Patents
Literature
355 results about "Reversed-Phase Liquid Chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reversed-phase chromatography (also called RPC, reverse-phase chromatography, or hydrophobic chromatography) includes any chromatographic method that uses a hydrophobic stationary phase. RPC refers to liquid (rather than gas) chromatography.
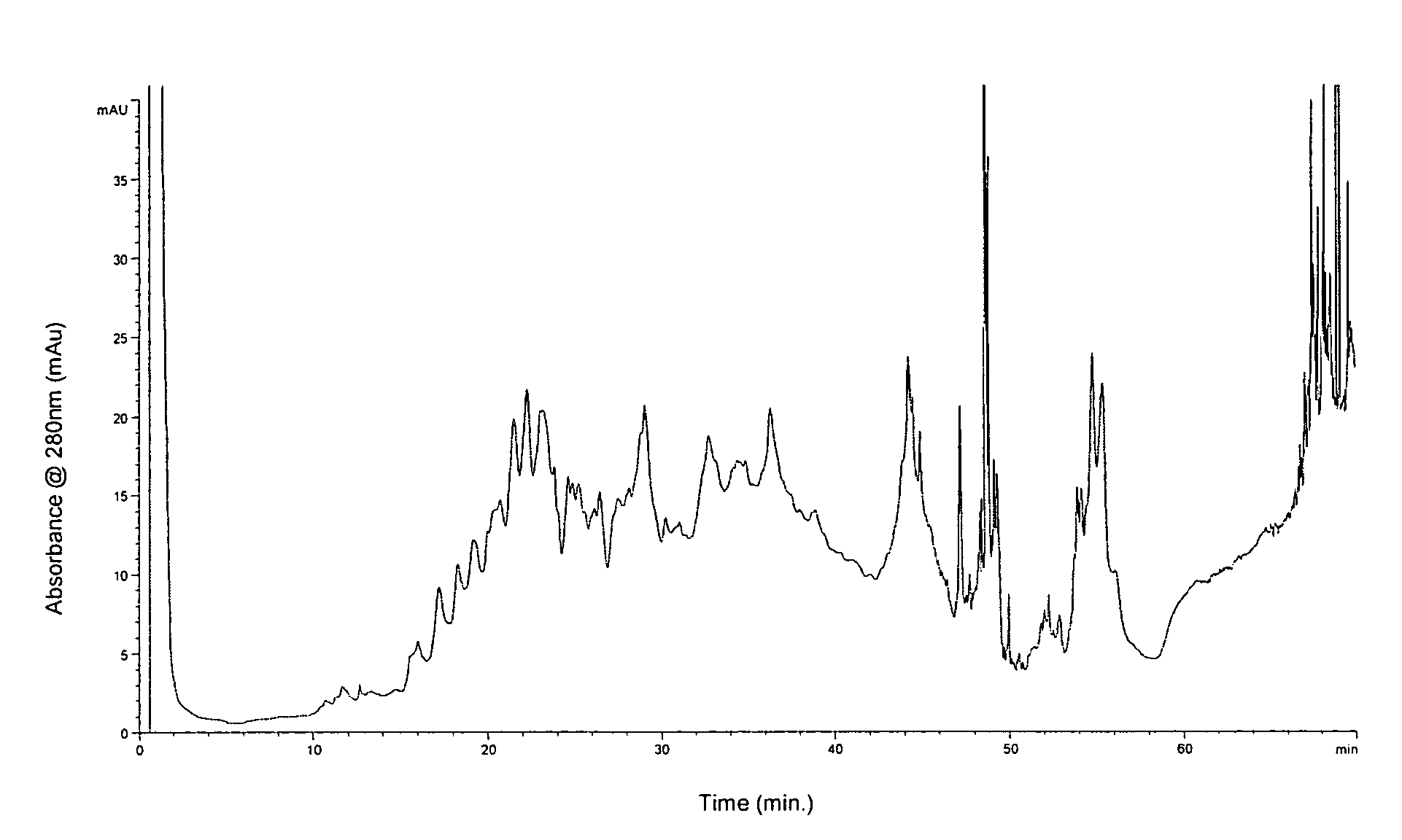
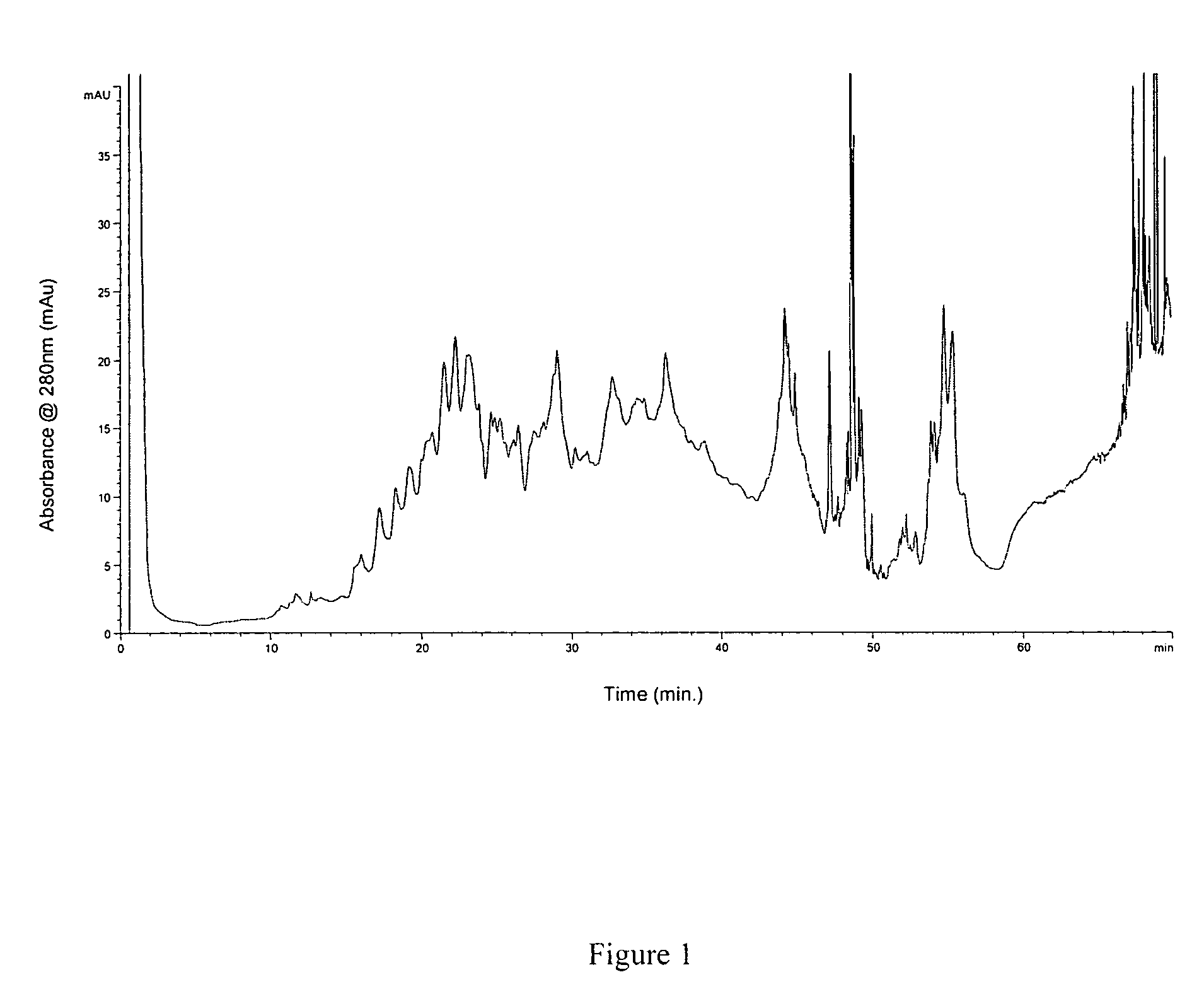
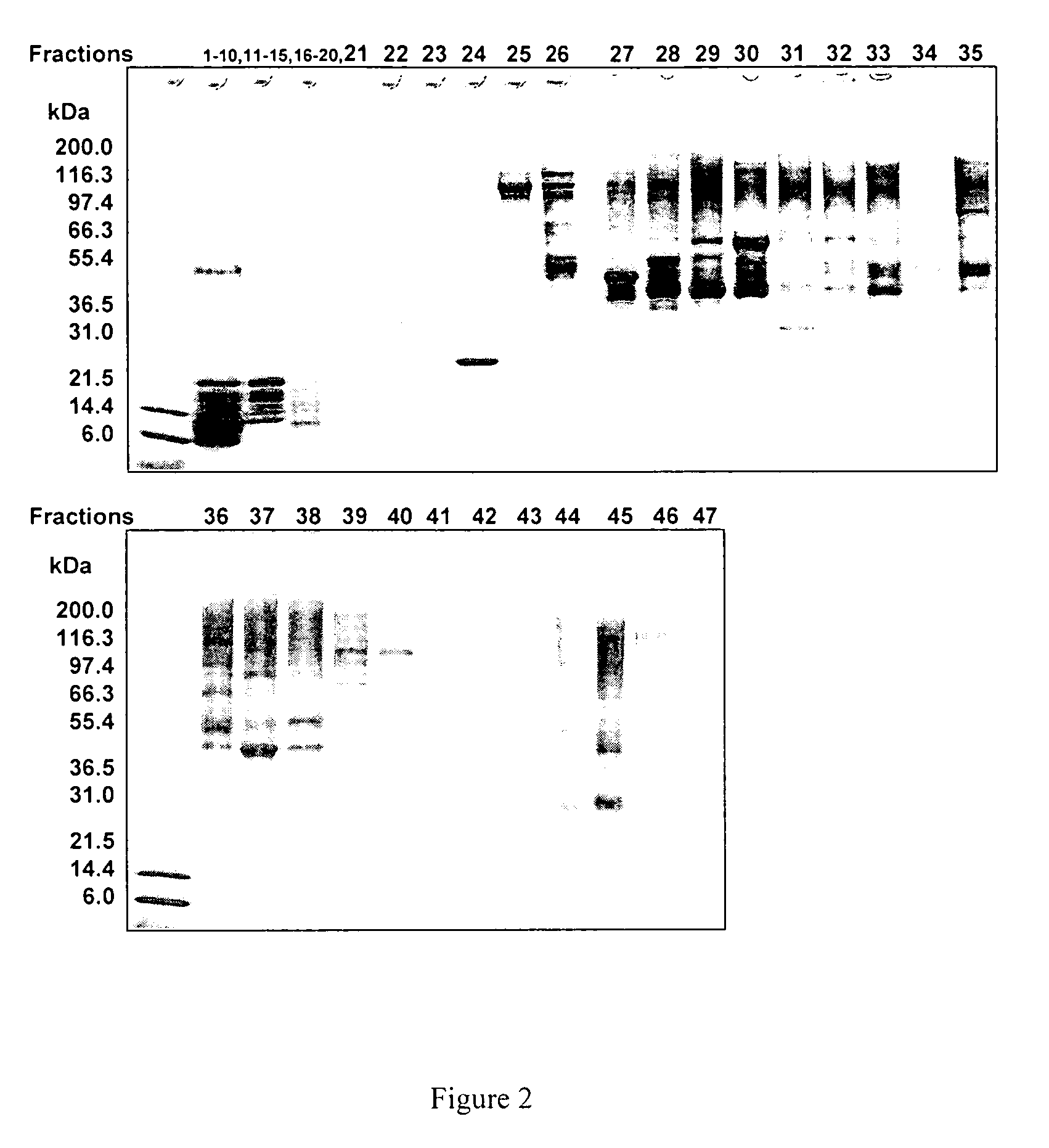
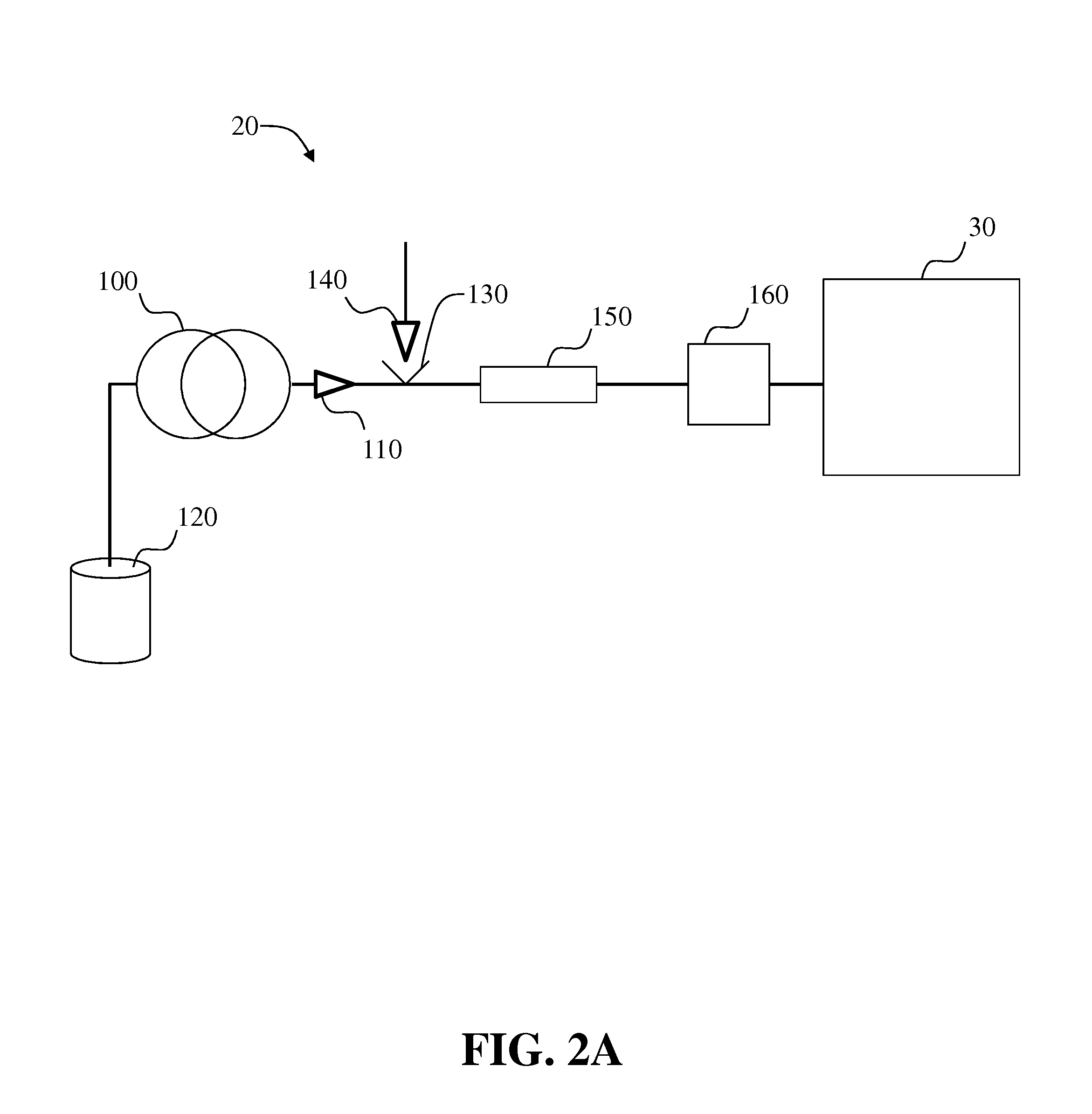
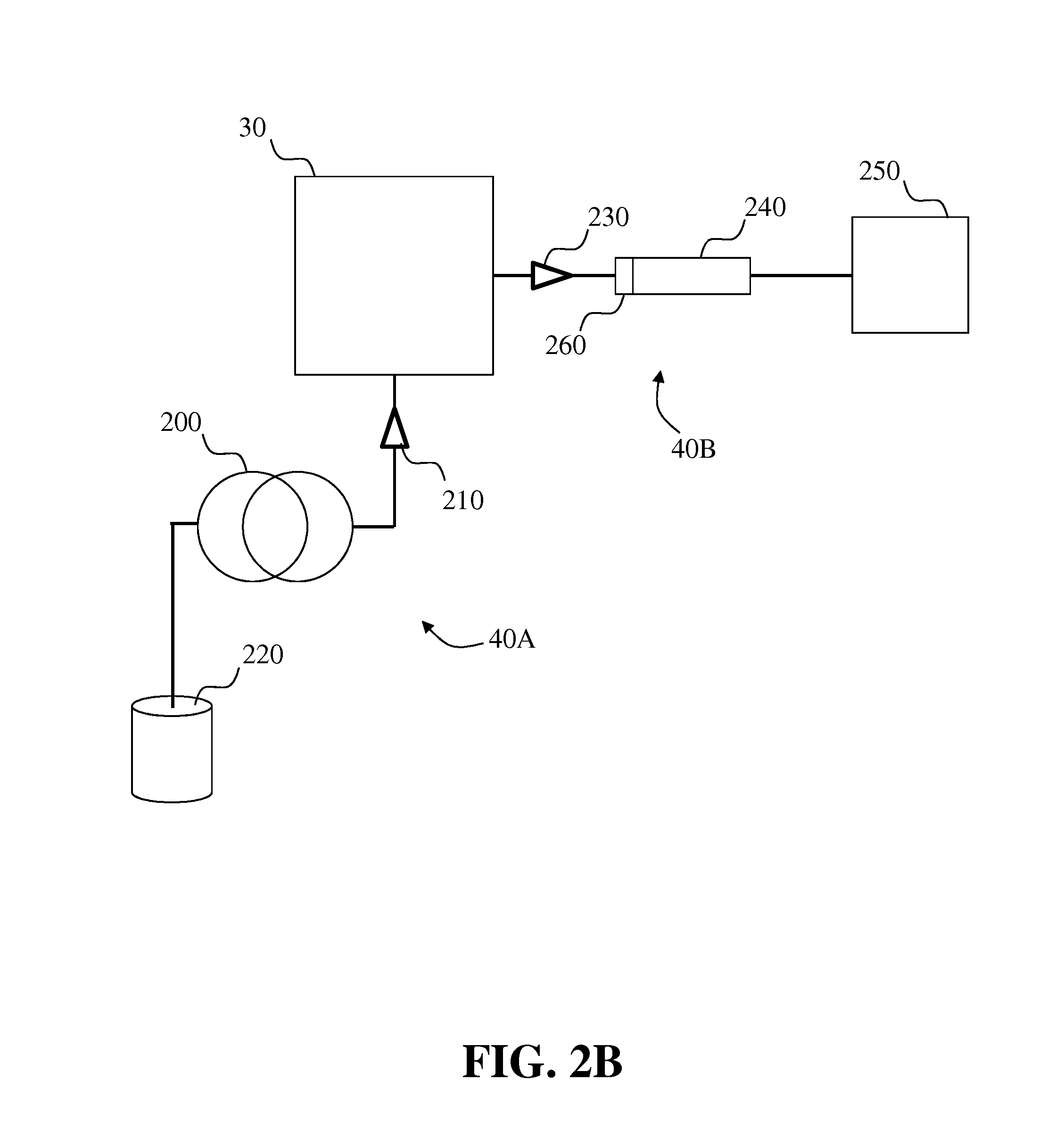
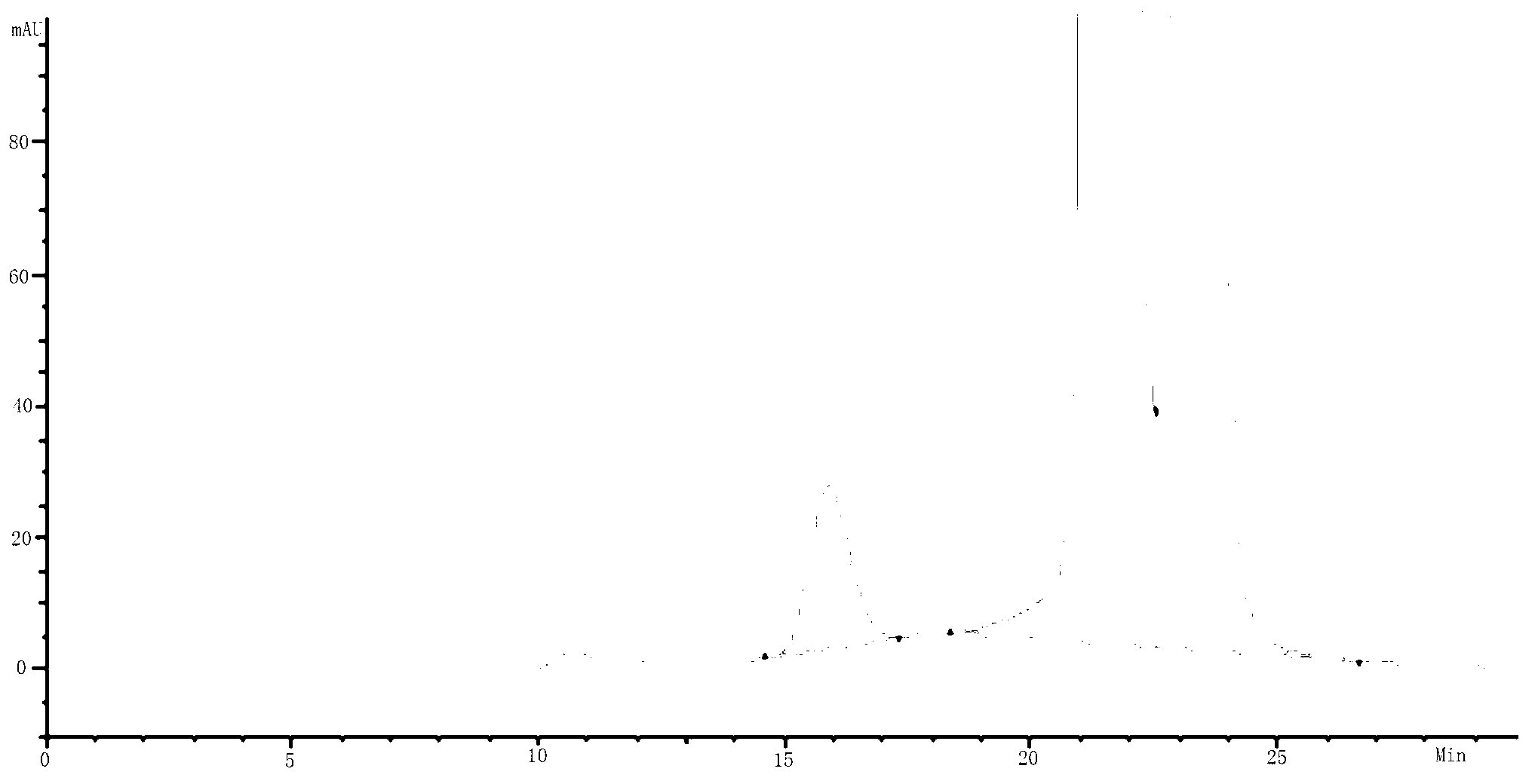
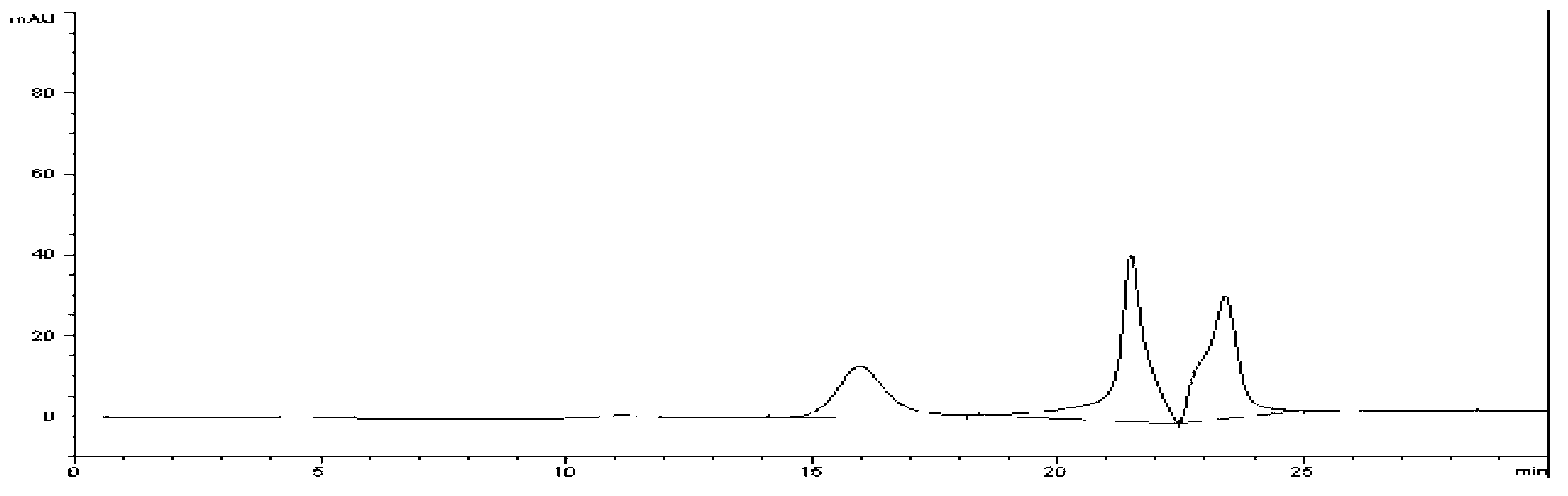
Methods and systems for on-column protein delipidation
ActiveUS7943046B2Minimal protein lossesGood reproducibilityIon-exchange process apparatusIon-exchanger regenerationStationary phaseAlcohol
Embodiments of the present invention provide a method of chromatographic delipidation comprising separating a lipid-containing sample on a superficially porous stationary phase at greater than about 70° C., at least about 80° C., having at least one mobile phase comprising an ion-pairing agent in water, an ion-pairing agent in an organic modifier, an acid in an organic modifier, and an alcohol. The invention provides minimal protein losses and high run-to-run reproducibility. The on-column delipidation method aventageously utilize reversed phase liquid chromatography.
Owner:AGILENT TECH INC
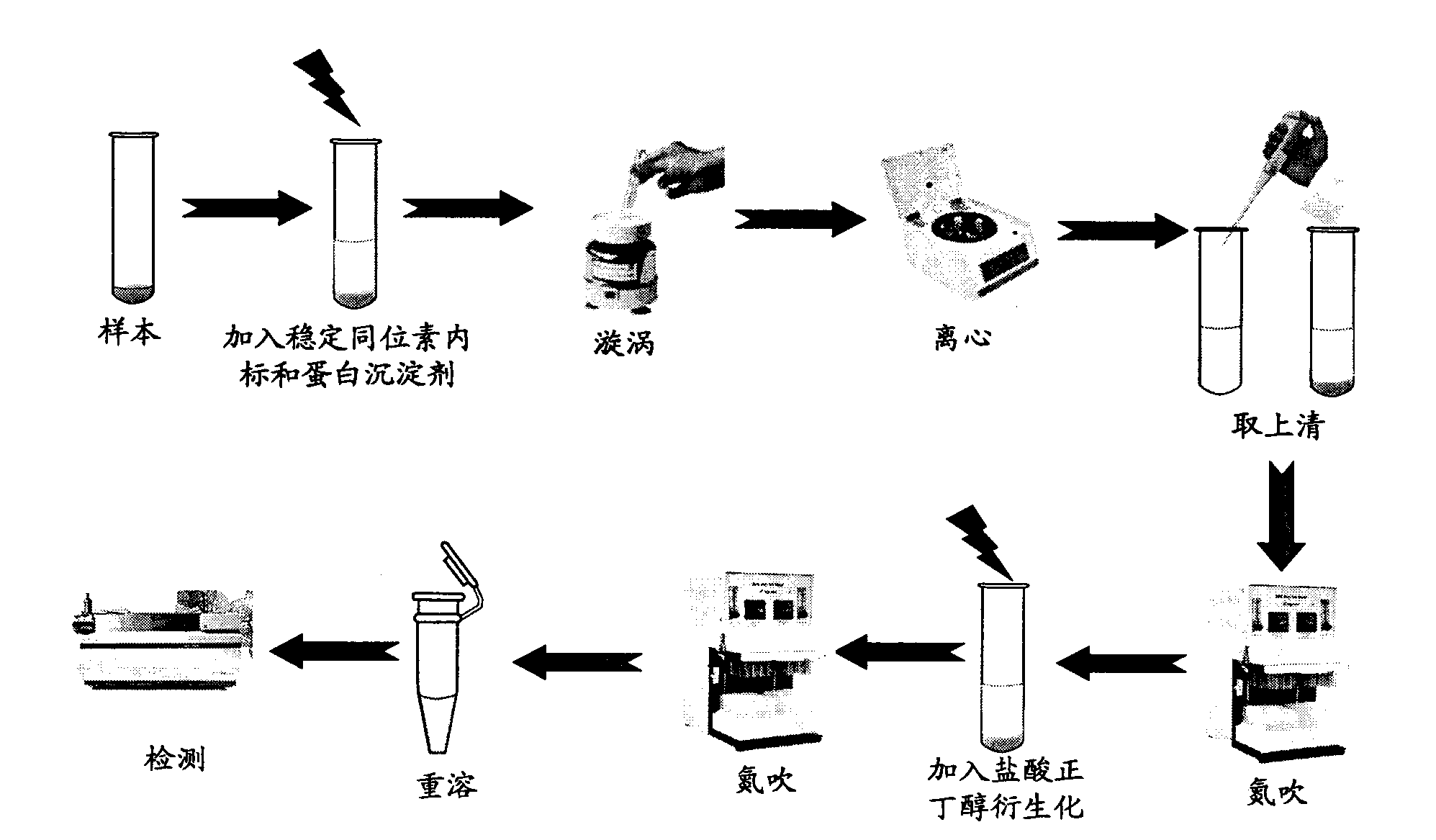
A simultaneous quantitative detection method of 30 amino acids and a preparation method thereof
