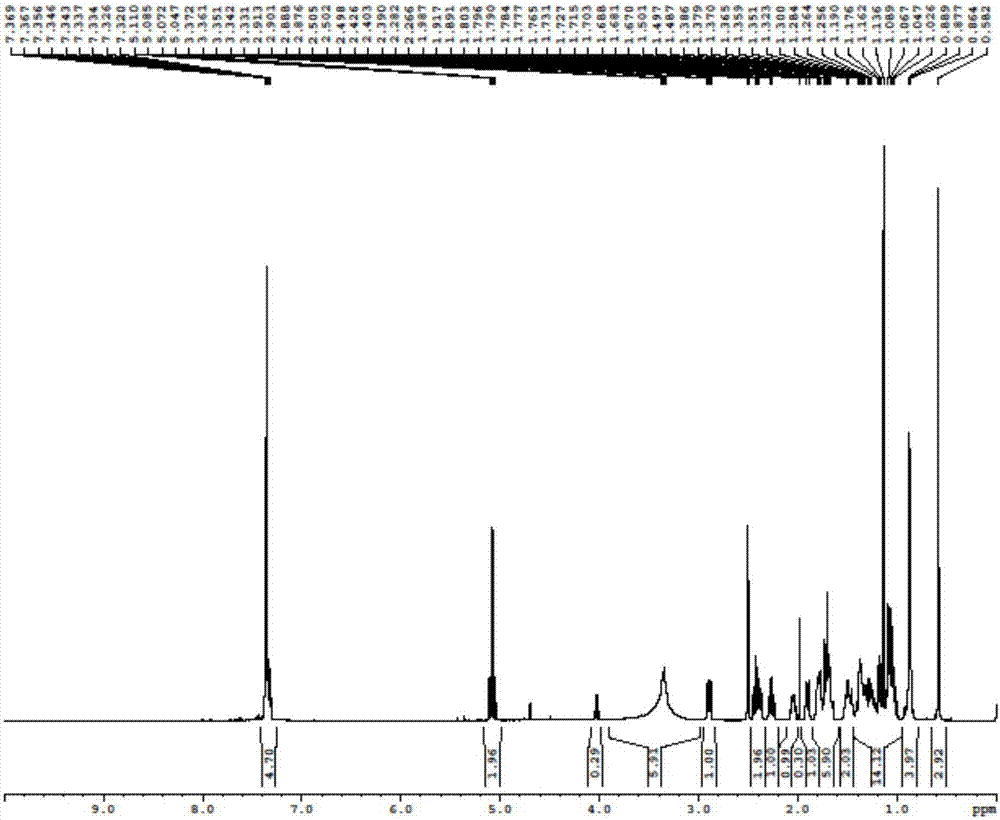
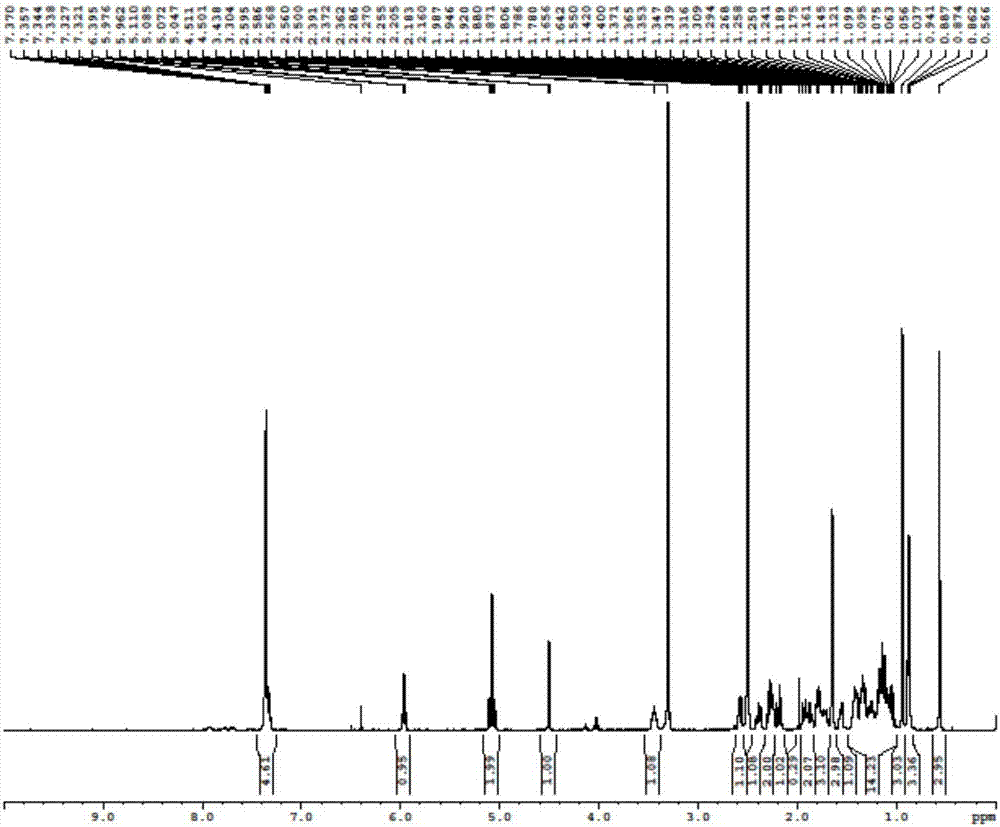
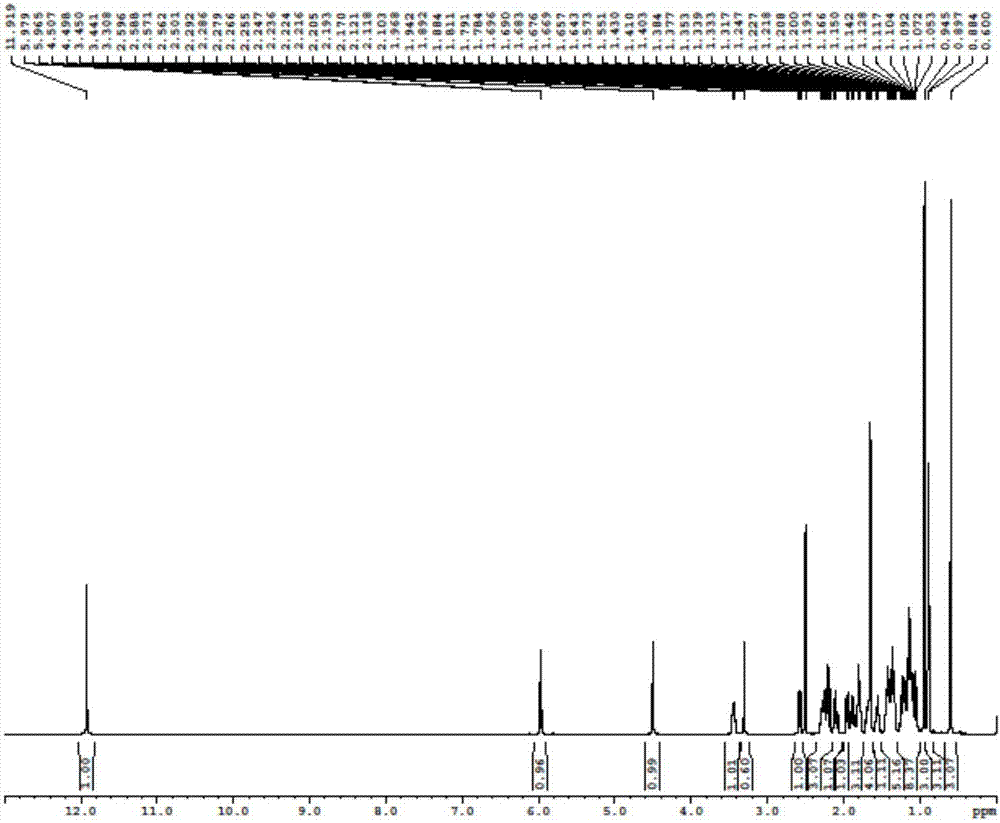
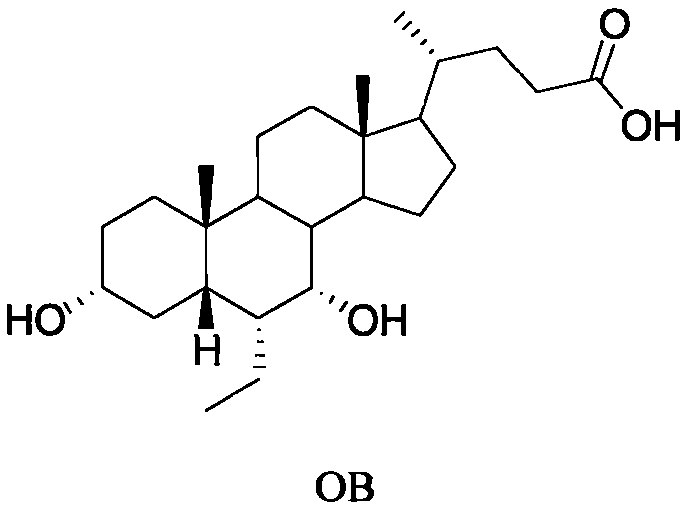
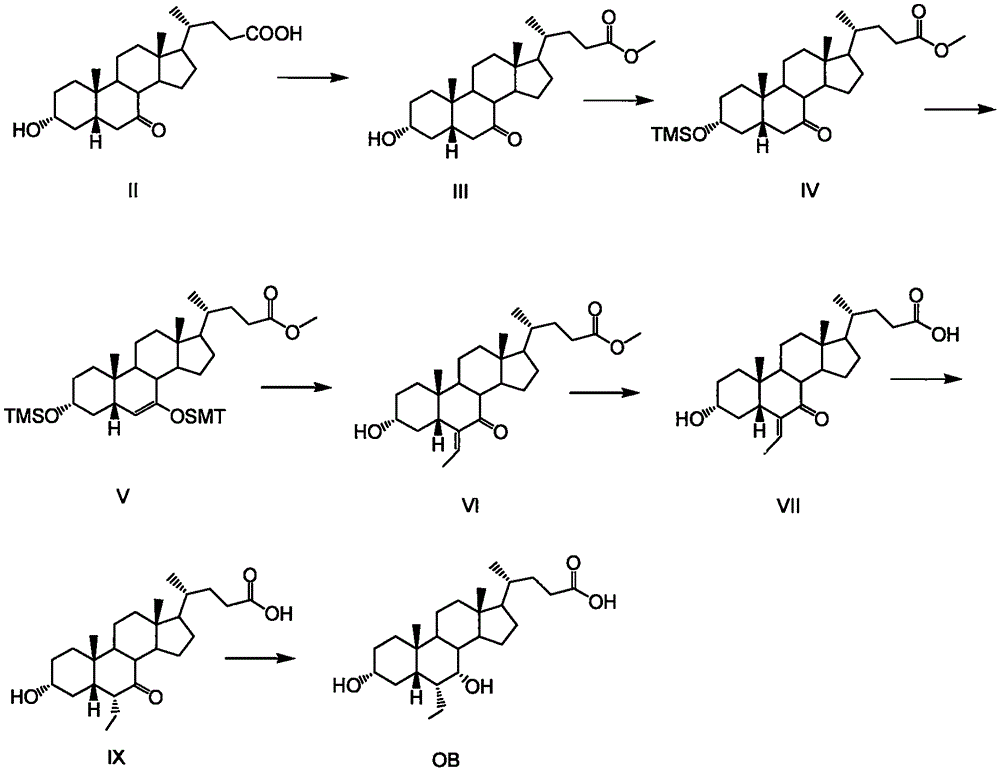
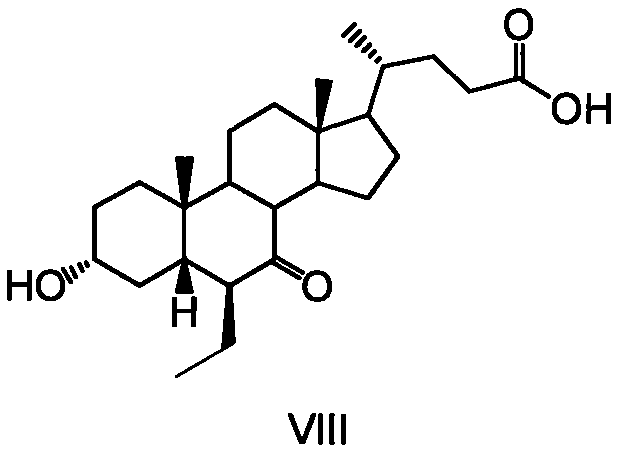
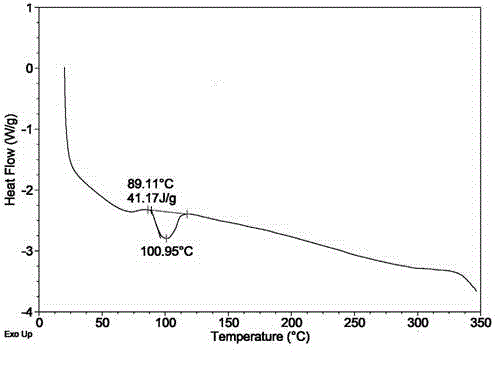
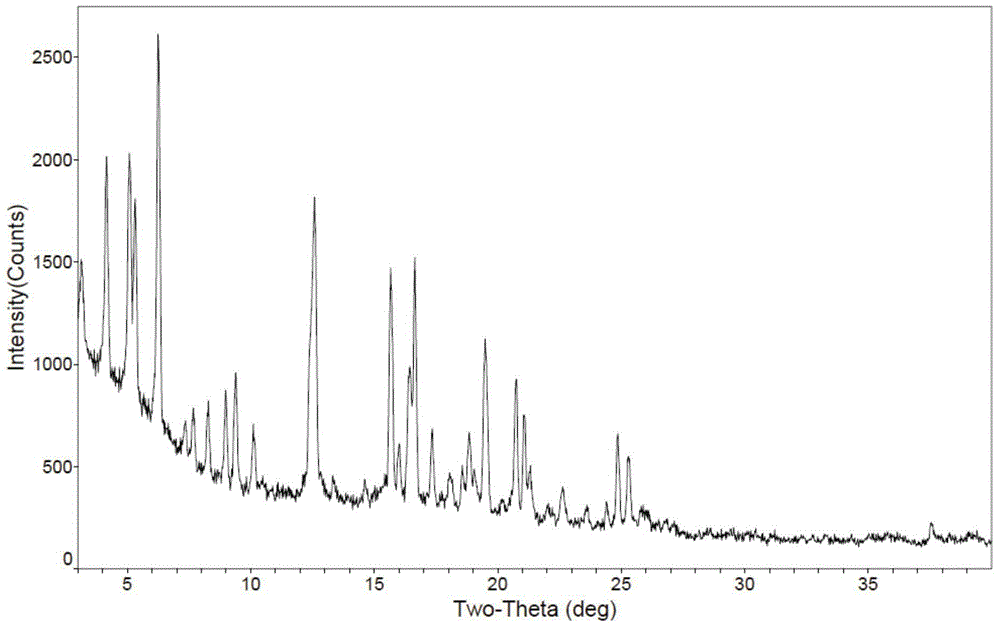
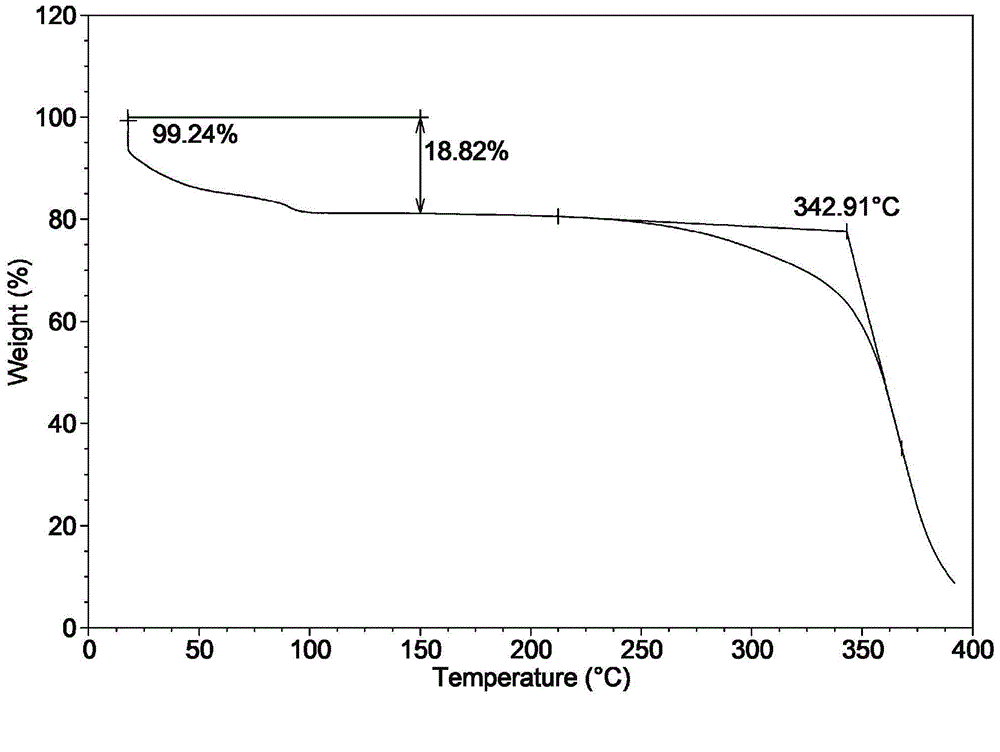
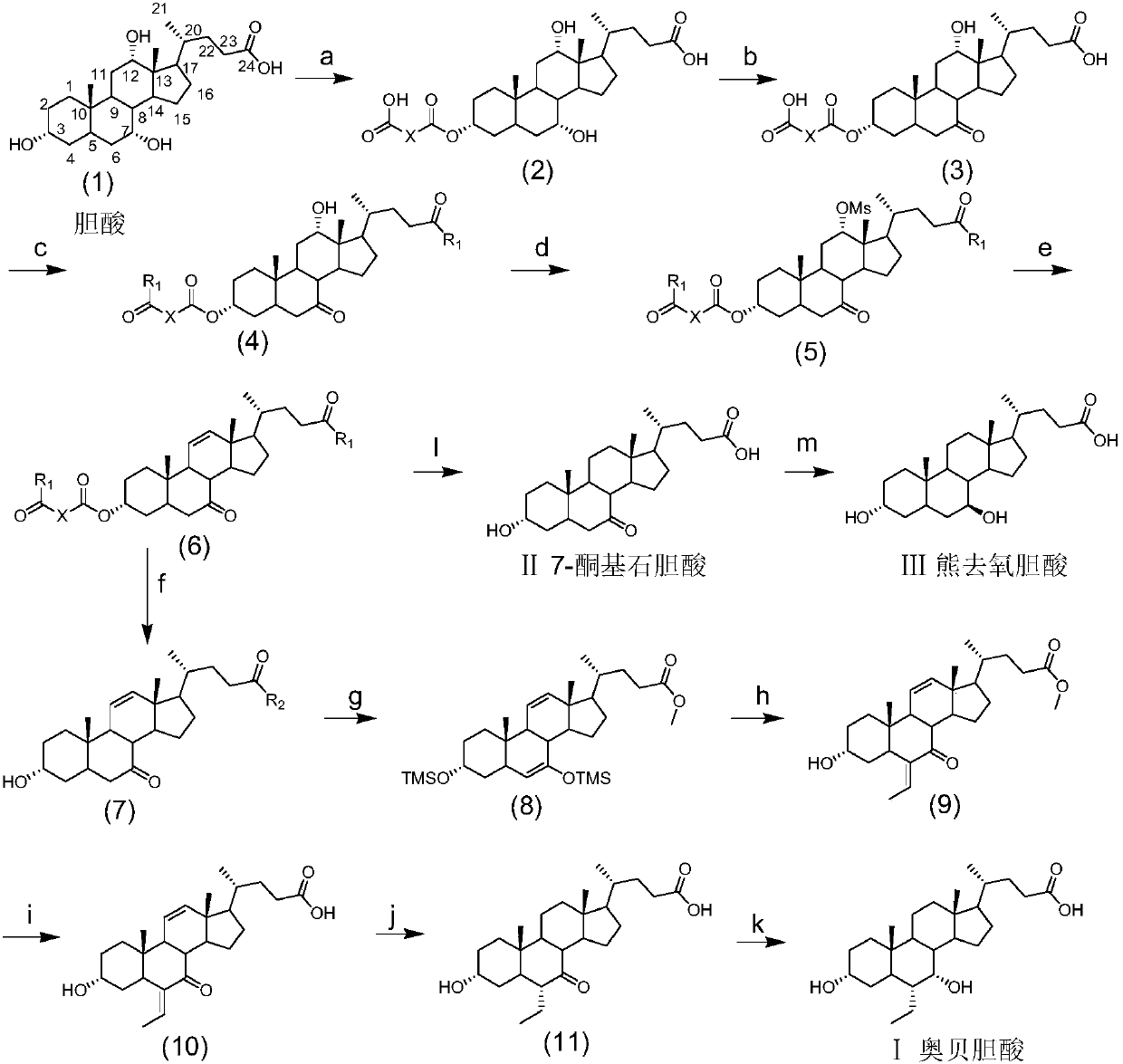
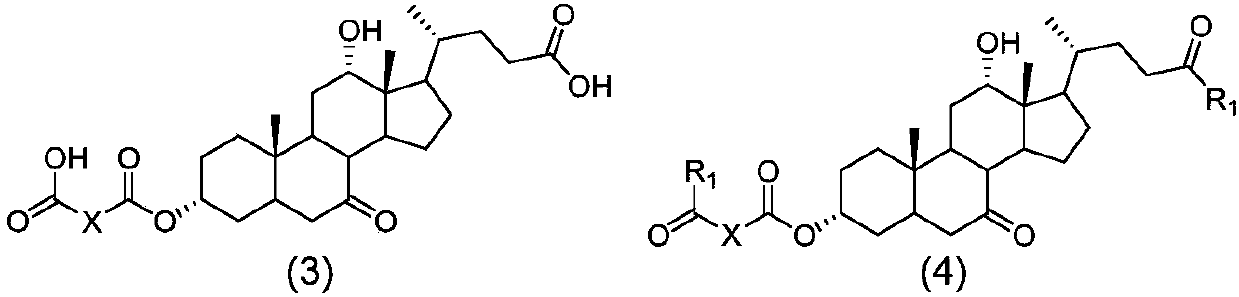
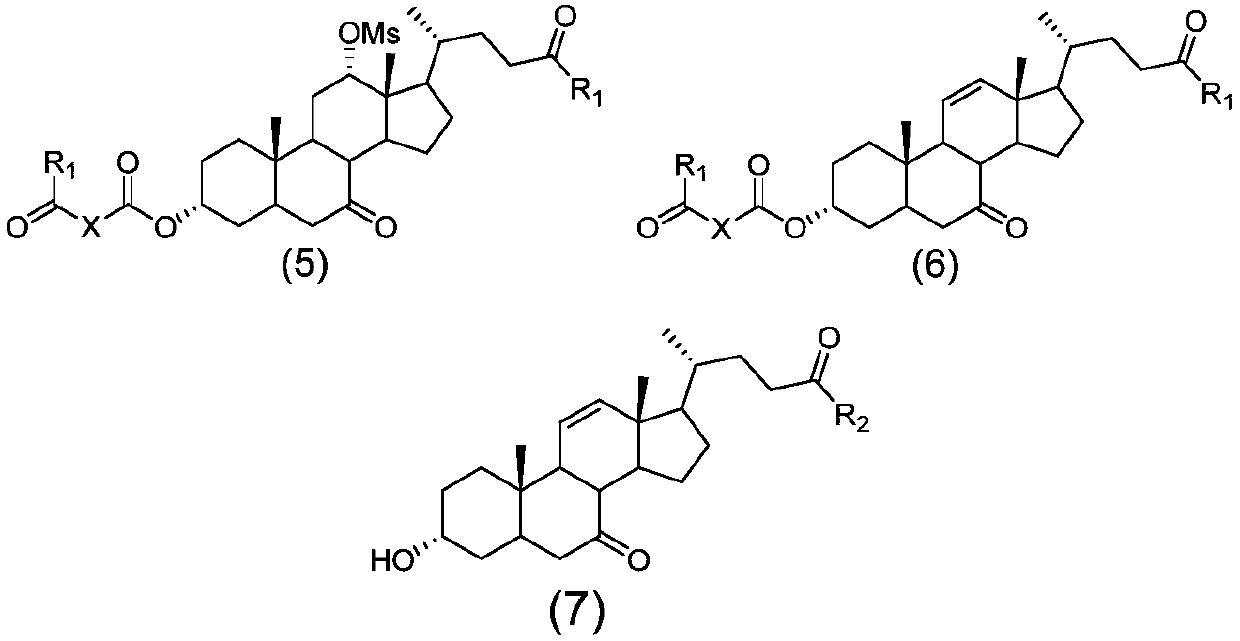
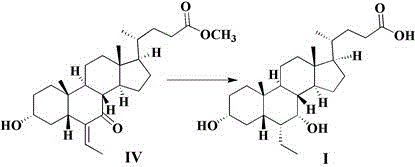
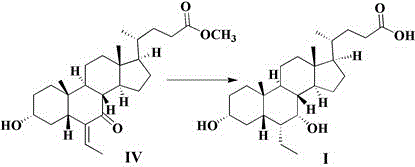
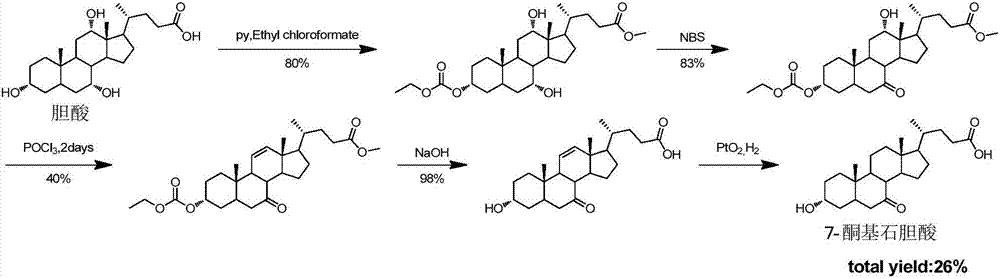
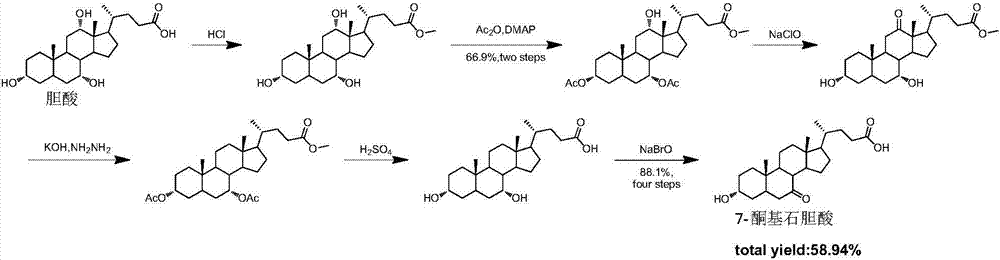
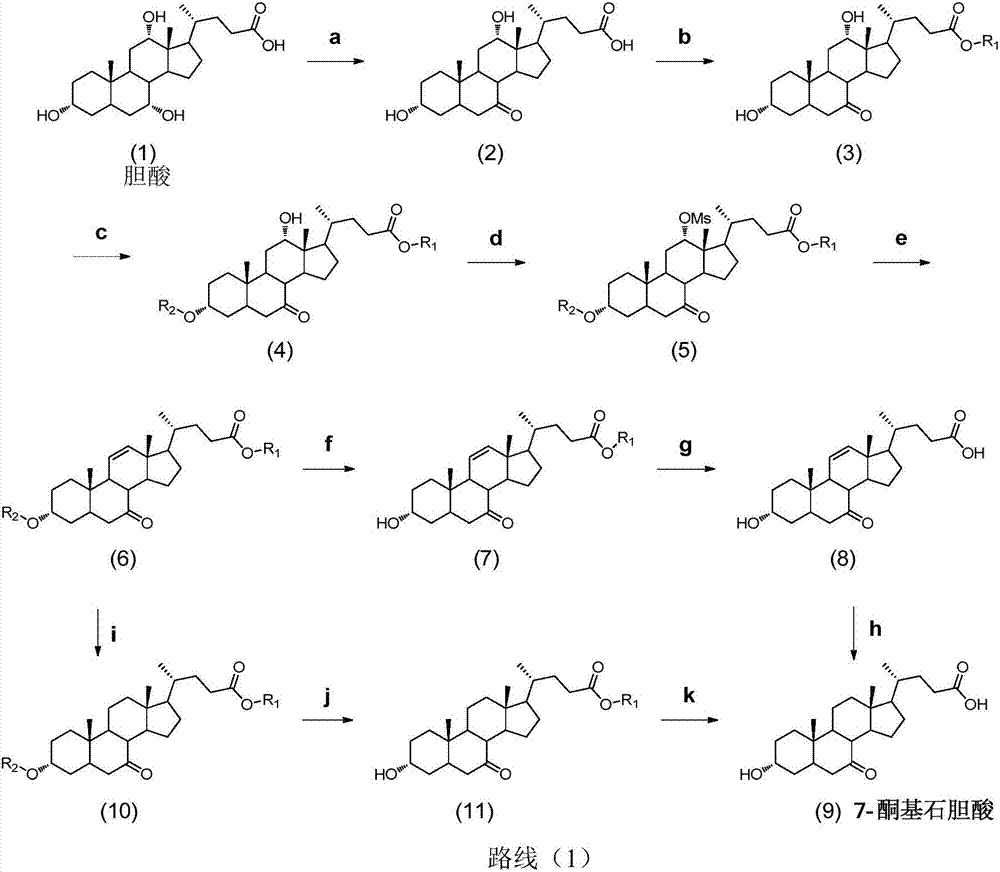
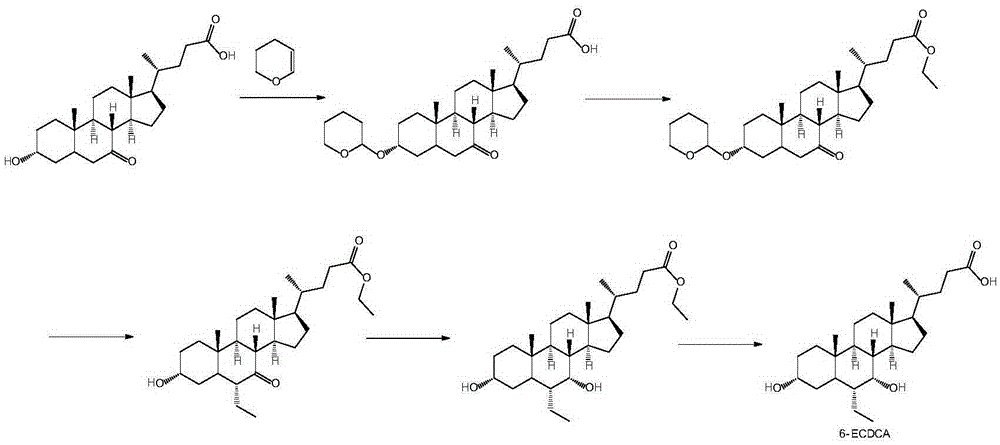
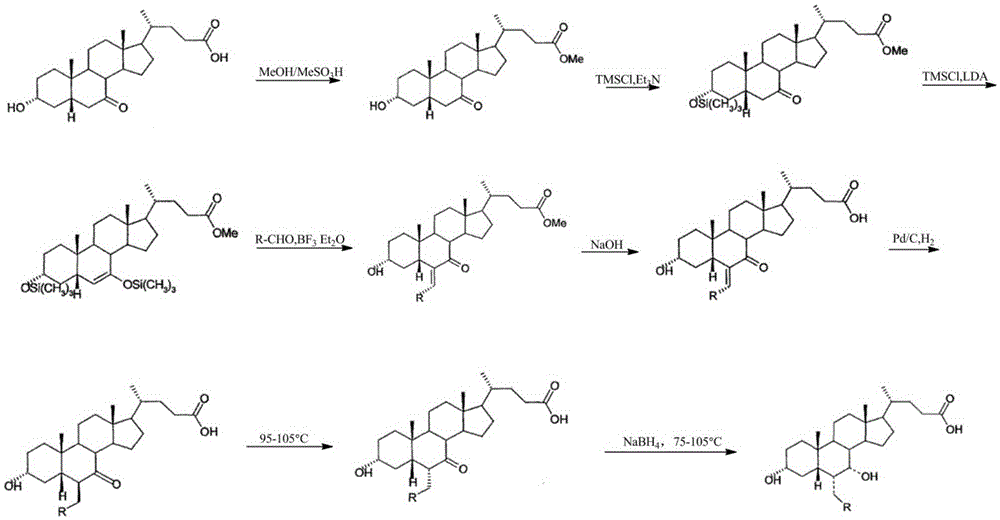
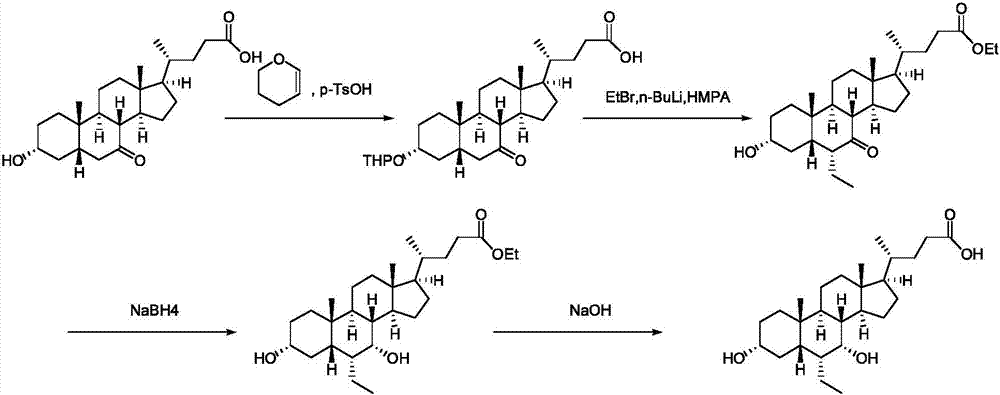
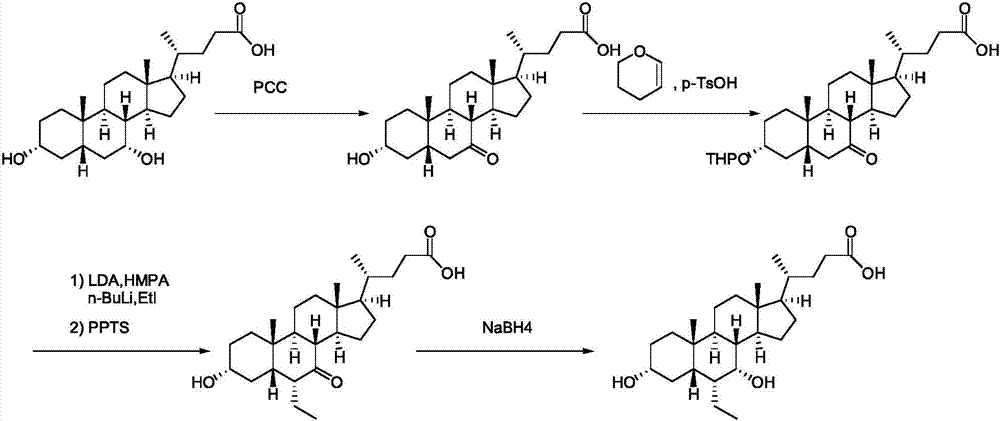
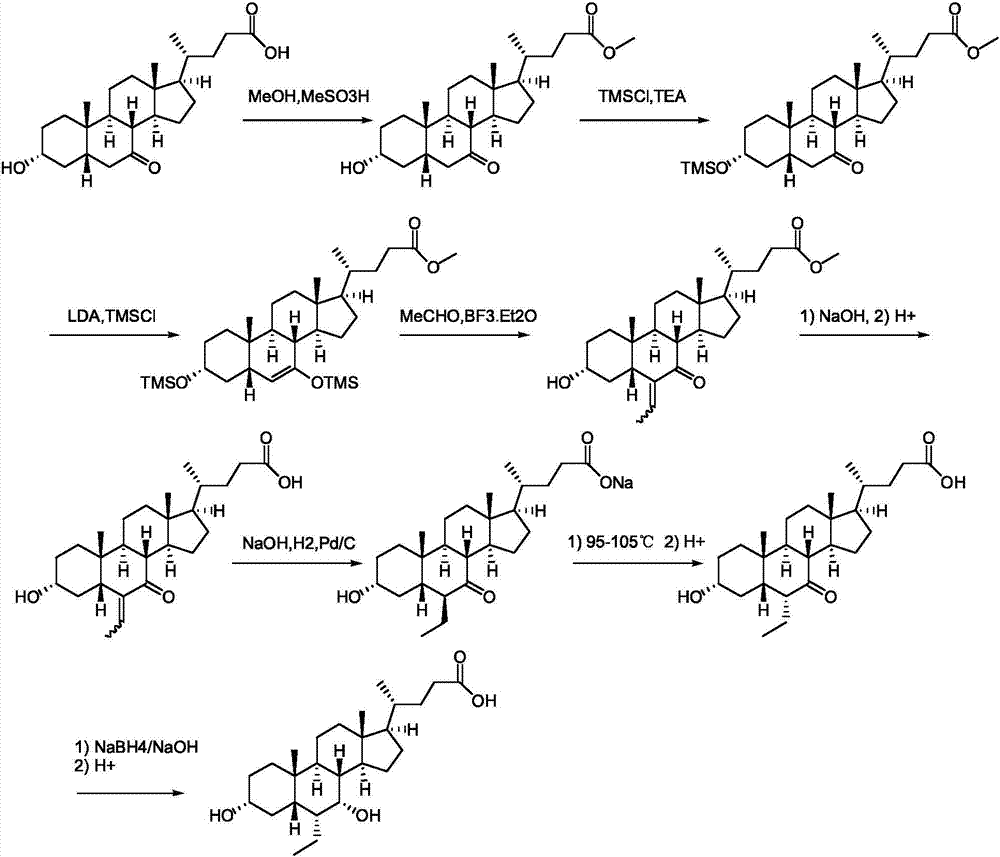
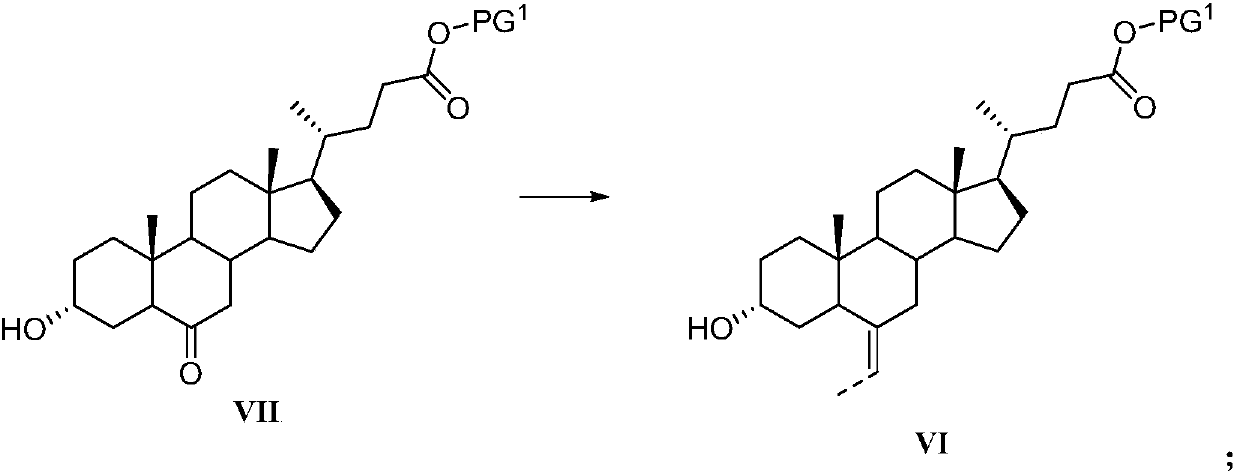
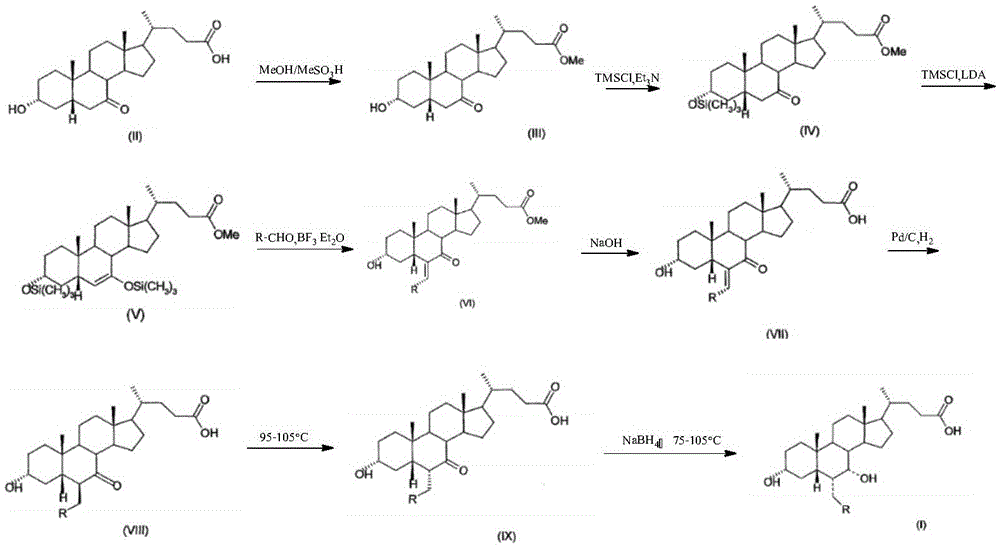
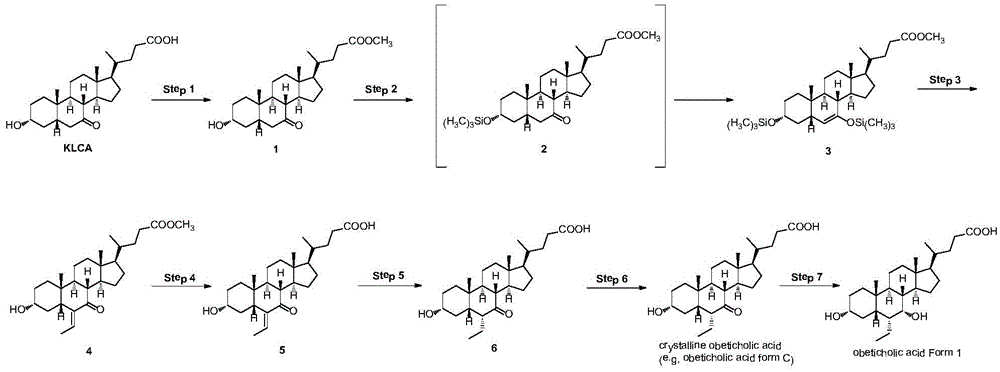
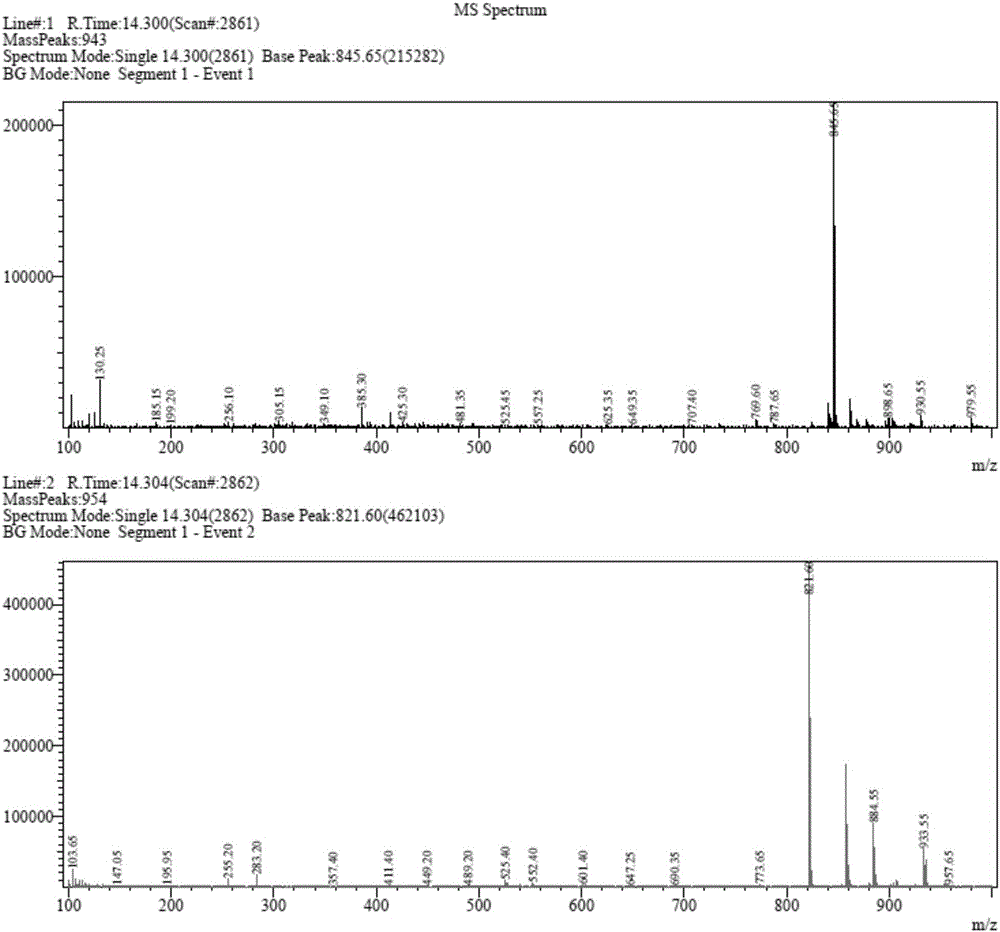
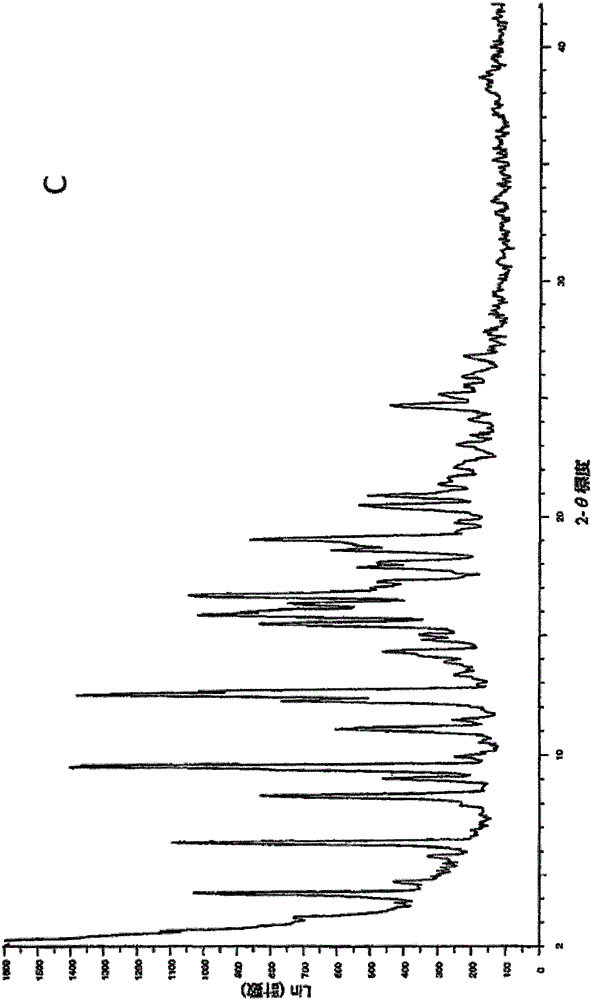
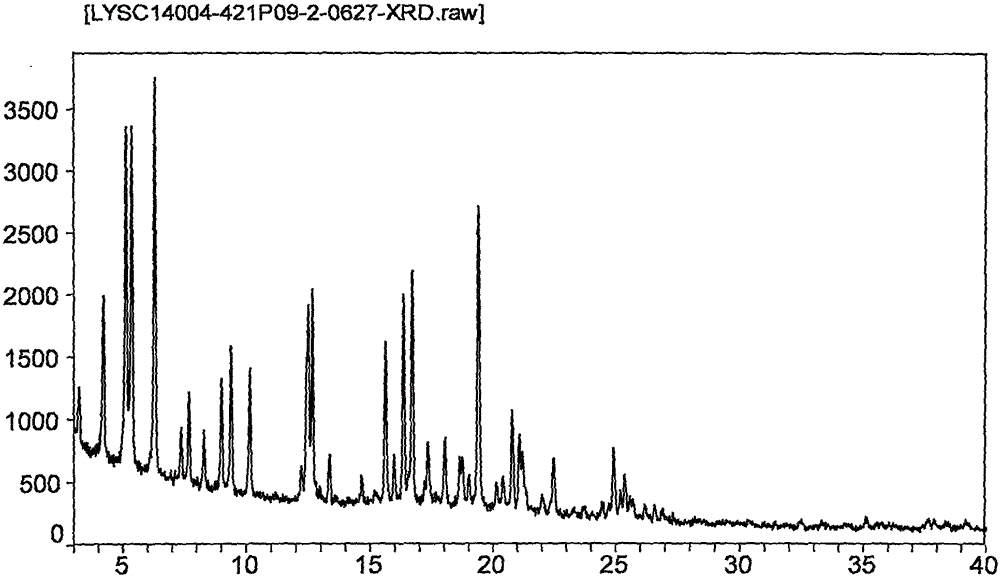
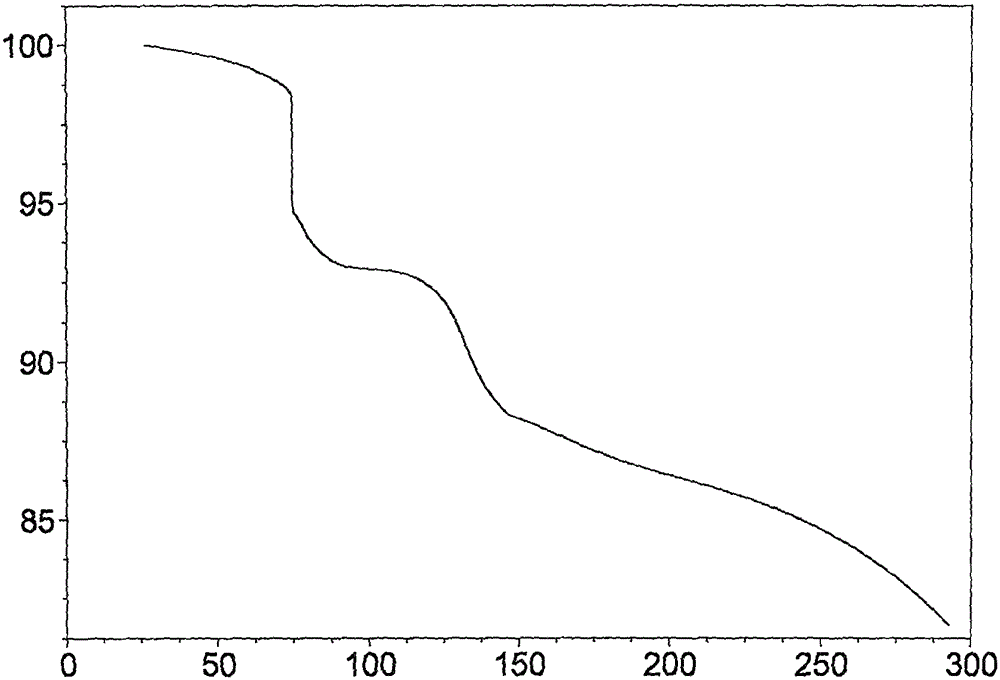
Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine
ActiveCN105669811ADifficult to purifyHigh purityMetabolism disorderSilicon compound active ingredientsKetoneMedicinal chemistry
The invention provides a preparation method of a 7-keto-6[alpha]-alkyl cholanic acid derivative. According to the preparation method provided by the invention, a 7-keto-6[beta]-alkyl cholanic acid derivative, as shown by a formula II, is used as a raw material, and the 7-keto-6[alpha]-alkyl cholanic acid derivative is prepared by converting a 6[beta] configuration into a 6[alpha] configuration under an acid or alkali condition. The invention also provides a 7-keto-6[beta]-alkyl cholanic acid derivative and an application thereof in preparation of 3[alpha],7[alpha]-dihydroxy-6[alpha]-alkyl-5[beta]-cholanic acid. The preparation method provided by the invention is simple and convenient, and is high in configuration conversion rate, and the product, the 7-keto-6[alpha]-alkyl cholanic acid derivative, is easy to purify, so that the purification difficulty for preparing the 3[alpha],7[alpha]-dihydroxy-6[alpha]-alkyl-5[beta]-cholanic acid is reduced.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for synthesizing, separating and determining obeticholic acid (OCA) isomer
The invention relates to an obeticholic acid (OCA) isomer namely an OCA-alpha alpha beta body, a synthetic method of the OCA-alpha beta alpha body, and a method adopting the reversed phase liquid chromatography condition to separate the OCA isomer. With adoption of the technical scheme disclosed by the invention, the OCA-alpha alpha beta body and the OCA-alpha beta alpha body with the HPLC purity greater than 98% can be obtained to meet quality control of the isomer in OCA.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Preparation method for obeticholic acid and intermediate thereof
The invention relates to a preparation method for obeticholic acid and an intermediate thereof. The intermediate 3alpha-hydroxy-6alpha-ethyl-7-keto-5beta-cholanic acid is obtained by subjecting 3alpha-hydroxy-6-ethylidene-7-keto-5beta-cholanic acid benzyl ester compounds to a reaction under the action of a catalyst and a hydrogen donor. The catalyst is selected from Pd / C or PtO2 or Raney Ni. The hydrogen donor is selected from cyclohexene or cyclohexadiene or tetrahydronaphthalene. According to the method, the yield is high, the stereoselectivity is high, safety is good, the reaction condition is mild, and the method is applicable to industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
Method for preparing obeticholic acid from new derivative of 3alpha-hydroxy-7-oxo-5beta-cholanic acid
The invention discloses a method for preparing obeticholic acid from a new derivative of 3alpha-hydroxy-7-oxo-5beta-cholanic acid. The synthesis method comprises the following steps: firstly, carrying out hydroxyl and carboxyl protection on the 3alpha-hydroxy-7-oxo-5beta-cholanic acid to prepare the corresponding new derivative; secondly, obtaining the obeticholic acid respectively according to two synthesis routes. According to the method disclosed by the invention, a safer protecting group reagent is utilized, and the problem that ultraviolet absorption of an intermediate is not strong is solved; the intermediate is easier to purify, the yield is improved, and the cost is reduced, so that the method is more suitable for industrialized amplification, and has remarkable creativity and actual application value.
