Method for synthesizing, separating and determining obeticholic acid (OCA) isomer
A technology of ethanol and compound, which is applied in the field of synthesis of obeticholic acid isomers, and can solve the problems such as a method for chromatographic separation of obeticholic acid without a synthetic method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of intermediate B
[0058] Dissolve 50g of compound A (mw: 416.3) in 500mL of 2.5% sodium hydroxide, add 5.0g of 10% Pd / C, control the temperature at 25°C, and pass in hydrogen to complete the reaction. After the reaction is completed, filter the reaction solution and concentrate the organic phase Afterwards, about 45 g of compound B was obtained, and the purity of the obtained compound B was 97%.
Embodiment 2
[0059] Example 2: Preparation of obeticholic acid αβα crude product
[0060] Compound B 20g (mw: 418.3) was dissolved in 250mL of 2% sodium hydroxide aqueous solution, 12.5g of sodium tetrahydroborate was added, and the temperature was raised to 35°C to complete the reaction. After the reaction, 1M hydrochloric acid solution was slowly added dropwise to the reaction solution to adjust the pH to about 3-4, during which time 500ml of water was added, and filtered to obtain 16g of white solid with an HPLC purity of about 89%.
Embodiment 3
[0061] Example 3: Separation and purification of obeticholic acid αβα
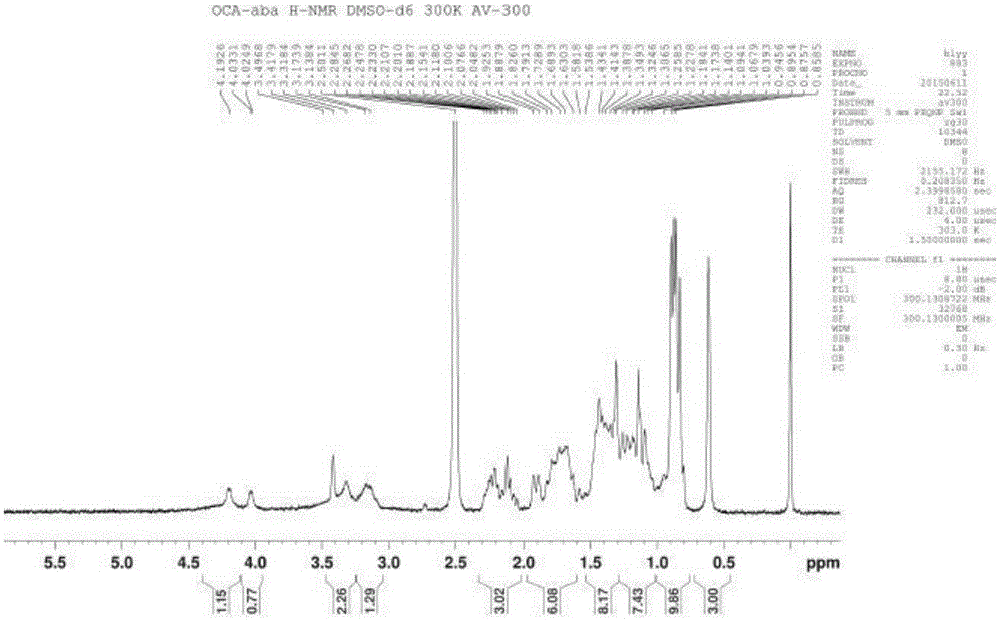
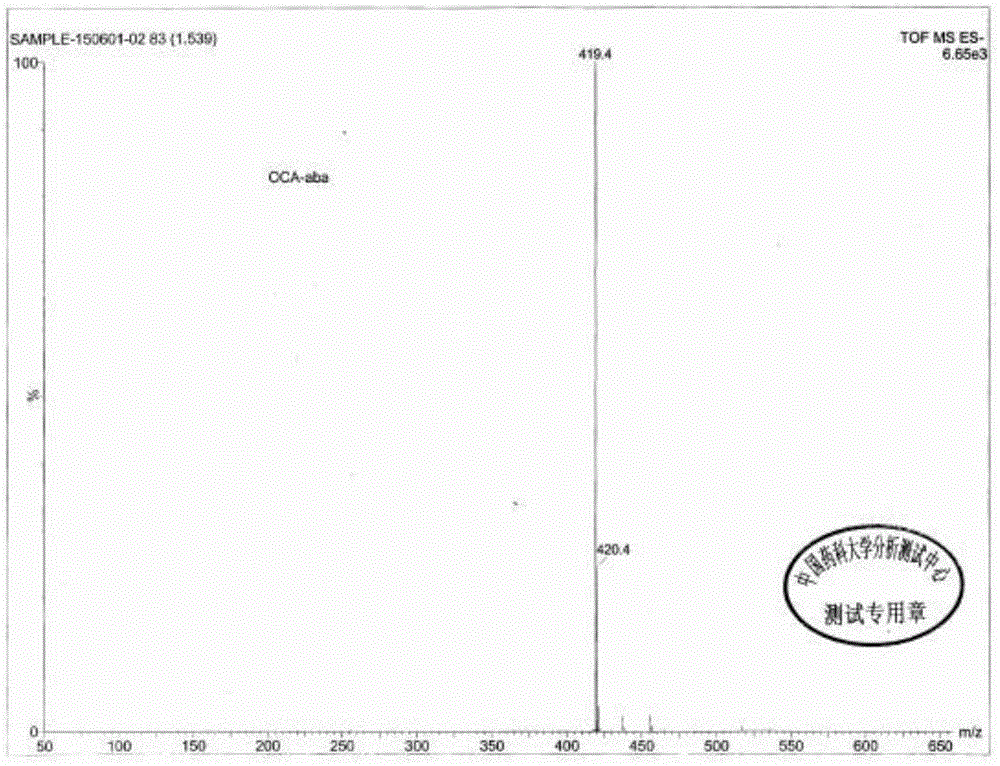
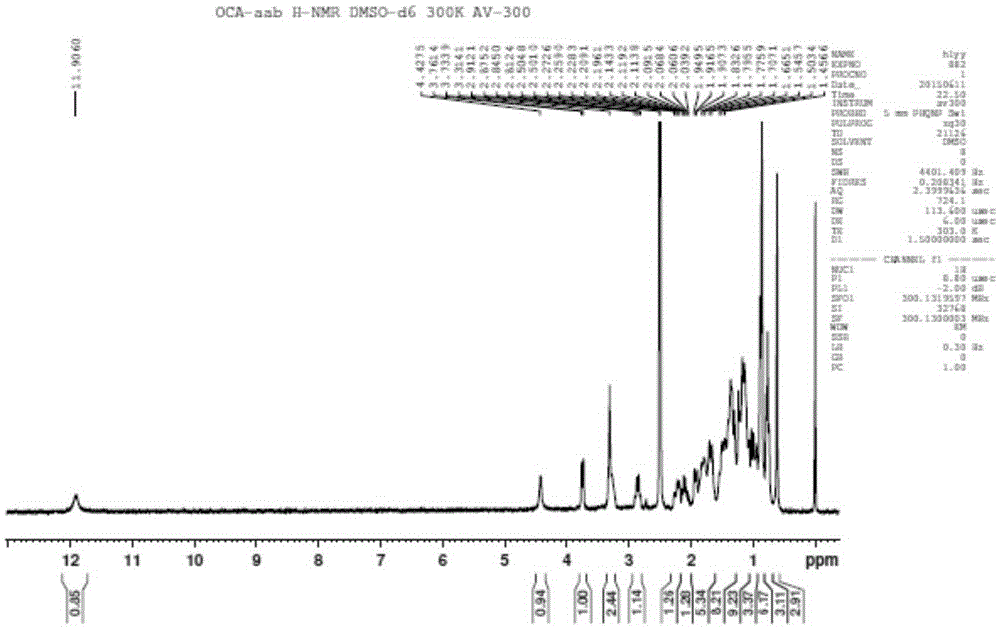
[0062] Soak HP20SS400mL resin in 1L acetone overnight, fill it into a glass chromatography column, and then rinse it with 10L water. Dissolve 10g of obeticholic acid ααβ crude product in 50mL of 5% sodium hydroxide solution, slowly flow the resulting solution through the chromatography column, and then carry out gradient elution with methanol / water mixed system, the ratio of methanol / water is 3:10 , using HPLC chromatograph to monitor the separation effect, and according to the monitoring results, collect fractions with more than 98% of the target components. After receiving, the methanol was concentrated under reduced pressure to remove methanol, and then the pH was adjusted to 3-5 with phosphoric acid to precipitate a solid, which was filtered and dried to obtain obeticholic acid ααβ, with an HPLC purity of about 97.7%. The obtained obeticholic acid ααβ was confirmed by HNMR and MS, see attached Figur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com