InactiveCN103163226AImprove detection qualityRealize localizationComponent separationMaterial analysis by electric/magnetic meansStable Isotope LabelingReversed-Phase Liquid Chromatography
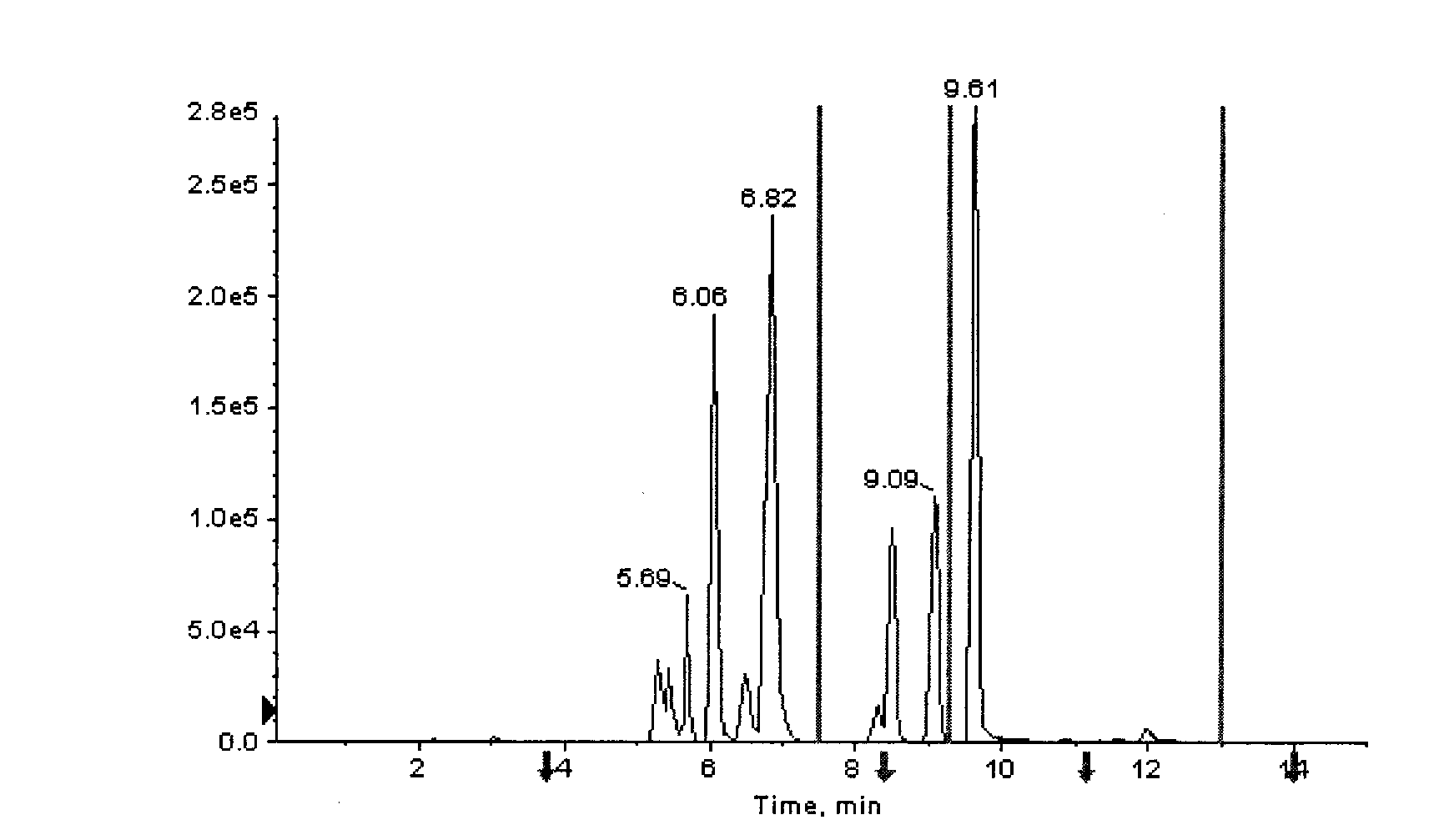
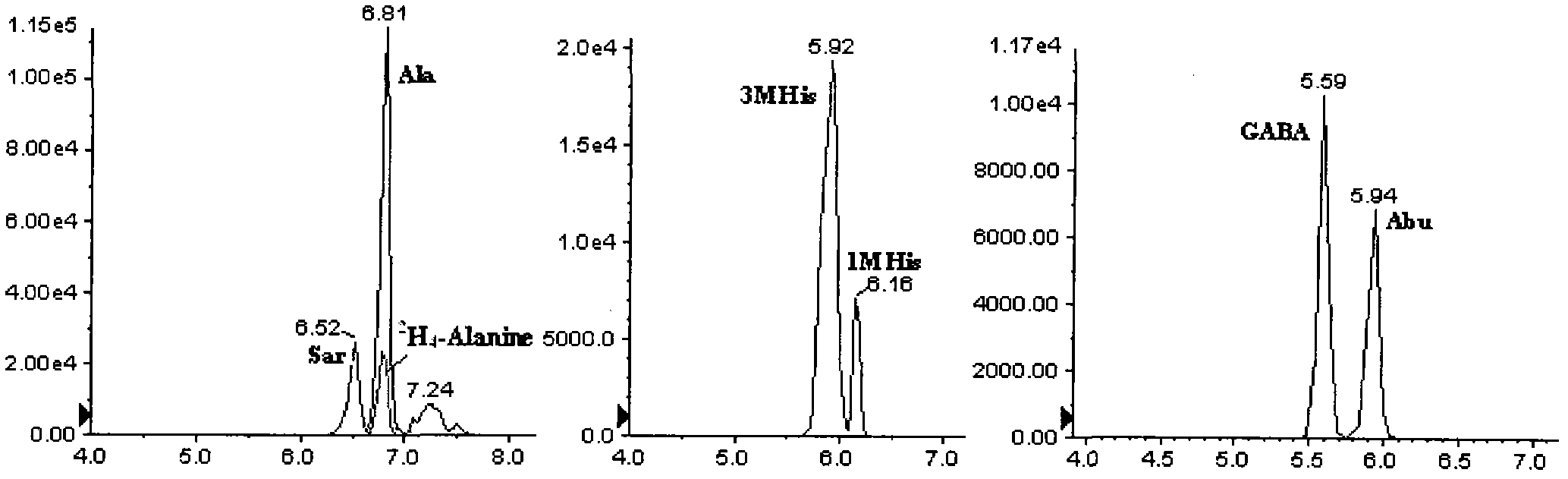
The invention provides a high-sensitivity and high-throughput kit for simultaneous quantitative detection of 30 amino acids and a preparation method thereof. According to the kit, after extraction treatment of amino acids in a complex sample by using organic solvents, precipitating by using methanol, adding stable isotope-labeled amino acid internal labels, performing derivatization treatment by using hydrochloric acid and n-butanol, drying and redissolving, carrying out reversed-phase chromatography gradient separation by adopting C18 bonded phase silica gel as a stationary phase and acetonitrile-water containing heptafluorobutyric acid and formic acid as a mobile phase, and carrying out quantitative detection by adopting a mass spectrometer. The kit of the invention has the advantages that detection sensitivity can reach 10ng / ml, and all methodological indexes can meet the requirements of quantitative detections of amino acids in clinical examinations, industrial productions and scientific researches.
Owner:刘丽宏 +2
Purification of oligomers
InactiveUS6921818B2Easy to separateEfficient purificationSilicon organic compoundsSilicon halogen compoundsChromatographic separationSilylene