Owner:HANGZHOU HEZE PHARMA TECH
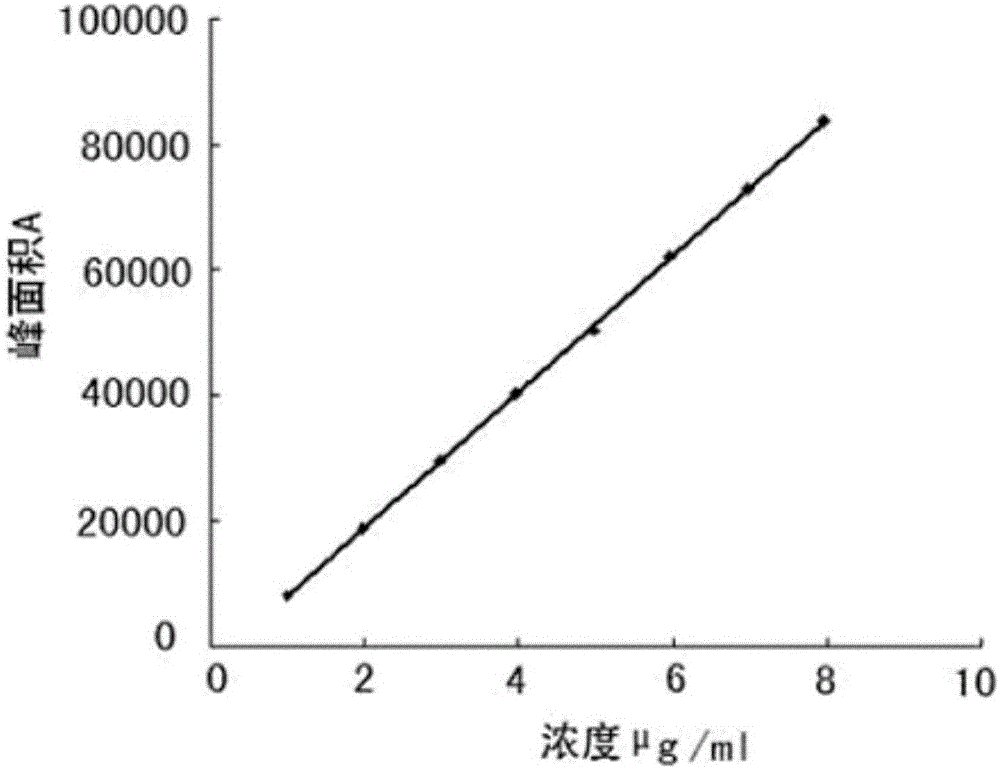
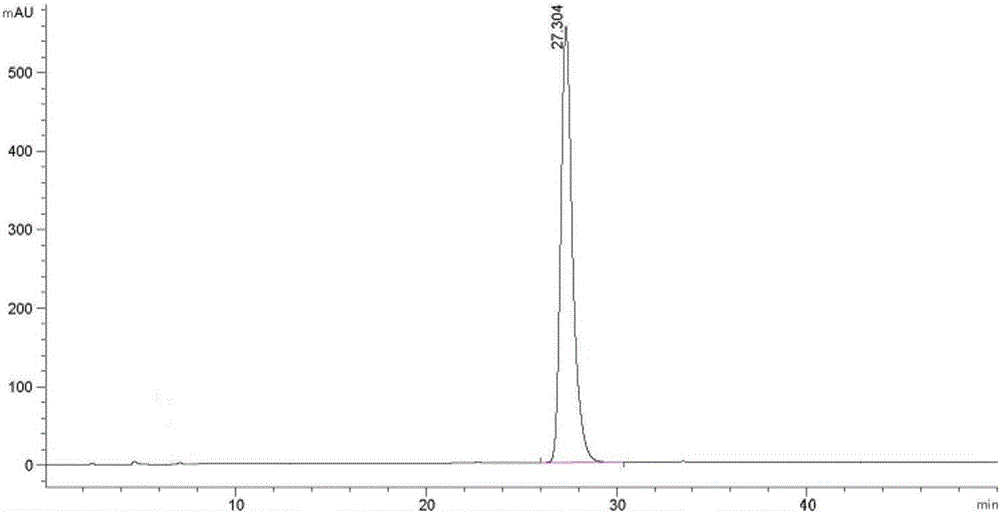
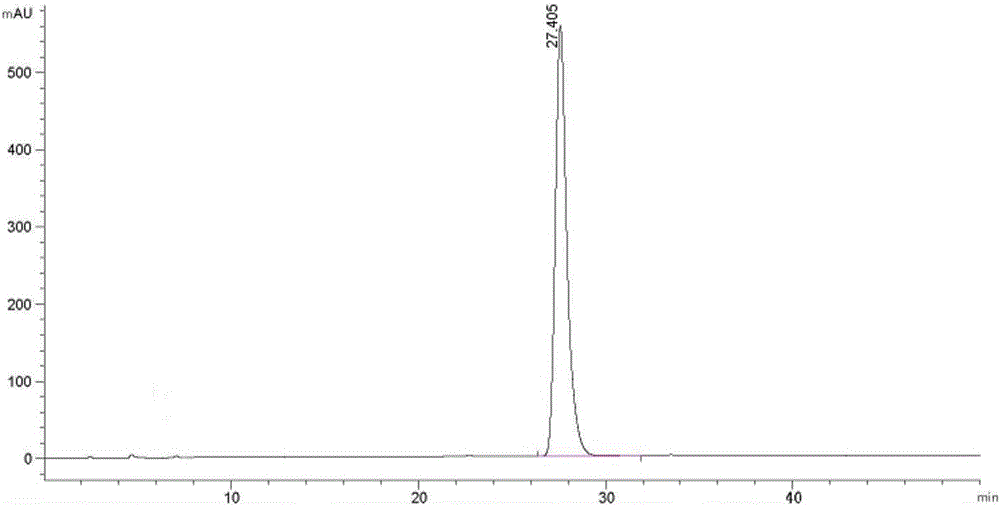
Obeticholic acid and detection method for related substances in preparation of obeticholic acid
The invention discloses an obeticholic acid and a detection method for related substances in a preparation of the obeticholic acid. The detection method includes the specific steps that the high performance liquid chromatography method is adopted, and a C18 column is adopted; a diode array detector is adopted, and the column temperature is 20 DEG C to 30 DEG C; a phosphate buffering liquid-acetonitrile-methyl alcohol solution serves as a moving phase for isocratic elution. By means of the detection method, the conditions of impurities and degradation products of the obeticholic acid can be rapidly and accurately detected, effective separation of the obeticholic acid and all known impurities can be achieved, the interference of all kinds of impurities generated in the synthesis and preparation processes of the obeticholic acid in the product purity is avoided, and the quality of raw material medicine and the quality of the preparation can be fully and reliably controlled. The detection method is easy and convenient to operate, high in sensitivity and good in separation degree, and an effectively analysis method is provided for controlling the quality of the product.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Synthesis method of obeticholic acid
InactiveCN106279336AHigh yieldLess impuritiesSteroidsBulk chemical productionSynthesis methodsCarboxylic acid
The invention discloses a synthesis method of obeticholic acid. The synthesis method takes 3alpha,7alpha-dihydroxyl-5beta-cholestane-24-acid as a starting material and comprises the following steps: carrying out hydroxyl oxidation and carboxylic acid ethyl esterification, and reacting with trimethylsilyl chloride to synthesize silyl enol ether; then enabling the silyl enol ether and acetaldehyde to subject to Mukaiyama hydroxyaldehyde condensation to obtain 6-ethylidene-3alpha-hydroxyl-7-one-5beta-cholestane-24-ethyl; carrying out catalytic hydrogenation, hydroxyl protection and ester group hydrolysis; carrying out selective reduction through sodium borohydride; finally, carrying out de-protection to obtain the obeticholic acid. By optimizing synthesis steps and selecting different protection reagents to protect hydroxyl and carboxyl for a plurality of times, and adopting a selective hydrogenation reduction reaction, the problems in a synthesis reaction of the obeticholic acid that more impurities are caused, a structure is easy to overturn, the yield in a 6alpha-ethylation process is low, purification is difficult to realize and the like are effectively solved; the total yield of an obeticholic acid product is greatly improved; the synthesis method has good economical efficiency and is suitable for industrial production.
Owner:合肥诺瑞吉医药科技有限公司
Preparation method of non-shaped obeticholic acid
ActiveCN105085597AReduce moisture contentFor long-term storageOrganic chemistry methodsSteroidsOrganic solventSolvent
The invention discloses a preparation method of non-shaped obeticholic acid. The preparation method comprises the following steps of a. dissolving the obeticholic acid into an organic solvent, and filtering, so as to obtain a filtering liquid, wherein the mass and volume ratio of the obeticholic acid and the organic solvent is 1:5-50g / ml; b. removing the solvent in the filtering liquid, so as to obtain the non-shaped obeticholic acid. The preparation method has the advantages that the water content of the prepared non-shaped obeticholic acid is low, the product stability is good, and the obeticholic acid product can be stored for a long time; the number of procedures is fewer, the operation is simple and convenient, the safety and environment-friendly effects are realized, the byproduct is avoided, the used solvent can be recycled and reutilized, the production cost is low, the production cycle is short, and the preparation method is very suitable for being applied into industry.
Owner:CHENGDU BAIYU PHARMA CO LTD
Recrystallization purification method for high-purity obeticholic acid
InactiveCN105541953AHigh purityAdapt to industrial productionSteroidsPurification methodsOrganic solvent
The invention discloses a recrystallization purification method for high-purity obeticholic acid. Obeticholic acid and organic amine are salified and then re-crystallized in an organic solvent to obtain high-purity obeticholic acid. The method includes the specific steps that crude obeticholic acid reacts with organic amine in the organic solvent to obtain obeticholic acid organic amine salt; the obeticholic acid organic amine salt is subjected to acid adjustment to be re-crystallized in an obeticholic acid mode. According to the purification method, high-purity (purity is larger than 98.5% and the individual impurity rate is smaller than 0.1%) obeticholic acid can be obtained, the steps of column chromatography and the like which cannot produce a large amount of high-purity obeticholic acid are not needed for the method, and therefore the purification method can better adapt to industrial production.
Owner:四川新功生物科技集团有限公司
Method for preparing obeticholic acid intermediate
The invention discloses a method for preparing an obeticholic acid intermediate 3-alpha-hydroxyl-6-ethylidene-7-keto-5-beta-cholanic acid (I) by taking 7-oxo-lithocholic acid (II) as a raw material as well as its derivative (IA). According to the invention, 7-oxo-lithocholic acid (II) is protected or directly unprotected through 3-hydroxy or 3-hydroxy and 24-hydroxy, the is further subjected to an aldol condensation reaction with acetaldehyde to obtain the 3-alpha-hydroxyl-6-ethylidene-7-keto-5-beta-cholanic acid (I) and the derivative, and the method is used for preparing the obeticholic acid. The method has the advantages of simple process and high yield, and is suitable for industrial production.
Owner:CHONGQING PHARMA RES INST
Compound composition of obeticholic acid and berberine and applications thereof
ActiveCN105168228AImprove efficacyEliminate side effectsOrganic active ingredientsMetabolism disorderBerberineMedicinal chemistry
The invention provides a pharmaceutical composition which comprises obeticholic acid or pharmaceutically acceptable salts thereof and berberine or pharmaceutically acceptable salts thereof. In addition, the invention also provides preparations involved in the pharmaceutical composition and preparation methods and applications thereof and the like.
Owner:BEIJING KAWIN TECH SHARE HLDG
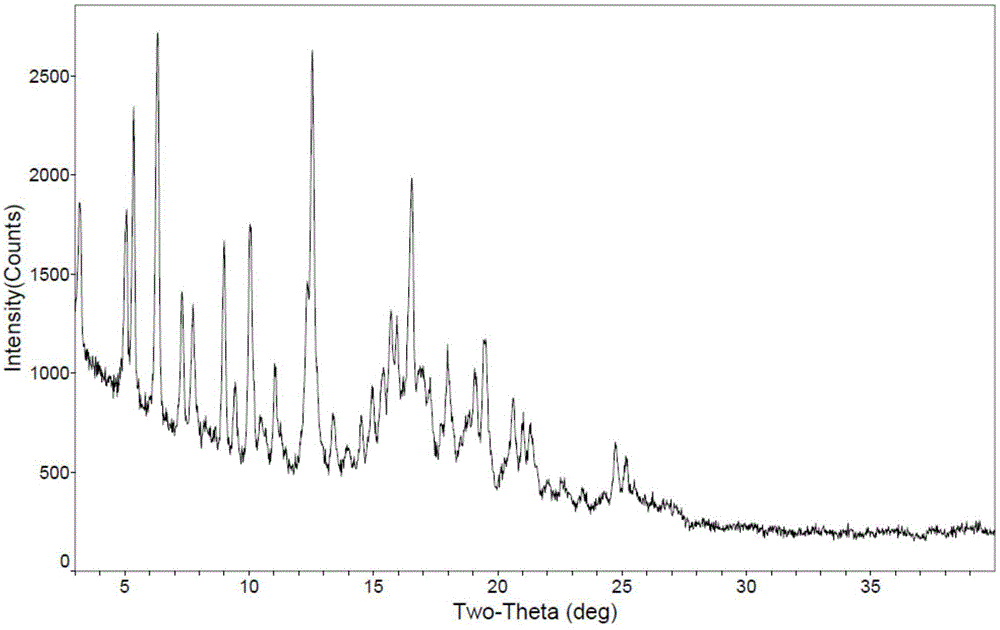
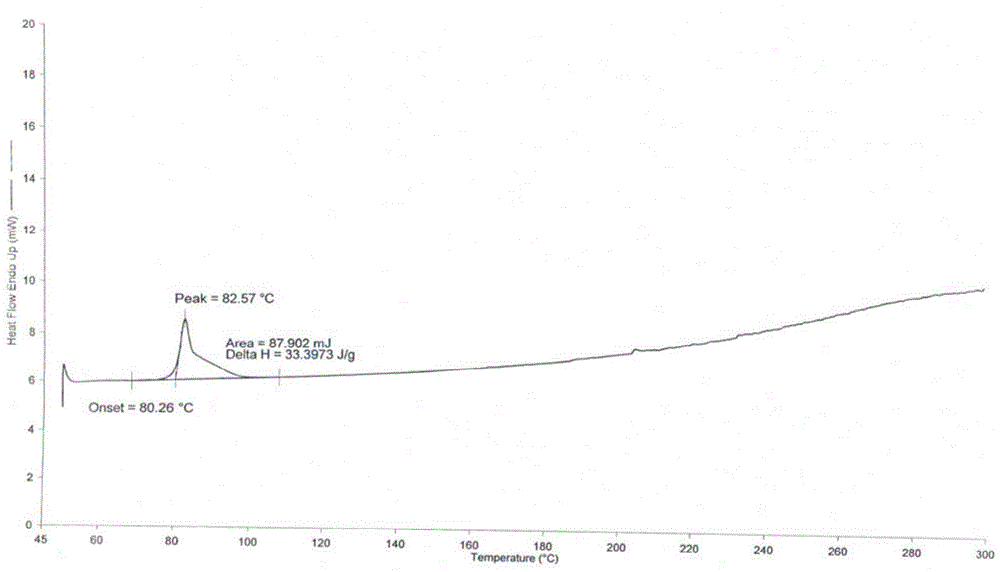
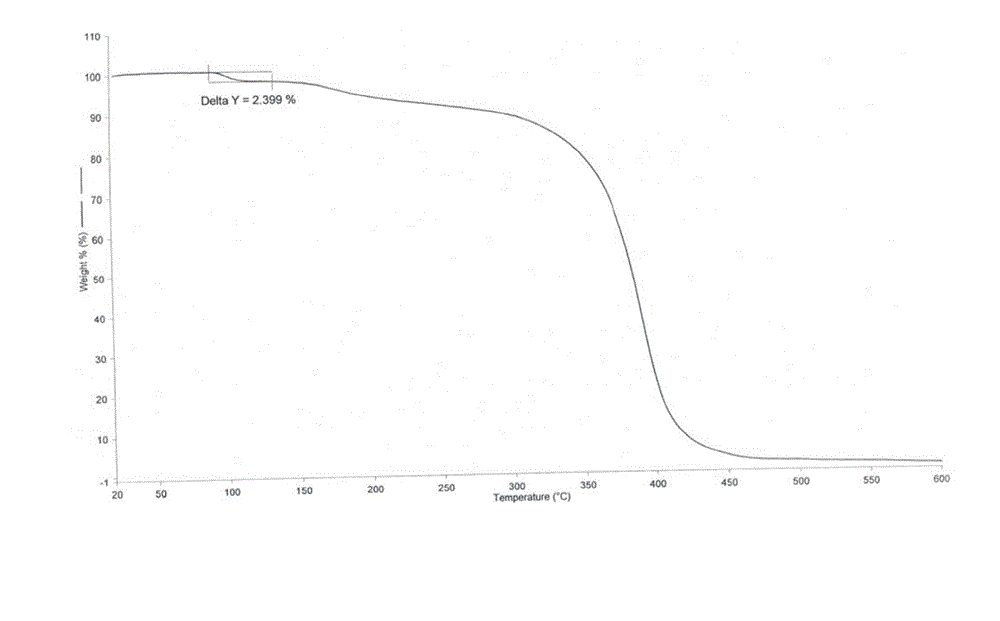
Polymorphic substances of obeticholic acid and preparation method thereof
The invention relates to polymorphic substances of obeticholic acid and a preparation method thereof. According to the invention, a novel crystallization method is adopted to prepare obeticholic acid polymorphic substances. The preparation method for a crystal form I comprises the following steps: dissolving obeticholic acid in a good solvent, adding a certain proportion of a poor solvent when complete dissolving is just realized under the condition of refluxing, decreasing a temperature, carrying out cooling so as to allow a crystal to be precipitated, and carrying out filtering and drying. The preparation method for a crystal form II comprises the following steps: dissolving obeticholic acid in an organic solvent, carrying out refluxing, decreasing a temperature, carrying out cooling so as to allow a crystal to be precipitated, and carrying out filtering and drying. The method provided by the invention has the following advantages: the crystallized obeticholic acid polymorphic substances are stable; meanwhile, operation is simple and practicable, and a conventional solvent can be used; the disadvantages of complex steps, difficult crystallization, poor repeatability and instability of a disclosed preparation method are overcome; and the method is suitable for industrial production.
Owner:厦门蔚扬药业有限公司 +1
Intermediate for preparing obeticholic acid, preparation method of intermediate and preparation of obeticholic acid
The invention relates to the technical field of medicine, and particularly relates to a intermediate for preparing obeticholic acid, a preparation method of the intermediate and a preparation of obeticholic acid. A route is provided to take acetaldehyde as electrophilic reagent, compared with a method of introducing ethenyl and ethyl iodide through an aldol condensation reaction, the yield is sharply improved; cyclohexylamine salt is refined to form a compound (V), the product quality can be sharply improved; double bonds are hydrogenated and then carbonyl is reduced so that ethenyl can be reduced, and by controlling appropriate temperature, by-products of deethylation can be sharply reduced. The method is mild, green and environmentally friendly, besides, the yield is higher than an existing preparation method, the route reaction condition is economical and effective, and suitable for industrial production on a large scale.
Owner:CHANGZHOU PHARMA FACTORY
A method for preparing obeticholic acid and related compound
ActiveCN106589039AImprove refining efficiencyReduce the introductionSteroidsBulk chemical productionProtecting groupCholane
This invention provides a new method for preparing obeticholic acid which belongs to the technical field of medicine. Its raw material is E / 2 -3alpha-hydroxy-b-ethylidene-keto-5beta-cholane-24-acid methyl ester(OB-4)which can be gained easily. First, OB-3 can be produced through hydroxy-protection with tetra hydropyrane protecting group. Then, OB-2 can be produced through hydrogenation-reduction in alkaline aqueous solution. Then, OB-1 can be produced through reducing again. At last, the target product--obeticholic acid can be got through catalyzing and removing tetrahydropyrane. The method is simple in production process, the content of isomer impurity is low, and the method is a new synthetic method of obeticholic acid suitable for industrial production.
Owner:SUZHOU LANXITE BIOTECH
Obeticholic acid compound, and medicinal composition containing compound
InactiveCN105985395AThe preparation process is stableGood reproducibilityOrganic active ingredientsDigestive systemObeticholic acidCrystal
The invention provides an obeticholic acid compound with a crystal form, and a preparation method and a medicinal composition thereof. A result of investigation of the crystal form of the obeticholic acid compound obtained in the invention from the hygroscopicity and the stability shows that the obeticholic acid compound accords with medicinal requirements. The preparation method has the advantages of stable process, good reappearance, and meeting of requirements of industrial large-scale production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Obeticholic acid compound and pharmaceutical composition thereof
The invention provides a crystal-form obeticholic acid compound, and a preparation method and a pharmaceutical composition thereof. A crystal form of the obtained obeticholic acid compound is investigated in the aspects of hygroscopicity, stability and the like, and the crystal form is found out to be in compliance with medicine requirements. The preparation process provided by the invention is stable, has good reproducibility, and meets the requirements of industrialized mass production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing obeticholic acid, ursodeoxycholic acid and 7-ketolithocholicacid
InactiveCN108676049AEasy to manufactureHigh yieldSteroidsBulk chemical productionCholic acidSynthesis methods
The invention discloses a method for preparing obeticholic acid, 7-ketolithocholicacid and ursodeoxycholic acid. Cholic acid is used as a raw material for preparing obeticholic acid through selectiveprotection by a 3-alpha-hydroxyl group, selective oxidation of a 7-alpha-hydroxyl group, esterification of a 24th carboxyl group, methanesulfonation of a 12-alpha-hydroxyl group, elimination, hydrolysis, silylation, condensation, hydrolysis, catalytic hydrogenation, carbonyl reduction and other reactions; an intermediate is subjected to catalytic hydrogenation to prepare the 7-ketolithocholicacidand then is reduced to prepare the ursodeoxycholic acid. The method provided by the invention uses cheap cholic acid as the raw material, and has advantages of novel synthesis method, low cost, high yield, mild reaction condition, high simplicity in operation, environmental friendliness and high convenience in industrial production.
Owner:EAST CHINA NORMAL UNIV
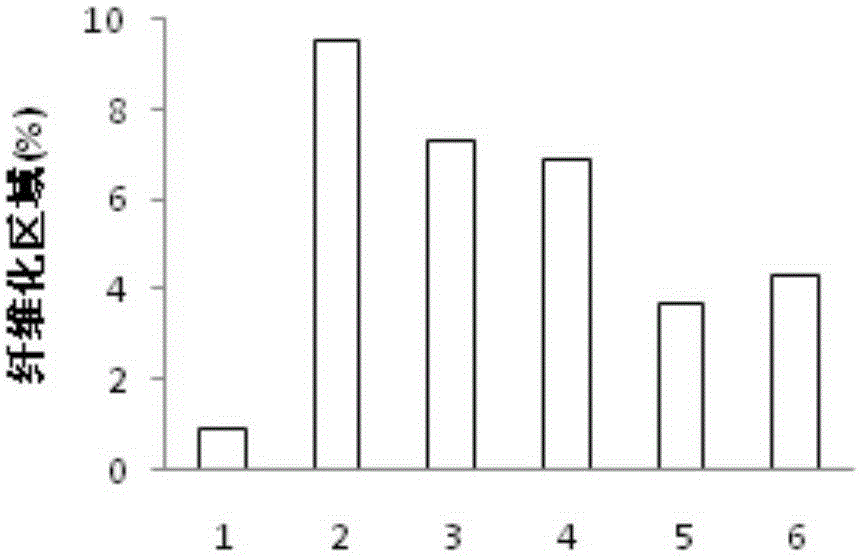
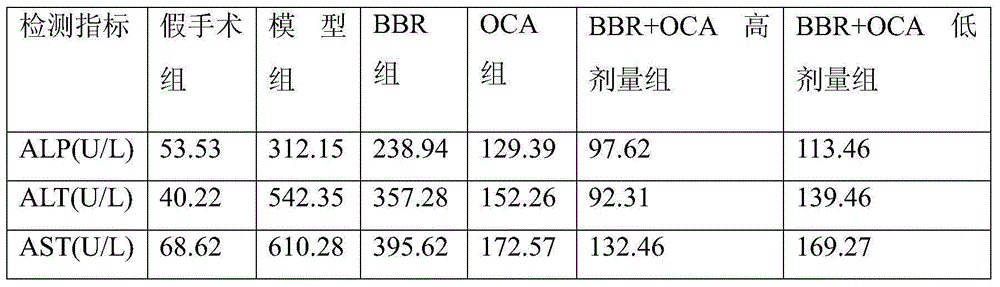
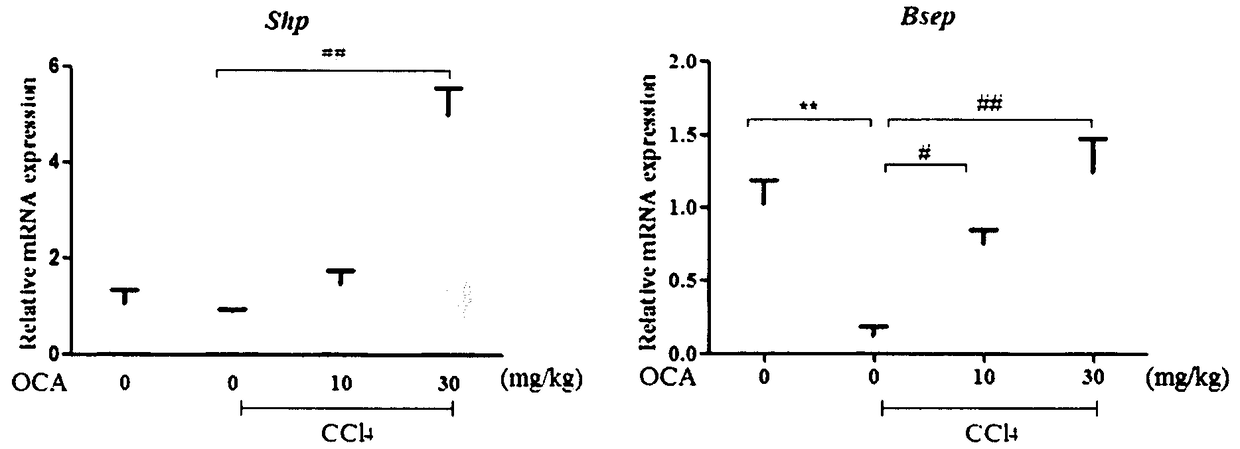
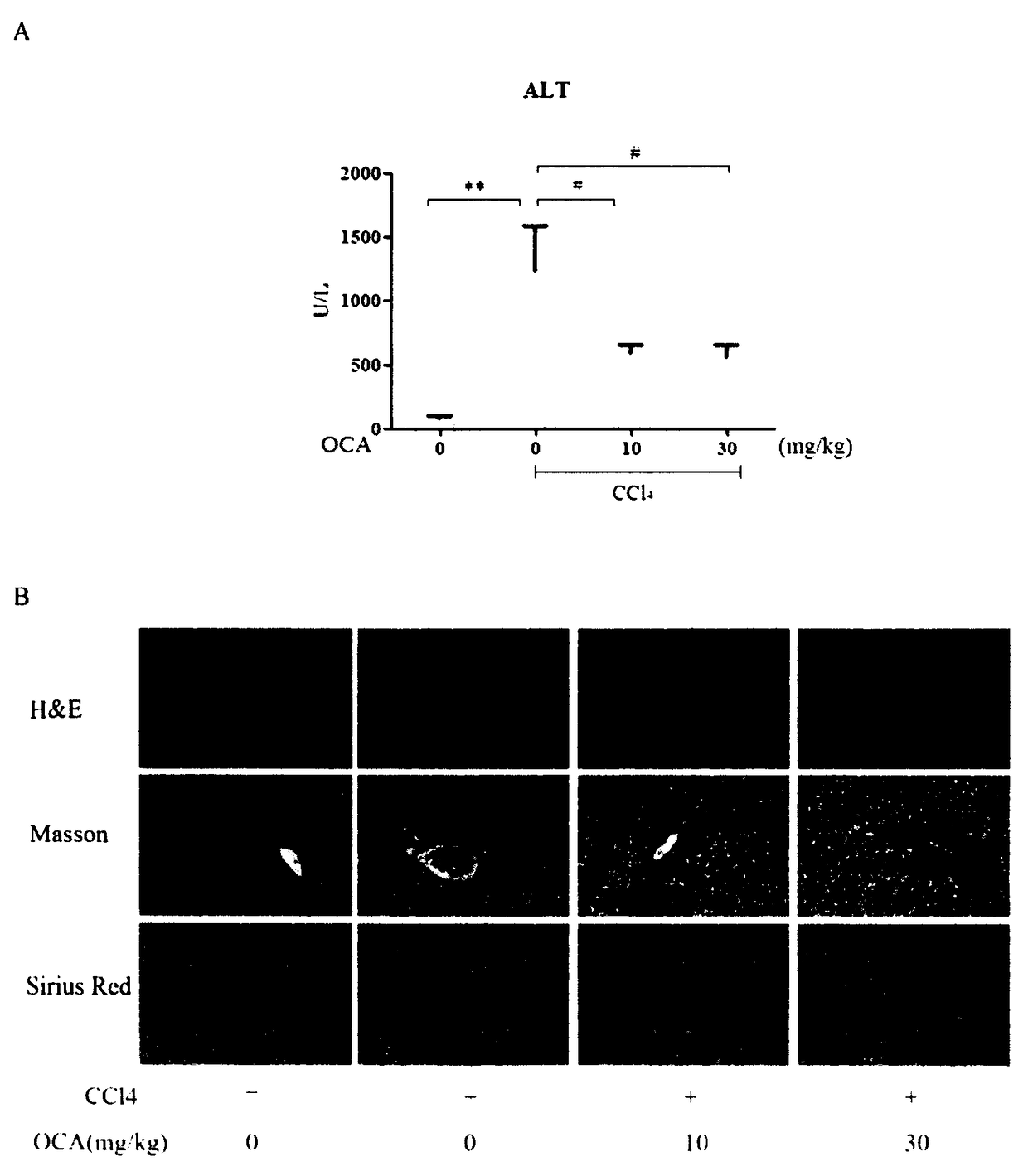
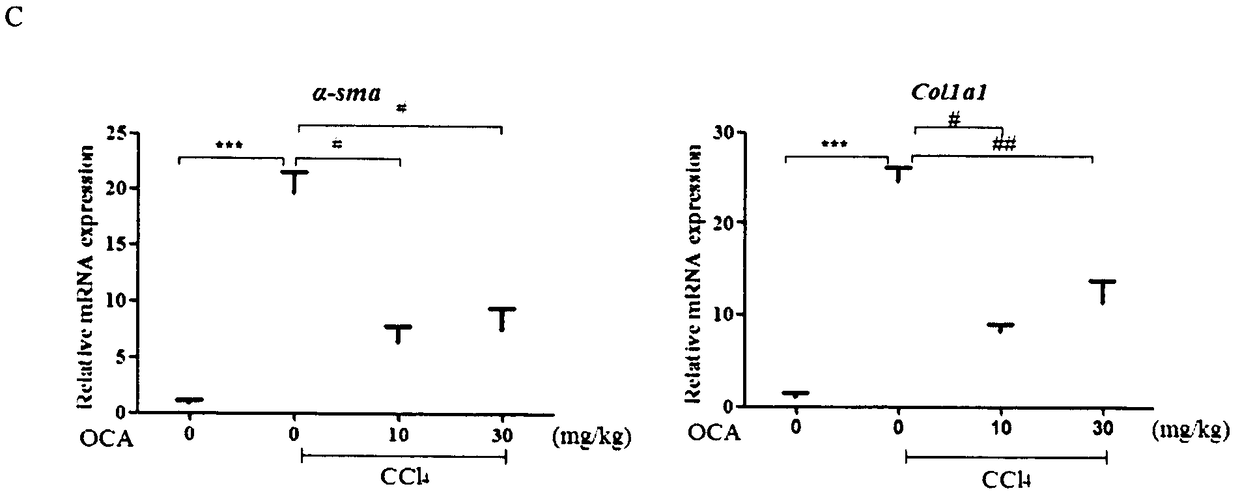
Application of combination of Farnesoid XReceptor (FXR) agonist and apoptotic inhibitor in preparation of superior anti-hepatic fibrosis drugs
InactiveCN108114284AAvoid side effectsProtectiveOrganic active ingredientsDigestive systemInhibitor of apoptosisHepatocyte structure
The invention belongs to the field of biomedicines, and provides a scheme of combination of an apoptotic inhibitor and Farnesoid XReceptor (FXR) agonist for exerting the synergetic liver protection anti-fibrosis effect. Particularly, the invention relates to therapeutic medication measure for liver fibrosis at an important stage according to chronic liver disease. The invention provides application of combination of FXR agonist and the apoptotic inhibitor in preparation of superior anti-hepatic fibrosis drugs, and particularly the FXR agonist, namely obeticholic acid and the apoptotic inhibitor IDN6556 are selected for use, when the FXR agonist and the apoptotic inhibitor are subjected to low-dosage combination, the glutamic-pyruvic transaminase level in serum can be obviously reduced, thestructural forms and functions of liver cells can be improved, and the fibrotic symptoms is reduced, and due to drug combination, the liver protection effect is obviously superior to that of the single use of the FXR agonist and the apoptotic inhibitor. The invention provides a preparation method of a new superior liver protection anti-fibrosis drug.