Compositions and methods are disclosed which facilitate purification of oligomers and other compounds. The disclosed compositions are silyl compositions that can be directly coupled, or coupled through a linking group, to a compound of interest, preferably to an oligomer at the end of oligomer synthesis. The silicon atom includes between one and three sidechains that function as capture tags. In one embodiment, the capture tags are lipophilic, which allows a derivatized oligomer to be separated from failure sequences by reverse phase chromatography. In another embodiment, the capture tags are compounds with a known affinity for other compounds, which other compounds are preferably associated with a solid support to allow chromatographic separation. Examples include haptens, antibodies, and ligands. Biotin, which can bind to or interact with a streptavidin-bound solid support, is a preferred capture tag of this type.
Owner:YALE UNIV
HPLC method for separation and detection of hydromorphone and related opioid pharmacophores
InactiveUS20080206883A1Component separationColor/spectral properties measurementsHplc methodPharmacophore
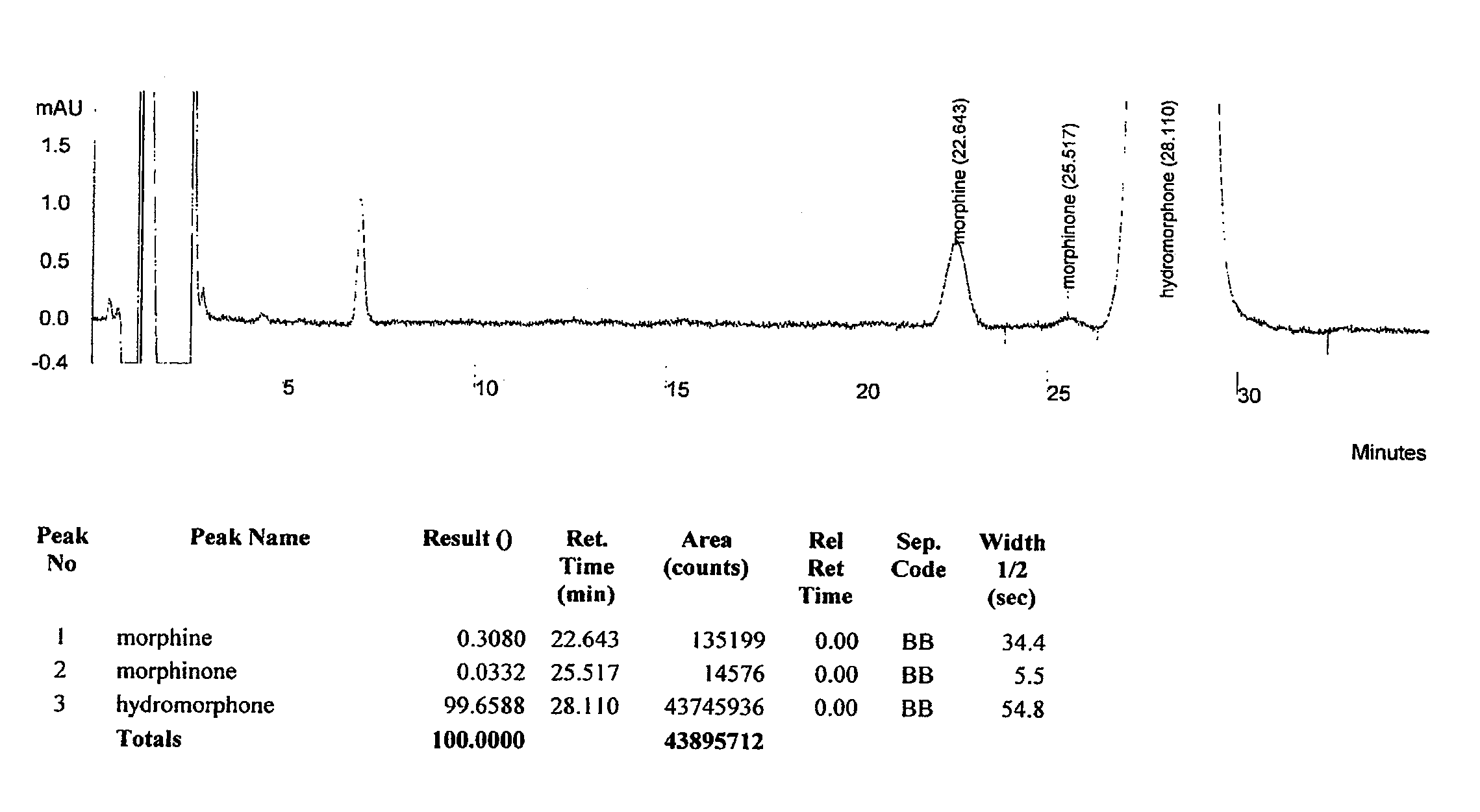
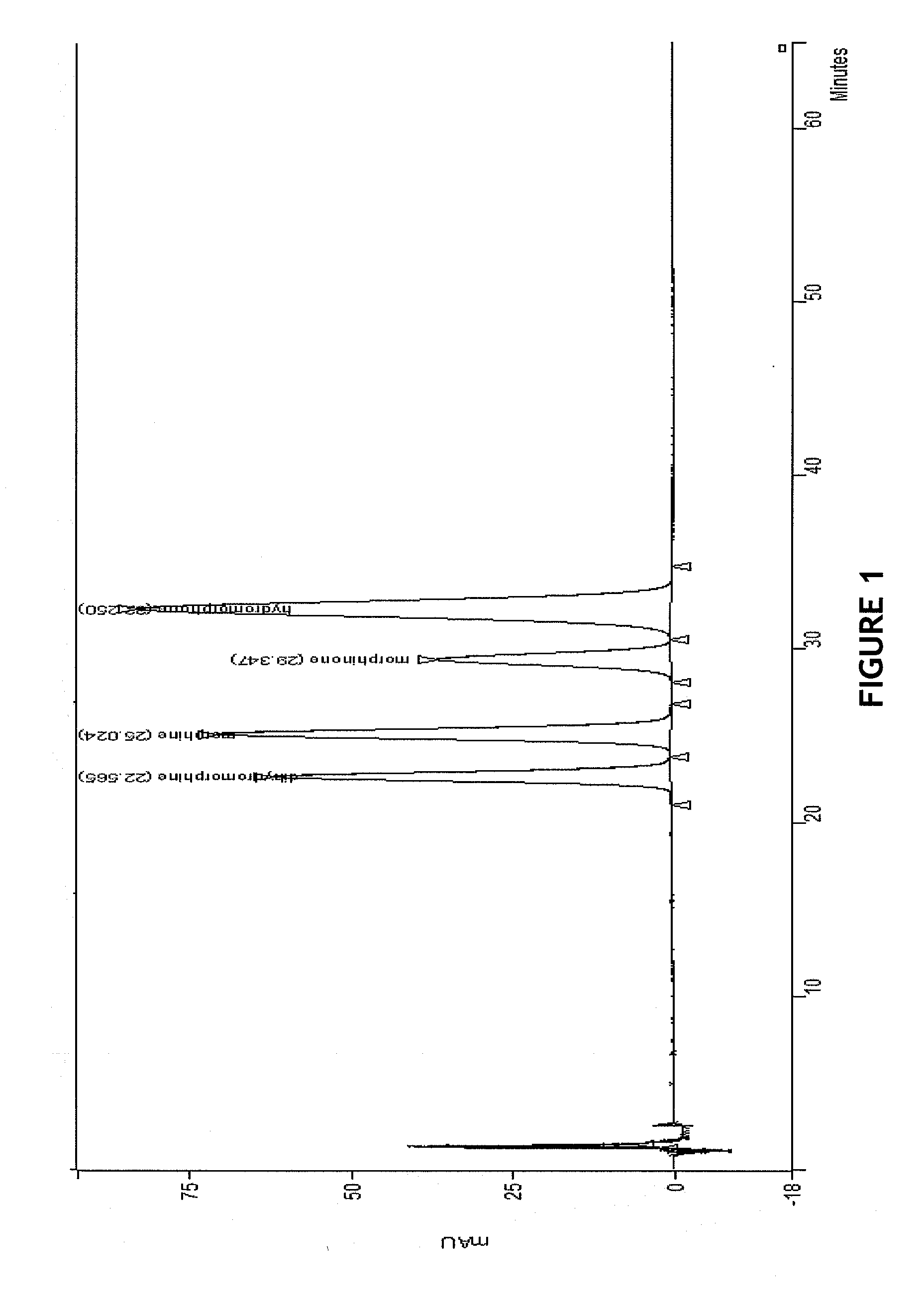
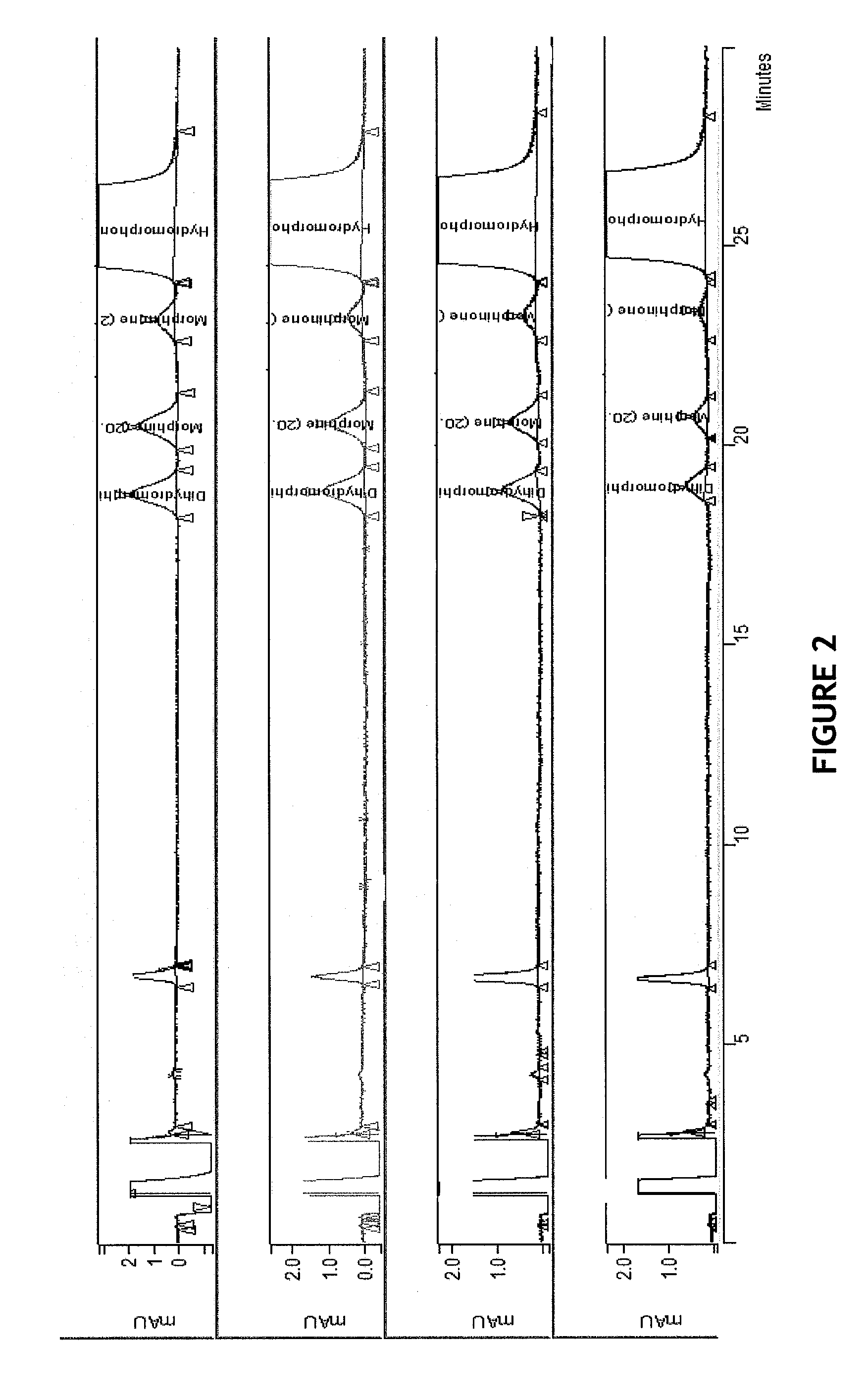
HPLC methods are provided to separate and detect morphinone, morphine, and dihydromorphine in the presence of hydromorphone. The isocratic HPLC methods employ ion-pair solute-solute ion-exchange mobile phase techniques in reversed phase chromatography. Method conditions in the disclosure provide separation and quantification of opioid pharmacophores in accordance with federal guidelines for obtaining resolution between analytes R≧2.0; tailing factor T≦2.0, capacity factor 2<k′≦50, and theoretical plate number N≧2000 for each opioid analyte peak.
Owner:CODY LAB
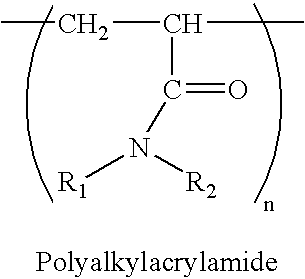
Polymeric adsorbents and method of preparation
InactiveUS6387974B1Significant compressibilitySignificant pressurePowder deliveryComponent separationPorosityStationary phase
Macroporous polymers having selected porosity and permeability characteristics that provide rigid polymer matrices suitable for use in medium and high pressure reversed phase liquid chromatography (RPC) are disclosed. A method for preparing the polymers using selected mixed porogens in selected proportions relative to the monomer phase is also disclosed. The polymers are especially useful as stationary phases in large scale chromatography columns without developing increased pressures during prolonged use, while maintaining good chromatographic performance for targeted biomolecules, such as insulin.
Owner:ROHM & HAAS CO
Packing material for chromatography having novel characteristic and method for isolation of substance using the same
InactiveUS6706187B1Improve hydrophobicityIon-exchange process apparatusChromatographic cation exchangersStationary phaseSubstance use
To provide chromatographic packings whereby biological components, etc. which cannot be separated by either ion-exchange chromatography or reversed phase chromatography employed alone can be efficiently separated without deteriorating their activities. Use is made of a packing which contains a charged copolymer and makes it possible to change the effective charge density on the surface of a stationary phase by a physical stimulus while fixing a mobile phase to an aqueous system.
Owner:TEURO OKANO +3
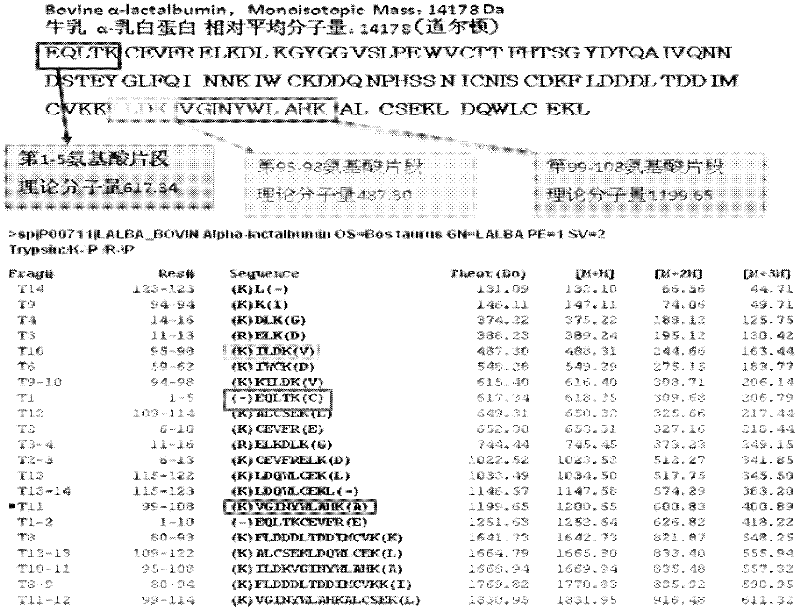
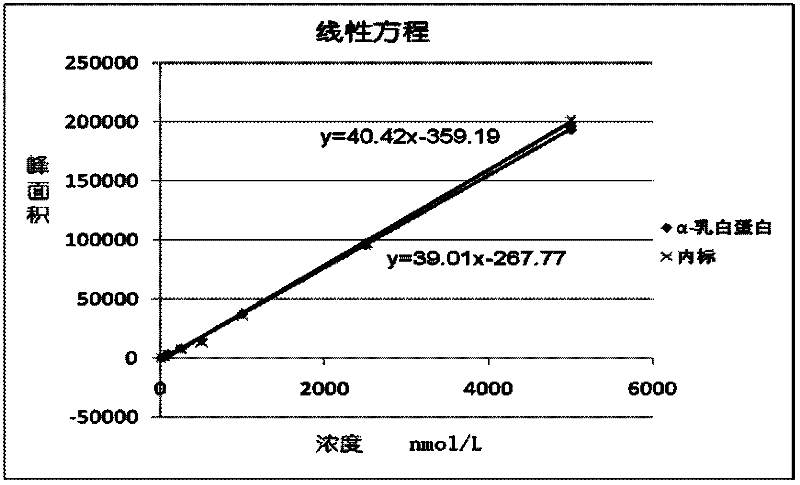
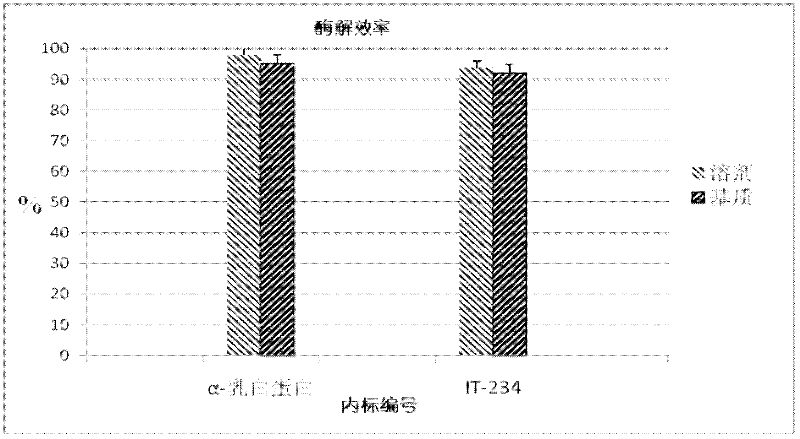
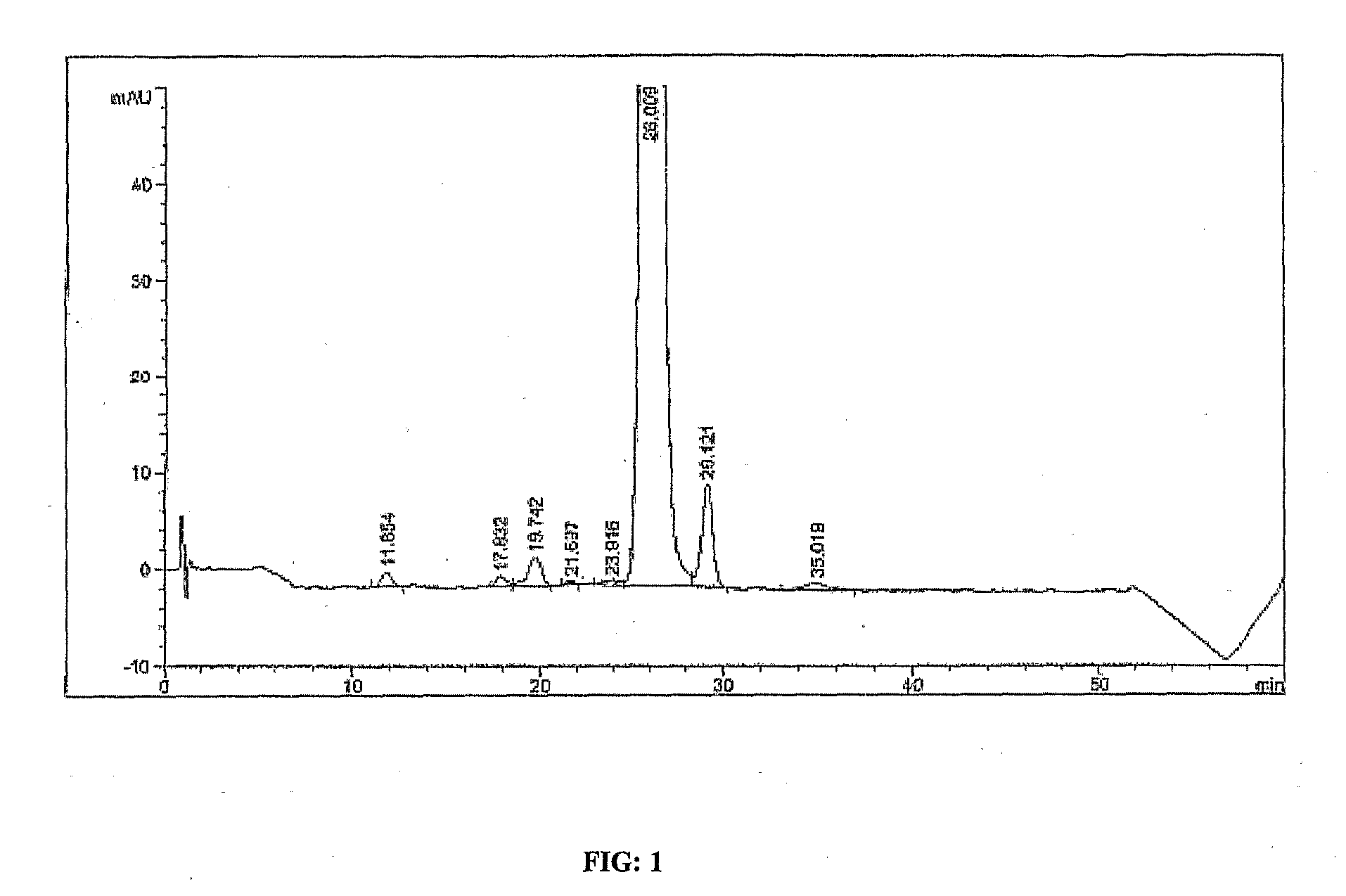
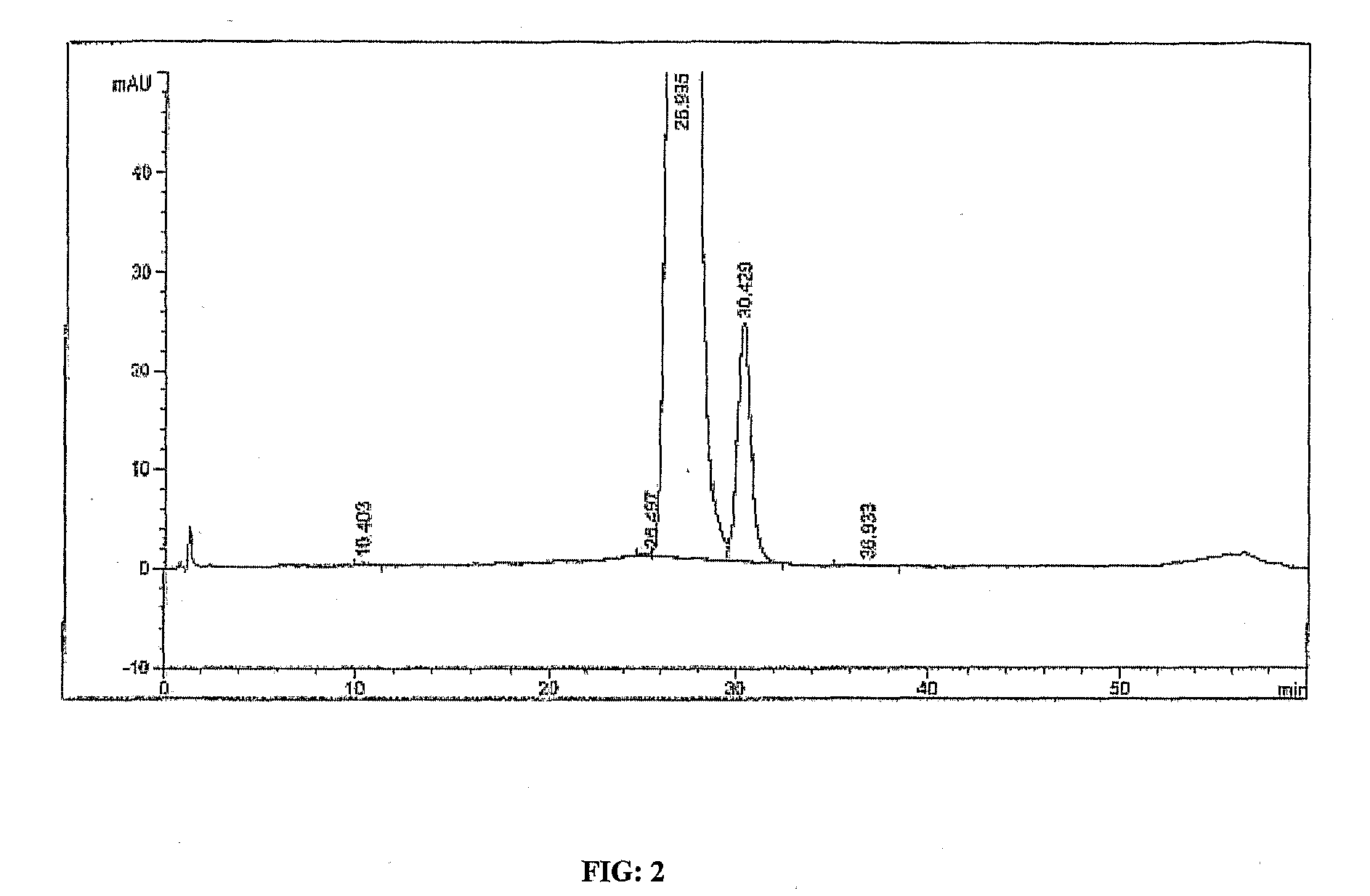
Quantitative detection method for bovine alpha-lactalbumin
InactiveCN102590413AEasy to operateGuaranteed reproducibilityComponent separationRelative standard deviationReversed-Phase Liquid Chromatography
The invention relates to a quantitative detection method for thermal-denaturation and non-denaturation bovine alpha-lactalbumin in milk and milk products by applying an enzymolysis-liquid chromatography and mass spectrometry combination technology. The quantitative detection method comprises the steps as follows: taking a certain amount of milk or milk samples, dissolving and diluting the milk or milk samples in water to obtain solution with total protein content being about 1mg / mL; after volume metering, correctly sucking 500 mu L, adding an internal standard substance, reacting disulfide bond with dithiothreitol (DTT), alkylating to protect sulfydryl produced in reaction by iodoacetamide (IAA), and then conducting constant-temperature and constant-time enzymolysis with trypsin; and separating enzymolysis products by reversed phase liquid chromatography, conducting detection with a mass spectrum multiple reaction monitoring (MRM) manner, and calculating the result by an internal standard method. The quantitation limit of the method is 0.001g / 100g; when adding amount is 0.2, 1.7 and 5.0g / 100g, the recovery rate is 98.9-110.8% (n is equal to 6) and repeatability: RSD (Relative Standard Deviation) is smaller than 7.6%; and the quantitative detection method can be applicable to the quantitative detection of samples with different contents of bovine alpha-lactalbumin.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Pure form of rapamycin and a process for recovery and purification thereof
InactiveUS20100029933A1Organic chemistryFermentationPurification methodsReversed-Phase Liquid Chromatography
The present invention relates to a pure form of rapamycin with a total impurity content less than 1.2%; a process for recovery and purification of rapamycin comprising steps of (a) treating the fermentation broth, extracts or solutions containing rapamycin with water immiscible solvent and concentration; (b) addition of a water miscible solvent to effect separation of impurities present; (c) optionally, binding of the solvent containing the product from step (b) to an inert solid, washing the solid with a base and acid, followed by elution; (d) subjecting the elute from step (c) or the solvent containing the product from step (b) to silica gel chromatography; (e) crystallization of the product obtained from step (d); (f) subjecting a solution of the product from step (e) to hydrophobic interaction or reversed phase chromatography; and (g) re-crystallization to afford rapamycin in substantially pure form.