Owner:CHINA PHARM UNIV
Method for preparing obeticholic acid and intermediate thereof
The invention belongs to the technical field of medical chemistry, particularly relates to a new method for preparing obeticholic acid and an intermediate thereof. The method comprises the steps that a compound III is subjected to catalytic hydrogenation to produce an intermediate II, and then the obeticholic acid is prepared. The method has the advantages of being simple in operation, mild in reaction conditions, and reduces the reaction temperature, reduces side reactions such as the dehydration of hydroxyls, improves yields and is suitable for a large-scale production.
Owner:QILU PHARMA
Preparation method for obeticholic acid
InactiveCN104926909AEasy to operateHigh yieldSteroidsBulk chemical productionEtherSodium borohydride
The invention provides a preparation method for obeticholic acid. The preparation method comprises the following steps of: adding 3alpha-hydroxyl-7-keto-5beta-cholanic acid and methanol into a reaction container and carrying out esterification reaction in an acidic environment to obtain 3alpha-hydroxyl-7-keto-5beta-methyl cholanate; adding 3,4-dihydro-2H-pyran and dioxane and protecting hydroxyl by tetrahydropyrane ether; adding bromoethane, lithium diisopropylamide and site alpha of diastereomeric methylated keto-carbonyl; then adding methanol, removing the protecting group which protects tetrahydropyrane ether and reducing hydroxyl in the acidic environment; adding sodium borohydride and reducing the mixture to 3alpha-hydroxyl-6beta-methyl-7-hydroxyl-5beta-methyl cholanate; and adding methanol and carrying out alkali hydrolysis to obtain 3alpha-hydroxyl-6beta-methyl-7-hydroxyl-5beta-cholanic acid, namely the target product obeticholic acid. According to the preparation method provided by the invention, the process line is mild in reaction, simple to operate and high in yield.
Owner:ZHEJIANG TIANSHUN BIOTECH
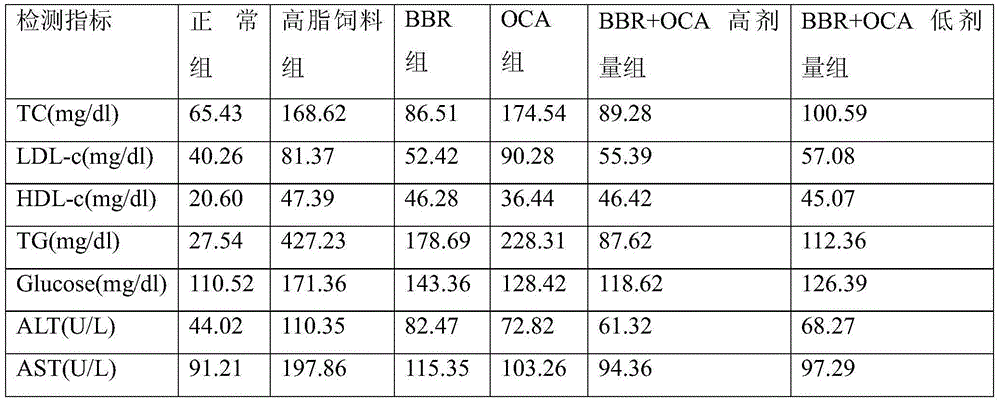
Obeticholic acid and metformin composition and application thereof
InactiveCN106974916AEliminate side effectsRelieve side effectsOrganic active ingredientsMetabolism disorderSide effectHigh fat
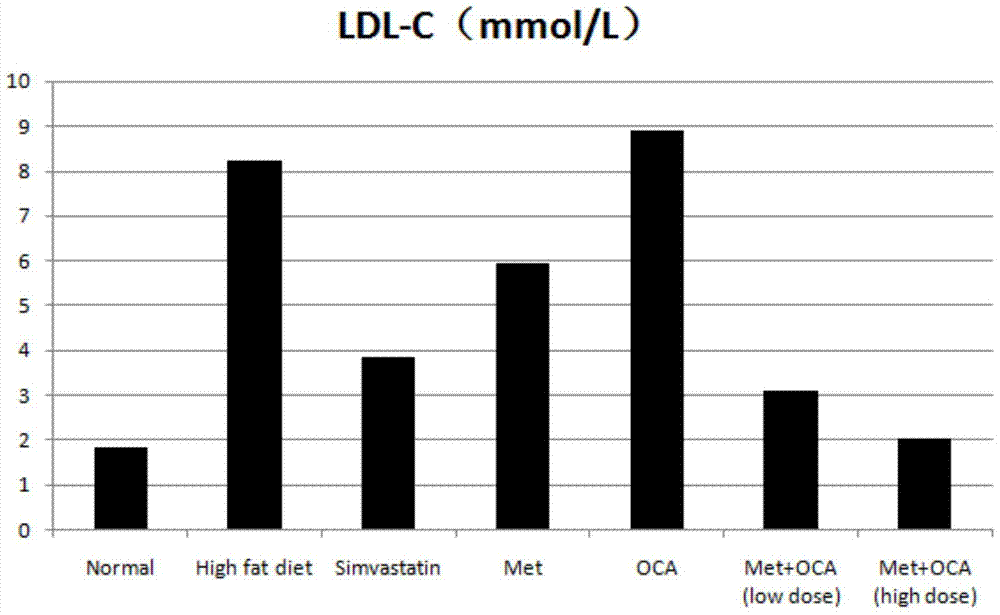
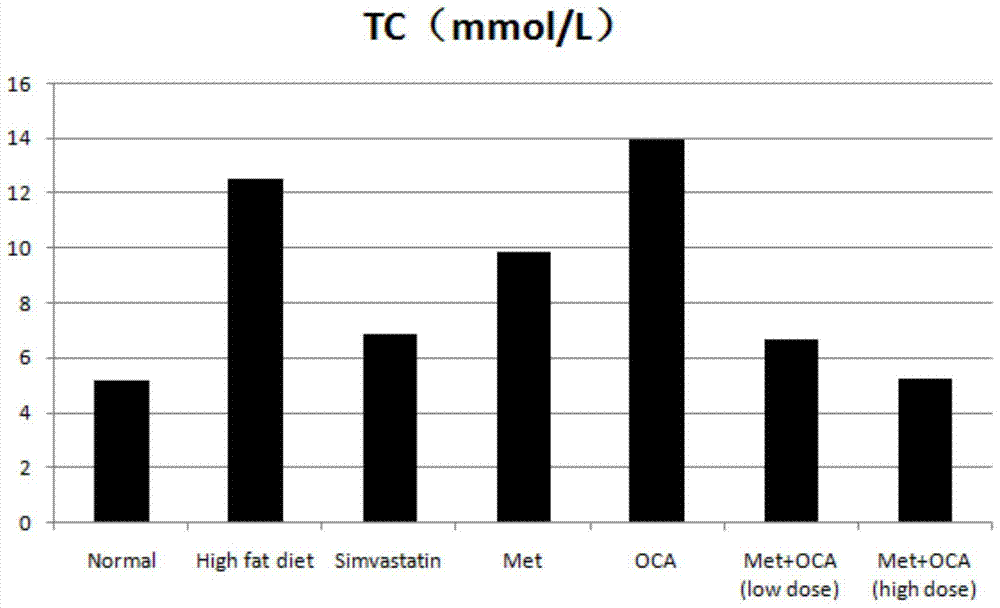
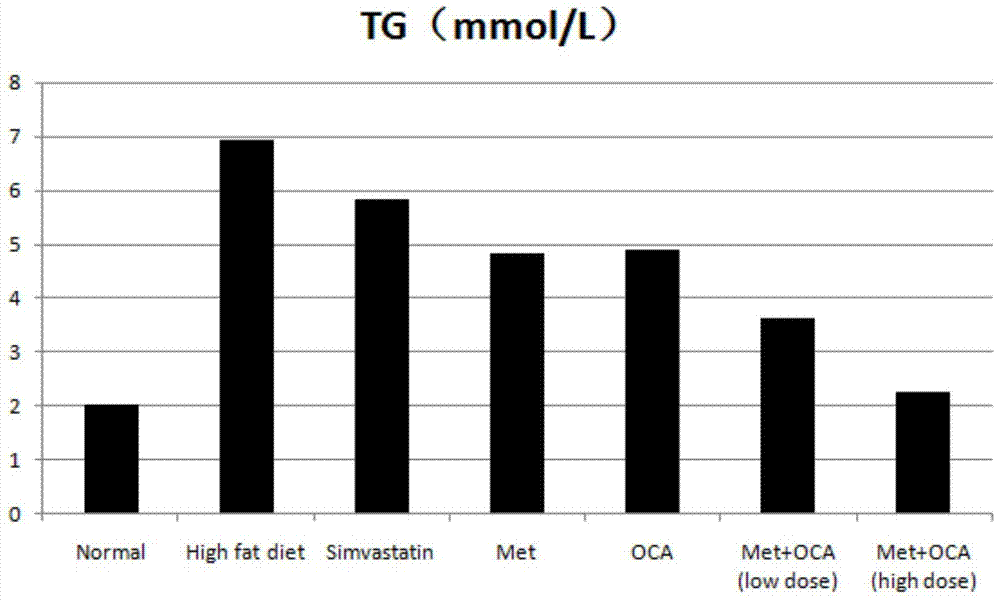
The present invention provides a pharmaceutical composition comprising obeticholic acid or a pharmaceutically acceptable salt thereof and metformin or a pharmaceutically acceptable salt thereof. In addition, the present invention also provides applications related to the pharmaceutical composition and the like. The compound combination can effectively eliminate the side effect of increasing LDL-C caused by obeticholic acid, significantly improve the blood biochemical indexes of high-fat and high-sugar animals, significantly improve fatty liver symptoms, and cure non-alcoholic fatty liver disease.
Owner:CHENGDU BESTCHIRALBIO LIMITED LIABILITY
Synthesis method for obeticholic acid intermediate 7-ketolithocholic acid
The invention discloses a chemical synthesis method for an obeticholic acid intermediate 7-ketolithocholic acid (3alpha-hydroxy-7-keto-5beta-cholestane-24-acid), and belongs to the field of organic chemical synthesis. The method adopts cholic acid as a raw material, and through 7alpha-hydroxyl selective oxidation, side chain carboxyl esterification, 3alpha-hydroxyl etherification, 12alpha-hydroxyl methanesulfonic acid esterification, elimination, hydrogenation, hydrolysis and other reactions, the obeticholic acid intermediate 7-ketolithocholic acid is synthesized. The synthesis method for 7-ketolithocholic acid adopts cheap cholic acid as the raw material, and has the advantages of novel synthesis method, low cost, high yield, environmental friendliness and convenience in industrialized production.
Owner:EAST CHINA NORMAL UNIV
Preparation method of high-purity obeticholic acid
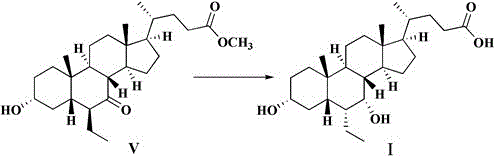
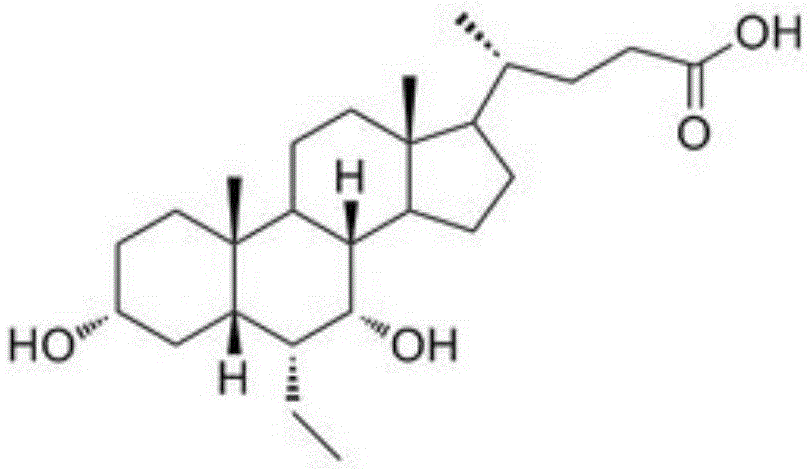
ActiveCN106749466AImprove protectionRaw materials are easy to getSteroidsChenodeoxycholic acidCarbonyl reduction
The invention relates to a preparation method of high-purity obeticholic acid. A compound chenodeoxycholic acid (CDCA) shown as a formula II is used as a starting raw material and subjected to oxidation, esterification, hydroxy protection, ethylidene formation, catalytic hydrogenation, carbonyl reduction and esterolysis reaction to obtain the high-purity obeticholic acid. The preparation method of the high-purity obeticholic acid, provided by the invention, has the advantages of low toxicity, low pollution, high purity, good stereoselectivity, low content of impurities, mild reaction conditions, high safety, simplicity and convenience in production operation and the like, and is suitable for industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
Obeticholic acid preparation method
The invention discloses an obeticholic acid preparation method, which comprises that (1) hyodeoxycholic acid II reacts with an alcohol compound III under the action of a catalyst to generate an ester compound IV; (2) the ester compound IV is subjected to PDC oxidation in dichloromethane to generate a compound V; (3) the compound V and trimethyl chlorosilane are subjected to a reaction at a temperature of -70 to -20 DEG C in tetrahydrofuran by using lithium diisopropylamide as an alkali to generate a silyl enol ether compound VI; (4) the silyl enol ether compound VI is subjected to m-chloroperoxybenzoic acid oxidation and deprotection in dichloromethane to generate a compound VII: (5) the compound VII and Yield generated from ethyltriphenylphosphonium bromide under the action of a strong alkali are subjected to a Wittig alkenylation reaction at a temperature of 0-70 DEG C to convert the ketone into the vinyl so as to generate a compound VIII; (6) the double bond of the compound VIII is subjected to catalytic hydrogenation reduction in a mixing solvent to generate a compound IX; and (7) the compound IX is hydrolyzed under an alkaline condition to generate the obeticholic acid.