Owner:BIOCON LTD
Method of preparing a plurality of active substances from ginkgo leaves
ActiveCN103130816AEasy high problemSolve the problem of highOrganic chemistryGinkgophyta medical ingredientsSeparation technologyReversed-Phase Liquid Chromatography
The invention discloses a method of preparing a plurality of activation materials from ginkgo leaves, and belongs to a manufacturing method of chemical substances or a manufacturing method of drugs. Strong polarity macroporous adsorption resin or polarity macroporous adsorption resin is adopted for separation, and is adopted to solve the problems that the amount of ginkgolic acid is high when the prior ginkgo extract is prepared, and solve the problems that a large amount of organic solvent is consumed when the prior ginkgo active substances are extracted and prepared, and accordingly the environment is polluted, or that a complex separation technology can not enlarge production, or that extraction efficiency is not high, and use ratio of raw materials is low and the like. The method of preparing the plurality of activation materials from the ginkgo leaves can simultaneously obtain a plurality of active substances such as ginkgo polysaccharide, ginkgol biloba extracts, ginkgol biloba general flavone, ginkgol biloba total lactones, gbilobalide A monosome, gbilobalide B monosome, gbilobalide C monosome and the like. The technical scheme is that the method comprises a raw material pretreatment step, an extraction step, a polysaccharide precipitation step, a macroporous resin separation step, a silica gel dry-column separation step, a reversed phase chromatography purification step, a crystallization step and the like, saves production cost, and improves work efficiency. The process of the method is simple and convenient to conduct. The method can be easily enlarged to the commercial process, and meanwhile greatly increase use ratio of raw materials.
Owner:HUBEI NUOKETE PHARMA
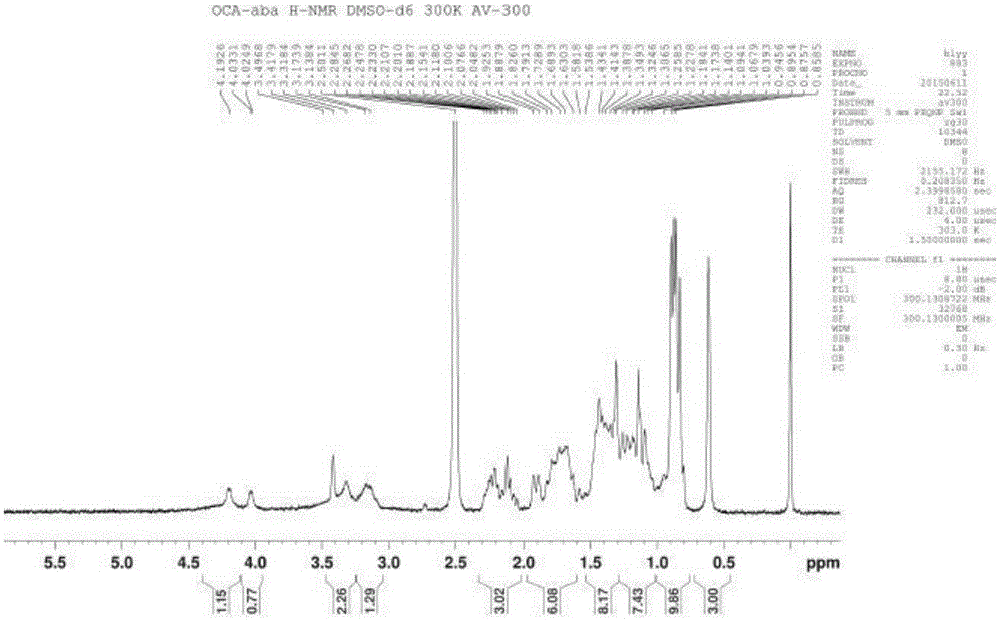
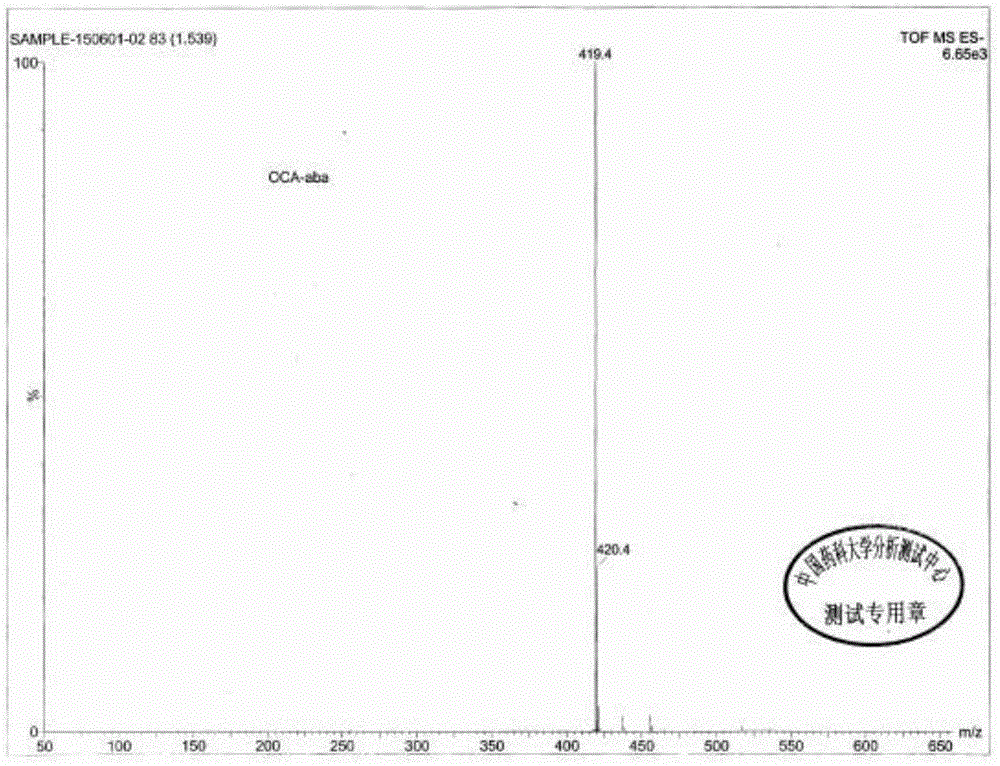
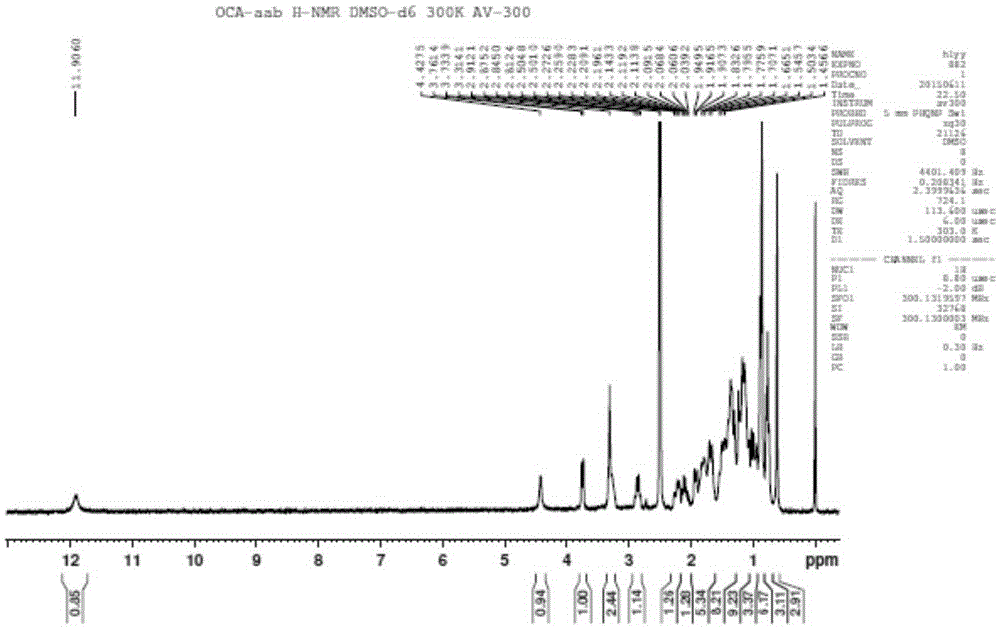
Method for synthesizing, separating and determining obeticholic acid (OCA) isomer
The invention relates to an obeticholic acid (OCA) isomer namely an OCA-alpha alpha beta body, a synthetic method of the OCA-alpha beta alpha body, and a method adopting the reversed phase liquid chromatography condition to separate the OCA isomer. With adoption of the technical scheme disclosed by the invention, the OCA-alpha alpha beta body and the OCA-alpha beta alpha body with the HPLC purity greater than 98% can be obtained to meet quality control of the isomer in OCA.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
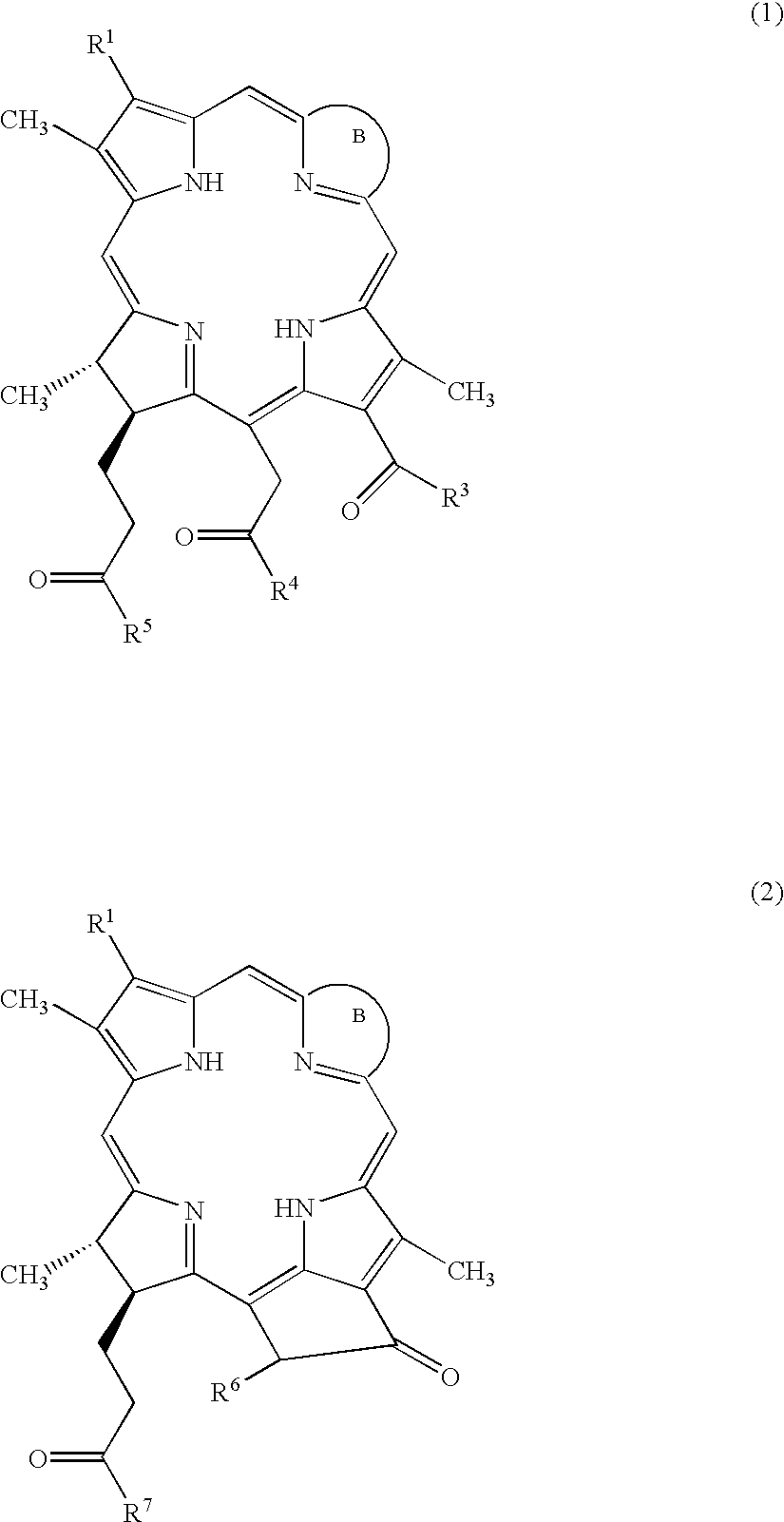
Water-soluble porphyrin derivatives for photodynamic therapy, their use and manufacture
InactiveUS6777402B2Easy and time-efficientBiocideEnergy modified materialsPorphyrinReversed-Phase Liquid Chromatography
Owner:BIOLITEC UNTERNEHMENSBETEILLIGUNGS II AG +1
Method for preparing vasopressin tannate
InactiveCN104672308AAvoid the problem of excessive volume and diluted concentrationSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDivinylbenzeneDesalination
The invention discloses a method for preparing vasopressin tannate. The preparation method of the vasopressin comprises the following step: sequentially carrying out reverse-phase purification and reverse-phase desalination on a vasopressin crude product solution by a high performance liquid chromatography, so as to obtain the vasopressin tannate, wherein packing of the high performance liquid chromatography, is a polystyrene-divinylbenzene (PS-DVB) copolymer. By combination of reverse-phase purification and reverse-phase desalination, the latest application of the polymer packing polystyrene-divinylbenzene is designed; and the vasopressin tannate can be prepared on a large scale.
Owner:QINGDAO KANGYUAN PHARMA
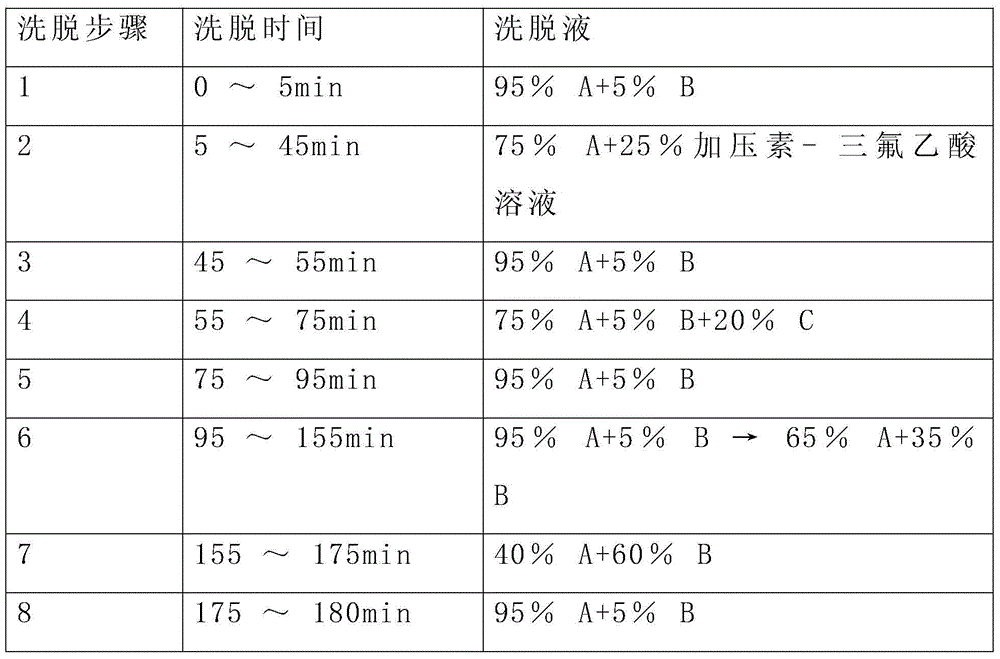
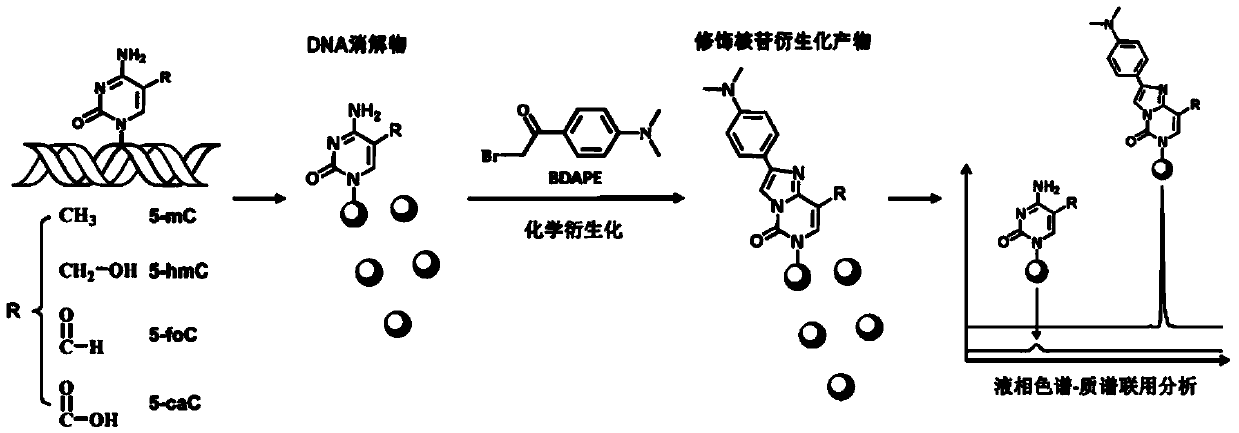
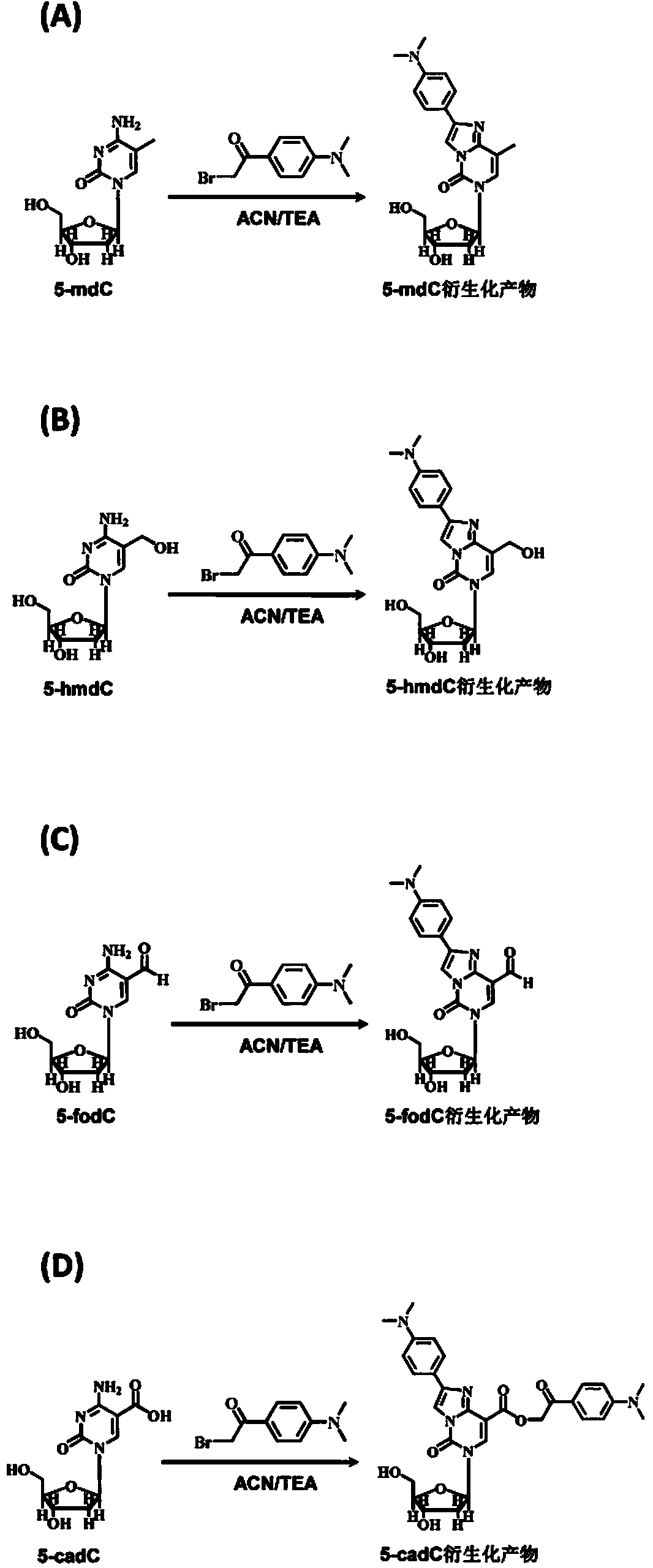
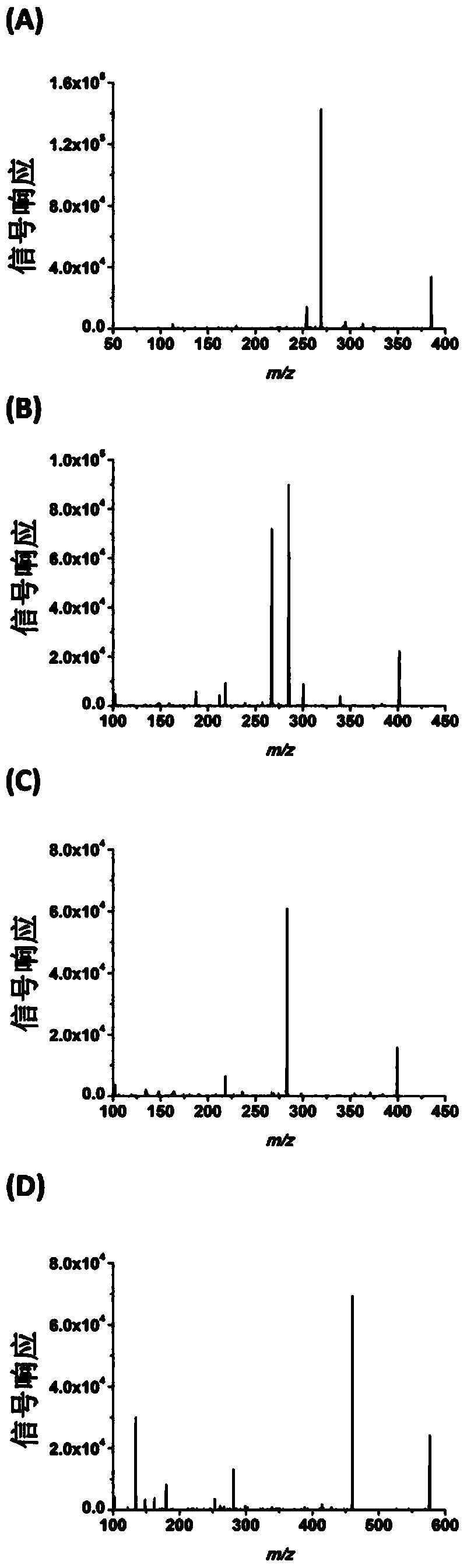
Chemical derivatization method and application thereof to nucleic acid modification detection by liquid chromatogram-mass spectrometer (LC-MS) method
InactiveCN104502492AThe method is easy to operateEasy to separateComponent separationMicrobiological testing/measurementReversed-Phase Liquid ChromatographyKetone
The invention discloses a chemical derivatization method and an application thereof to nucleic acid modification detection by a liquid chromatogram-mass spectrometer (LC-MS) method. A derivatization strategy is performed on 5-methylcystein, 5-hydroxymethylcytosine, 5-aldehyde cytosine and 5-carboxylcytosine in deoxyribonucleic acid (DNA) by means of 2-bromo-1-(4-dimethylamino-phenyl)-ethyl ketone (BDAPE). Retention behaviors of modified nucleosides in reversed phase liquid chromatography are remarkably improved, and meanwhile, the detection sensitivity of the modified nucleosides in mass spectra is greatly improved. The chemical derivatization method has the advantages that the sensitivity and the selectivity are high, the operation is simple and convenient, quantitative detection of cytosine modification with extremely low abundance in the DNA can be achieved without complicated sample pretreatment, and the method can be applied to analysis of diseases and study on mutual relation among the 5-methylcystein, the 5-hydroxymethylcytosine, the 5-aldehyde cytosine and the 5-carboxylcytosine.
Owner:WUHAN UNIV
Detection method of parecoxib sodium genotoxicity impurity and application thereof
ActiveCN105372376AAchieve separationHigh sensitivityComponent separationPhosphateReversed-Phase Liquid Chromatography
The present invention provides a detection method of parecoxib sodium genotoxicity impurity and application thereof. The method uses a reversed-phase liquid chromatography method. The chromatography conditions are as follows: the chromatographic column comprise a C18 column, a C8 column, a phenyl column and a Hilic column; a mobile phase consists of water-acetonitrile, dilute phosphoric acid-acetonitrile, and phosphate-acetonitrile; a flow phase comprises an aqueous phase and an organic phase in the ratio of 90:10-10:90; the column temperature is 25-40 DEG C; the flow rate of the mobile phase is 0.2-2ml / min; detection wavelength is 205-290nm; a detector is a UV detector or a photodiode array (PDA) detector; and the sample size is 0.1-40 mul. The detection method of parecoxib sodium genotoxicity impurity achieves the separation of parecoxib sodium and three genotoxicity impurities in a short period of time, has high sensitivity and specificity, and simple operation, reaches the separation rate of the main component and genotoxicity impurities, and the separation rate of genotoxicity impurities both greater than 1.5; and the method can be used for quality control of parecoxib sodium, and has practical value.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Systems and methods for two-dimensional chromatography
ActiveUS20160238573A1Reduce the amount requiredComponent separationSolid sorbent liquid separationAnalyteDrug compound
Provided are two-dimensional chromatography systems and methods for separating and / or analyzing complex mixtures of organic compounds. In particularly, a two-dimensional reversed-phase liquid chromatography (RPLC)-supercritical fluid chromatography (SFC) system is described including a trapping column at the interface which collects the analytes eluted from the first dimension chromatography while letting the RPLC mobile phase pass through. The peaks of interest from the RPLC dimension column are effectively focused as sharp concentration pulses on the trapping column, which is subsequently injected onto the second dimension SFC column. The system can be used for simultaneous achiral and chiral analysis of pharmaceutical compounds. The first dimension RPLC separation provides the achiral purity result, and the second dimension SFC separation provides the chiral purity result (enantiomeric excess).