Owner:XIAMEN HALOSYNTECH CO LTD
Preparation method of obeticholic acid and intermediate thereof
ActiveCN108264532ASimple and fast operationLow costSteroidsBulk chemical productionCombinatorial chemistryReagent
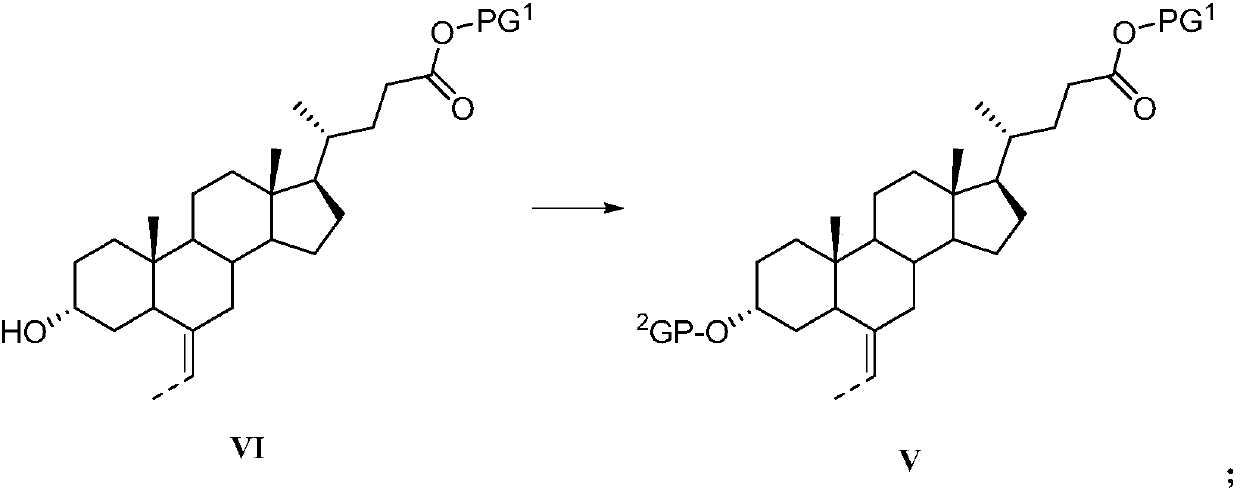
The invention discloses a preparation method of obeticholic acid and an intermediate thereof. The invention provides a preparation method of a compound V. The preparation method of the compound V comprises the following steps: carrying out hydroxyl protective reaction on a compound VI and a hydroxyl protective reagent to obtain the compound V. The preparation method is simple and convenient to operate, low in cost, gentle in condition, environmentally friendly and suitable for industrialization.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
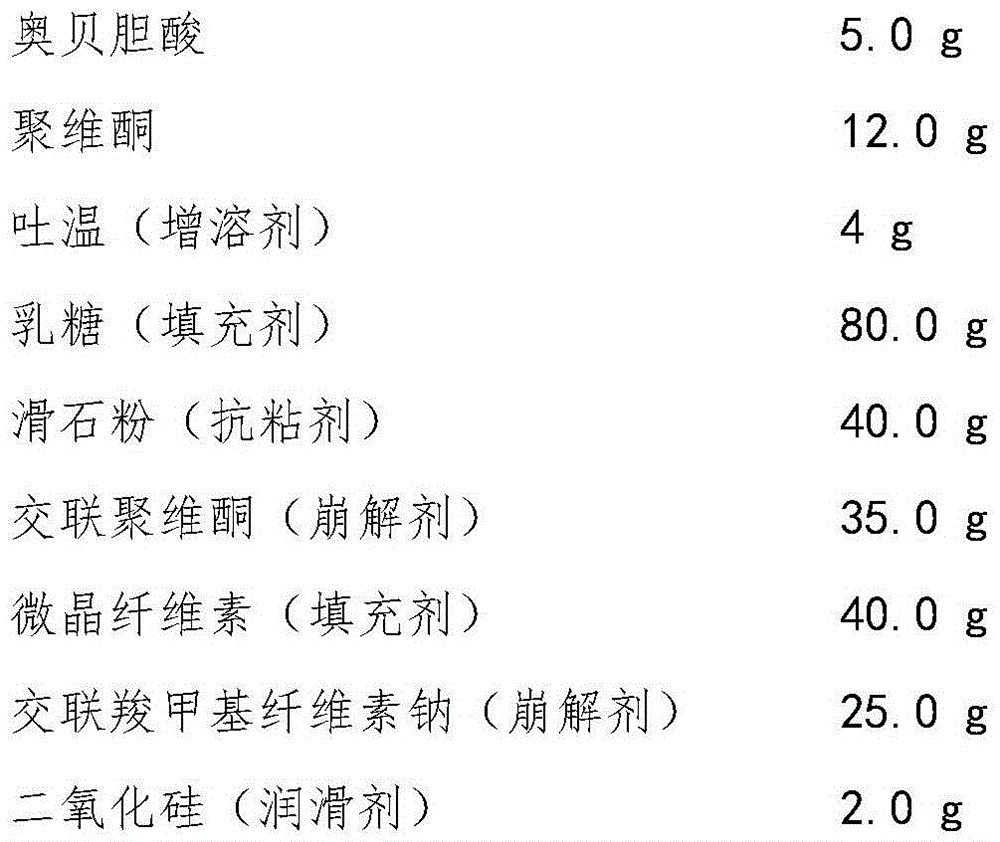
Oral disintegrating tablet of obeticholic acid, and preparation method thereof
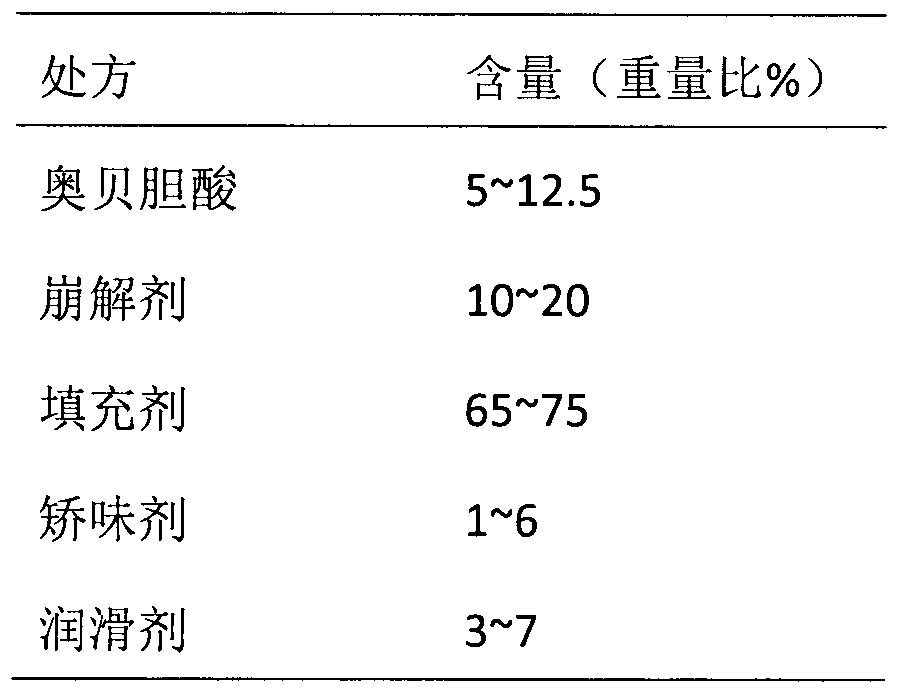
InactiveCN105997909AThe composition of the prescription is simplePromote dissolutionOrganic active ingredientsDigestive systemActive componentBULK ACTIVE INGREDIENT
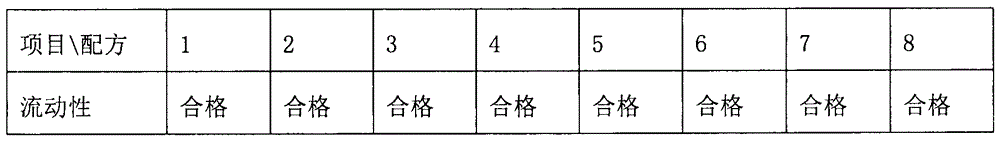
The invention belongs to the field of pharmaceutical preparations, and in particular relates to an orally disintegrating obeticholic acid tablet and a preparation method thereof. The orally disintegrating tablet of obeticholic acid of the present invention is composed of active ingredient obeticholic acid, filler, disintegrant, flavoring agent and lubricant, and its weight percentage is: 5‑12.5: 65‑75: 10‑20 : 1-6: 3-7. The invention adopts a powder direct tableting method to prepare obeticholic acid orally disintegrating tablets, and the process is simple and suitable for large-scale industrial production. The disintegration time, content uniformity, stability and the like of the obeticholic acid orally disintegrating tablet prepared by the present invention all meet the pharmacopoeia standards.
Owner:CHINA PHARM UNIV
Preparation for improving release efficiency
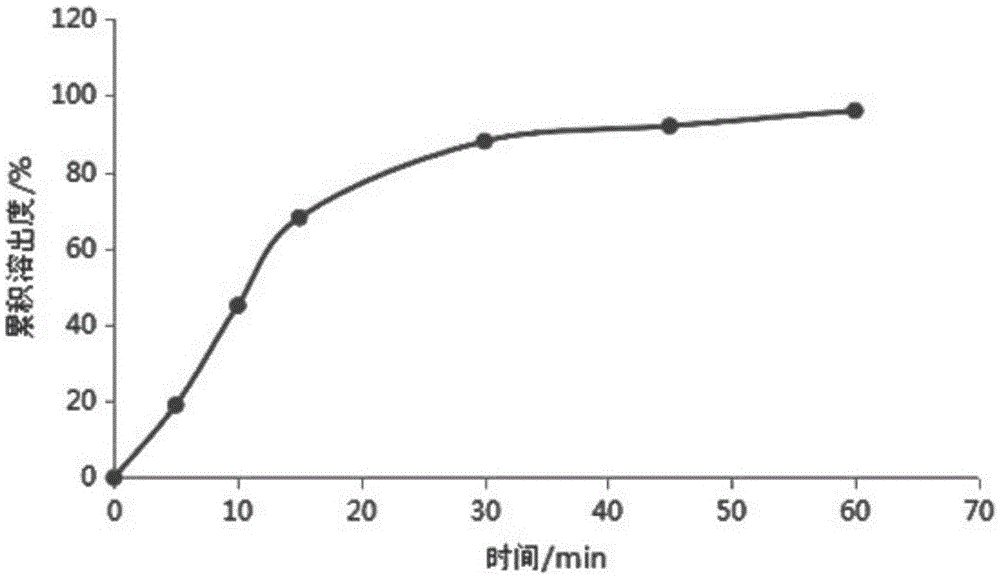
InactiveCN105534932AImprove solubilityImprove dissolution rateOrganic active ingredientsDigestive systemSolubilityAdditive ingredient
The invention discloses a preparation for improving release efficiency. The preparation takes obeticholic acid as an active pharmaceutical ingredient and povidone as a carrier material, and is prepared into a solid dispersion by adoption of a spray drying technology, and then the solid dispersion is further prepared into a dispersible tablet, so that the solubility and dissolving-out speed of a medicine are increased, the absorption of the medicine in a body is promoted, and the bioavailability of the medicine is improved. The solid dispersion is prepared into the dispersible tablet to enhance the medicine-taking compliance of a patient.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Method for refining obeticholic acid
The invention belongs to the field of medicine synthesis, and relates to a method for refining high-purity obeticholic acid. The method for refining the obeticholic acid comprises the following steps that crude obeticholic acid is added to an organic solvent, heating and stirring are carried out till the crude obeticholic acid is completely dissolved, activated carbon decoloration is carried out, natural cooling and devitrification are carried out, and filtering and drying are carried out to obtain the high-purity obeticholic acid. According to the method, the content of isomer impurities in the obeticholic acid can be reduced to 0.15% or below, the content of chenodeoxycholic acid impurities can be reduced to 0.1% or below, the product purity is 99.55% or above, and the refining yield is not lower than 85%. In addition, the refining method is simple in technological process, convenient to operate, low in production cost, high in product purity, stable in technology and suitable for industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
Compositions and methods of treating liver disease
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
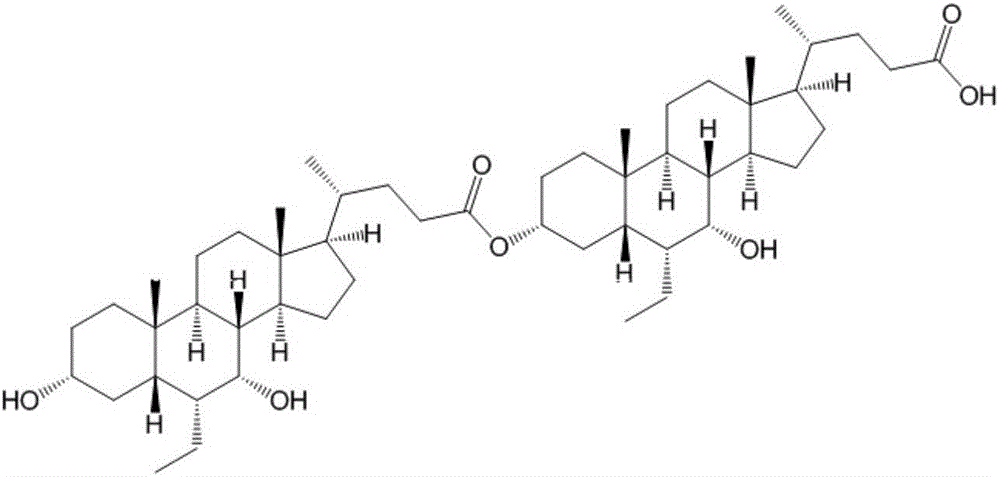
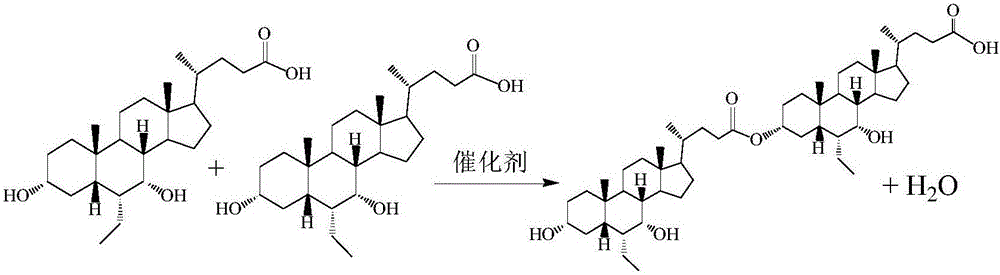
Obeticholic acid dimer impurities and preparation method thereof
The invention relates to obeticholic acid dimer impurities and a preparation method thereof. The preparation method sequentially includes the steps of S1, preparing a beticholic acid dimer impurity coarse compound; S2, performing column chromatography separation and purification on the beticholic acid dimer impurity coarse compound. The preparation method has the advantages that the method is used for preparing the dimer impurities generated during the synthesizing and degrading process of obeticholic acid, the quality of the obeticholic acid can be well controlled, and medicine safety can be increased.
Owner:FUJIAN COSUNTER PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka.patsnap.com/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000011.PNG)
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka.patsnap.com/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000021.PNG)
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka.patsnap.com/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000022.PNG)