Owner:GENENTECH INC
Method for preparing octreotide and octreotide acetate
ActiveCN103965291AAvoid the problem of excessive volume and diluted concentrationSuitable for continuous productionOxytocins/vasopressinsAngiotensinsOctreotide acetateDesalination
The invention discloses a method for preparing octreotide and octreotide acetate. The method for preparing octreotide comprises the following steps: sequentially performing reverse phase purification and reverse phase desalination on an octreotide crude product solution by using a high performance liquid reversion phase chromatography method, wherein the packing of the high performance liquid reversion phase chromatography method is a poly styrene-divinyl benzene (PS-DVB) copolymer. The method integrates reverse phase purification and reverse phase desalination, novel application of the copolymer packing, namely, the styrene-divinyl benzene, is designed, and octreotide and octreotide acetate can be prepared in a large scale.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Purification method for recombinant human follicle-stimulating hormone
ActiveCN103059125ALow costHigh purityDepsipeptidesPeptide preparation methodsPurification methodsRecombinant human follicle stimulating hormone
The present invention relates to a purification method for recombinant human follicle-stimulating hormone (FSH), including anion-exchange chromatography, affinity chromatography, and gel filtration chromatography. The method is low in cost, few in steps, simple and feasible, and stable in quality; and no complicated denaturation and renaturation processes are comprised in the method, and the use of reversed phase chromatography, metal chelate chromatography or hydrophobic interaction chromatography, which have a greater impact on protein activity, is prevented. According to the method, firstly, anion exchange of flow-through mode is used for removing a large number of contaminating proteins, pigments and some residual DNA, endotoxin and host proteins, which not only protects the subsequent affinity filler, but also achieves a coarse purification and concentration enrichment; affinity media are conjugated with camel-sourced antibodies, the carrying capacity is high, and only an intact FSH molecule, instead of a single subunit, can be bound specifically, so degraded monomer subunits are better removed; and gel filtration can further remove residual DNA, endotoxin and host proteins, and can also remove protein aggregates and inadequately glycosylated FSH proteins.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Reverse-phase chromatography and mass-spectrometry combined detection method for complete low-molecular-heparin degradation product through precolumn derivatization
ActiveCN103630647AEnsure safetySolve the problem that only 8 common heparin disaccharides can be detectedComponent separationReversed-Phase Liquid ChromatographyMass Spectrometry-Mass Spectrometry
The invention relates to a reverse-phase chromatography and mass-spectrometry combined detection method for a complete low-molecular-heparin degradation product through precolumn derivatization. All components of the complete low-molecular-heparin degradation product are detected by combining reversed-phase chromatography and a high-resolution mass spectrum; all the components in a sample are separated through the reversed-phase chromatography, and a precision molecular weight can be obtained through the high-resolution mass spectrum; besides detection of eight common heparin disaccharides, tail end modification structures and special structures such as 3-O-tetrose-hydrosulfates relevant to anticoagulation and trisaccharides obtained through peeling reaction can be also detected; therefore, the structure of each complete low-molecular-heparin degradation product can be precisely represented. According to the reverse-phase chromatography and mass-spectrometry combined detection method, the problem that only the eight common heparin disaccharides can be detected in the prior art is solved; the reverse-phase chromatography and mass-spectrometry combined detection method has an extremely large practical value in research, development and production control of low-molecular-heparin imitating drugs and guarantee of the safety of the drugs.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Polymeric adsorbents and method of preparation
InactiveUS20020042487A1Significant compressibilitySignificant pressureIon-exchange process apparatusComponent separationStationary phasePorosity
Macroporous polymers having selected porosity and permeability characteristics that provide rigid polymer matrices suitable for use in medium and high pressure reversed phase liquid chromatography (RPC) are disclosed. A method for preparing the polymers using selected mixed porogens in selected proportions relative to the monomer phase is also disclosed. The polymers are especially useful as stationary phases in large scale chromatography columns without developing increased pressures during prolonged use, while maintaining good chromatographic performance for targeted biomolecules, such as insulin.
Owner:ROHM & HAAS CO
Preparation method for oxytocin deamidation impurity
ActiveCN106749539APrevent elutionSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDesalinationReversed-Phase Liquid Chromatography
The invention discloses a preparation method for an oxytocin deamidation impurity. The preparation method comprises the following step of: successively subjecting a solution of a crude oxytocin deamidation impurity precursor to reversed-phase cyclization, reversed-phase purification and reversed-phase desalination by adopting high-performance liquid-phase reversed-phase chromatography, wherein a filling material for the high-performance liquid-phase reversed-phase chromatography is silica gel C18; and the crude oxytocin deamidation impurity precursor contains two free sulfhydryl groups. The preparation method provided by the invention innovatively adopts a reversed-phase adsorption method for cyclization, purification and desalination, solves the problems of cyclization, purification and desalination once for all, optimizes the production process, and is applicable to continuous industrial production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
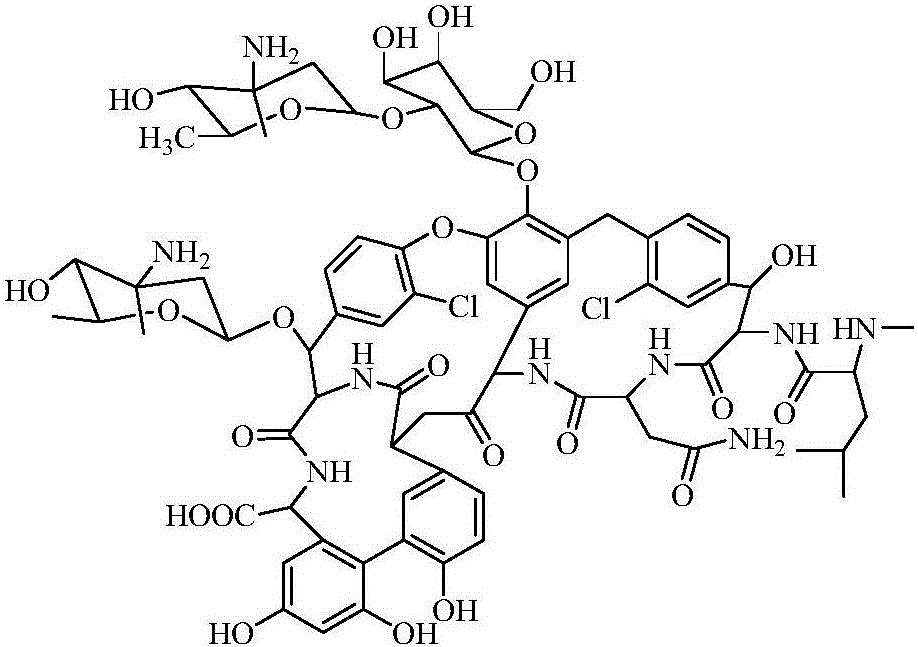
Purification method of Oritavancin intermediate A82846B
ActiveCN107434823AReduce usageReduce energy consumptionPeptide preparation methodsPurification methodsOritavancin
The invention provides a purification method of an Oritavancin intermediate A82846B. Specifically, the method comprises the following steps: loading an A82846B fermentation broth to an ion exchange resin chromatography column, and carrying out elution separation to obtain a first eluate; loading the first eluate toa reversed phase chromatography chromatography column with silica gel C18 or polystyrene polymer as a filler, carrying out impurity elution, carrying out sample elution and collecting a second eluate. Purity of the obtained A82846B is greater than 80%. By the method, quality of the product can be guaranteed; and the method is suitable for an expanded commercial market. The invention also provides a method for preparing the Oritavancin intermediate A82846B salt and a method for synthesizing Oritavancin by the use of the A82846B salt.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Methods and systems for on-column protein delipidation
ActiveUS20060240633A1Minimal protein lossHigh run-to-run reproducibilityIon-exchange process apparatusIon-exchanger regenerationLipid formationStationary phase
Embodiments of the present invention provide a method of chromatographic delipidation comprising separating a lipid-containing sample on a superficially porous stationary phase at greater than about 70° C., at least about 80° C., having at least one mobile phase comprising an ion-pairing agent in water, an ion-pairing agent in an organic modifier, an acid in an organic modifier, and an alcohol. The invention provides minimal protein losses and high run-to-run reproducibility. The on-column delipidation method aventageously utilize reversed phase liquid chromatography.
Owner:AGILENT TECH INC
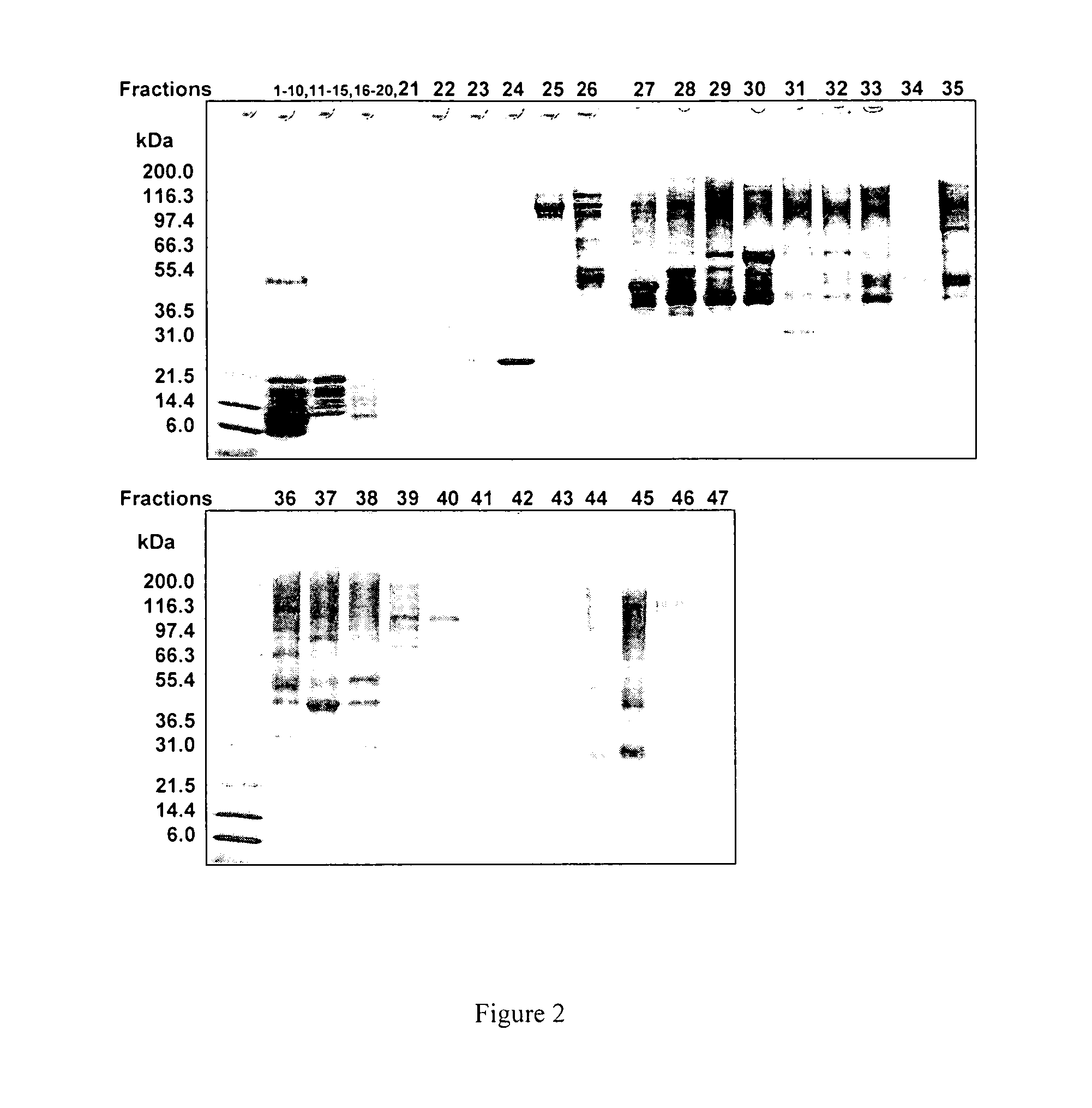
Calixarene crown ether bonded silica stationary phase and preparation method and application thereof
InactiveCN102107135AImprove hydrophobicityEasy to identifyIon-exchange process apparatusOther chemical processesBenzoic acidSilanes
Owner:ZHENGZHOU UNIV
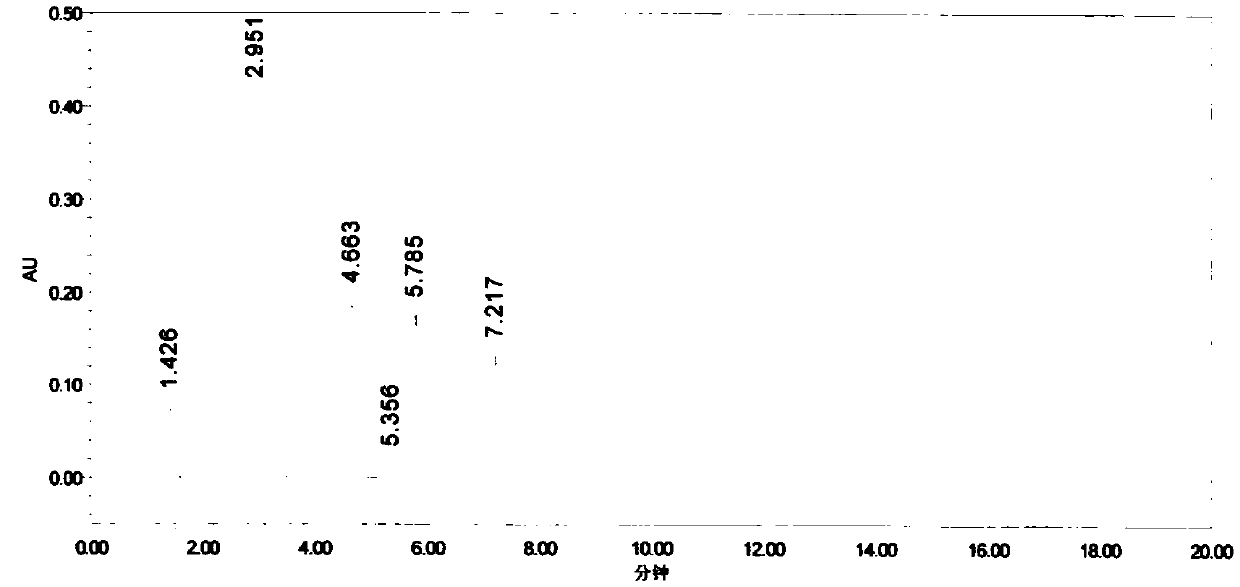
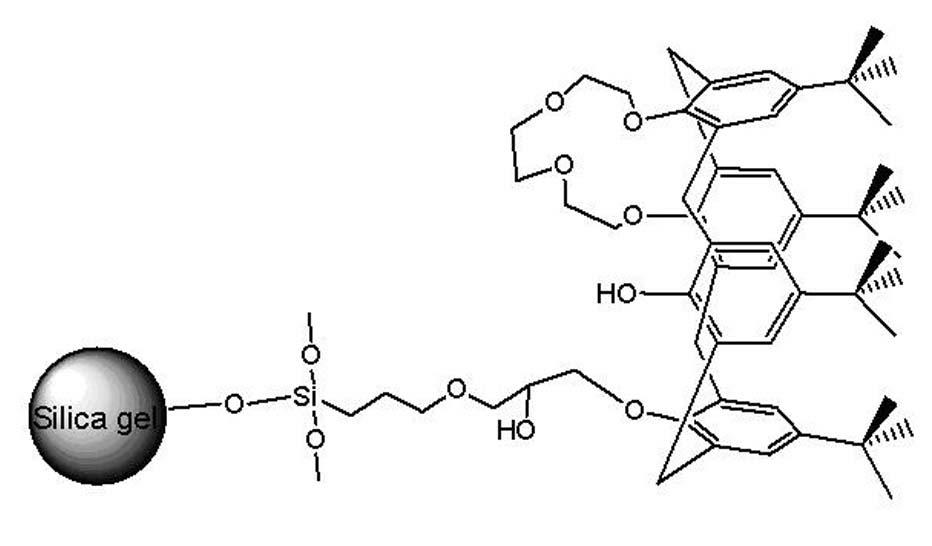
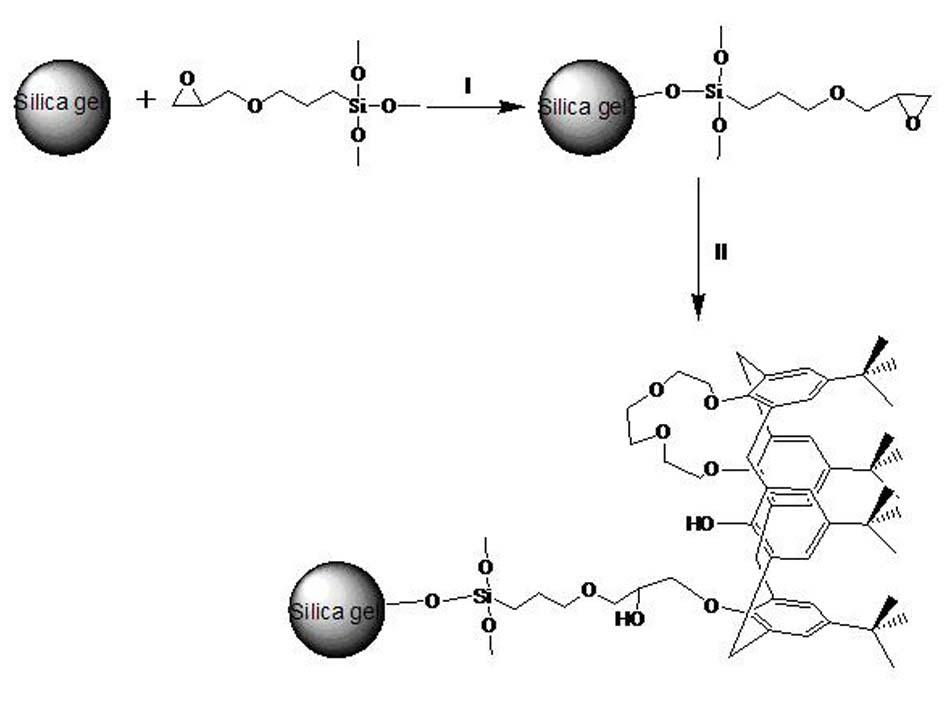
Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof
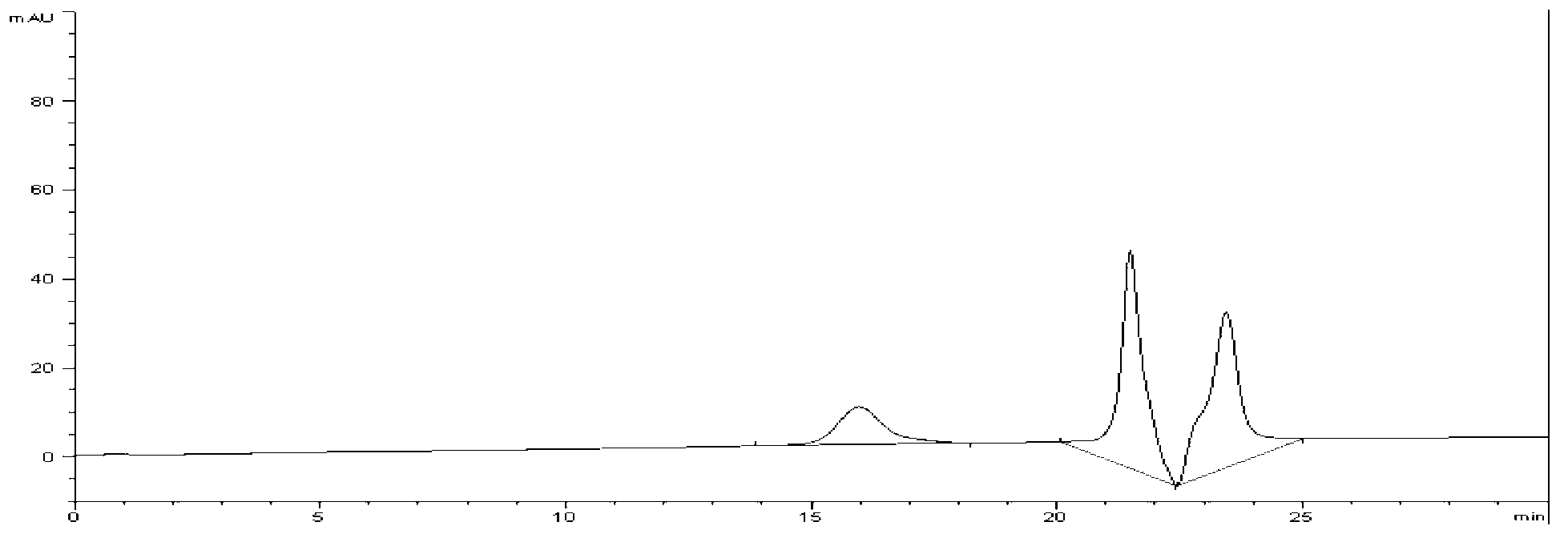
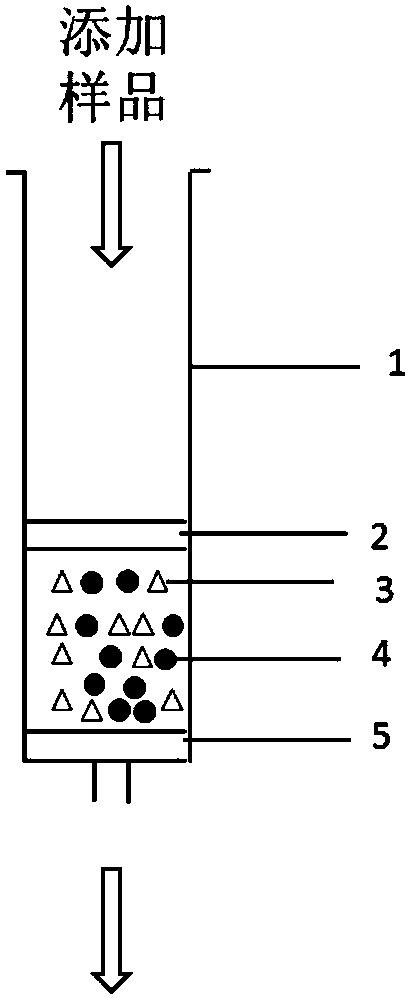
InactiveCN102489274AEasy to separateImprove hydrophobicityOther chemical processesSilanesReversed-Phase Liquid Chromatography
The invention relates to an alanine substituted calix[4]arene bonded silica stationary phase and a preparation method and application thereof. According to the invention, gamma-aminopropyl trimethoxy silane is used as a coupling agent and reacts with specially-produced silica gel under the protection of triethylamine and inert gas so as to prepare gamma-aminopropyl trimethoxy bonded silica which reacts with 25,27-di(L-alanine methyl ester-N-carbonyl-methoxy)-26,28-dihydroxy-p-tert-bytyl-calix[4]arene under the protection of triethylamine and inert gas so as to prepare the alanine substituted calix[4]arene bonded silica stationary phase. The alanine substituted calix[4]arene bonded silica stationary phase prepared in the invention has the advantages of high bonded amount, stable performance and better separation selectivity, has same performance as traditional ODS reversed phase chromatography does, provides a plurality of action sites for inclusion, complexation, hydrogen bonding, etc. and is advantageous in separation of specific substances difficult to separate.
Owner:ZHENGZHOU UNIV
Solid phase extraction column for simultaneous purification of various mycotoxins and application thereof
ActiveCN107727781ASimultaneous purificationShorten the timeComponent separationMycotoxinReversed-Phase Liquid Chromatography
The invention provides a solid phase extraction column for simultaneous purification of various mycotoxins and an application thereof. The solid phase extraction column comprises multi-walled carbon nanotubes and a reversed-phase chromatography filler. Through compounding of the multi-walled carbon nanotubes and the reversed-phase chromatography filler, simultaneous one-step purification of various mycotoxins is effectively realized, a cost and analysis time are saved and the solid phase extraction column is suitable for various fruits, vegetables, grains, feed and food samples. The solid phase extraction column is especially suitable for simultaneous purification of various fusarium toxins.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
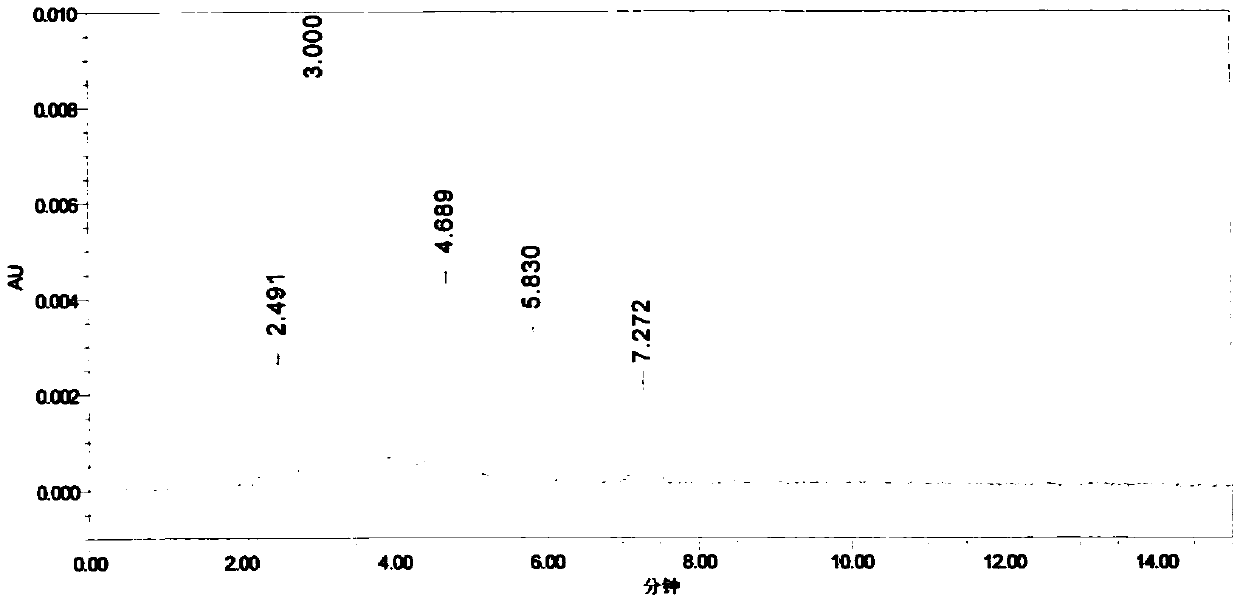
Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof
InactiveCN102107136ASimple methodHigh bonding capacityIon-exchange process apparatusOther chemical processesStationary phaseHydrogen
The invention discloses a tetraoxacalix[2]arene[2]triazine bonded silica stationary phase with large bonding quantity and a stable bonding layer. The concrete preparation method comprises the following steps: in the presence of anhydrous potassium carbonate, 3-aminopropyltriethoxysilane silica reacts with the tetraoxacalix[2]arene[2]triazine in N2 at the temperature of 30-90 DEG C to obtain the tetraoxacalix[2]arene[2]triazine bonded silica stationary phase. Experimental result indicates that the calixarene bonded stationary phase synthesized by the method has the characteristics of relatively large bonding quantity, stable bonding layer and the like; and the method is easy, the preparation cost is low, and the preparation method is widely applicable. The tetraoxacalix[2]arene[2]triazine bonded silica stationary phase not only has the traditional ODS (octadecylsilane bonded silica) reversed-phase chromatography performance, but also can provide sites for various actions such as complexing, hydrogen bonding, pi-pi interaction, space matching and the like. Therefore, the tetraoxacalix[2]arene[2]triazine bonded silica stationary phase can replace ODS to some degree, and provide possibility in separating substances difficult to separate at the same time.
Owner:ZHENGZHOU UNIV
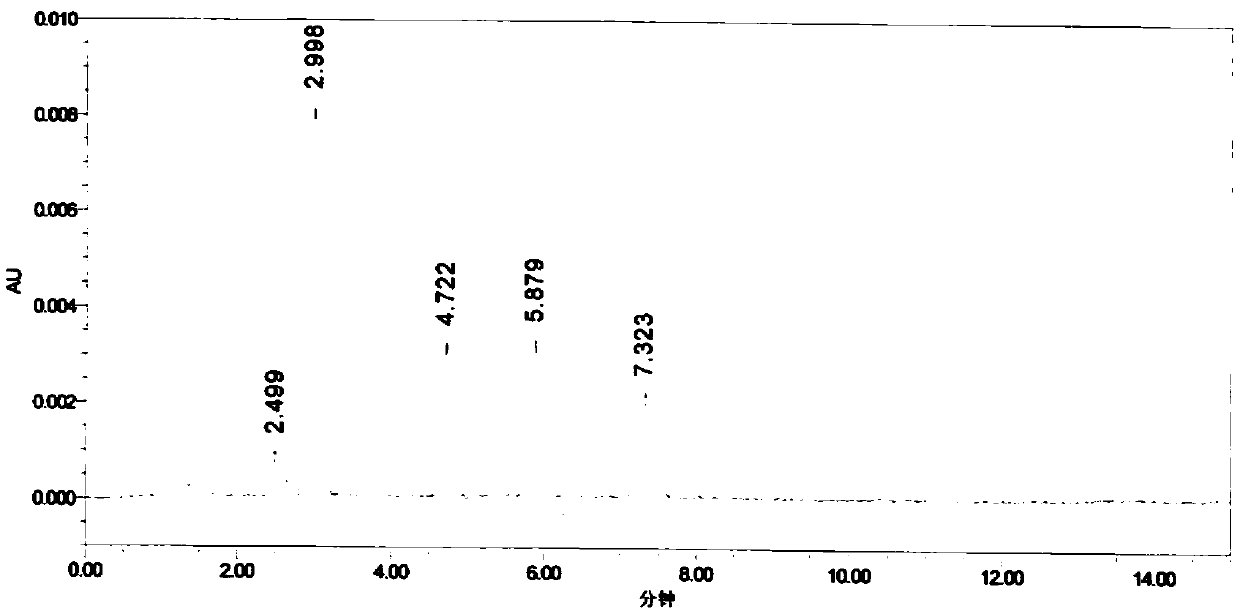
Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof
InactiveCN102101042AHigh bonding capacityReduce manufacturing costIon-exchange process apparatusOther chemical processesStationary phaseHydrogen
The invention discloses a tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase with high bonding quantity and stable bonding layers. A preparation method for the stationary phase comprises that: 3-aminopropyltriethoxysilane silica gel and tetraazacalix [2] arene [2] triazine undergo reflux reaction in N2 at the temperature of 130 DEG C in the presence of anhydrous potassium carbonate to form the tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase. The calixarene bonded stationary phase synthesized by adopting the method has high bonding quantity and stable bonding layers; and the preparation method is simple and convenient, has low preparation cost and wide application range. The stationary phase not only has the reversed phase chromatography performance of the traditional ozone depleting substance (ODS), but also can provide multiple acting sites of complexing, hydrogen bonding effect, phi-phi effect, spatial matching and the like, so the stationary phase can replace the ODS to a certain degree, and provides separation possibility for difficultly separated substances at the same time.
Owner:ZHENGZHOU UNIV
Preparation method of monosialotetrahexosyl ganglioside sodium with high purity
ActiveCN103524572ALow incidence of adverse reactionsSugar derivativesSugar derivatives preparationVirus inactivationMedicine
The invention relates to a preparation method of monosialotetrahexosyl ganglioside sodium with high purity. The method comprises the following steps: extracting and purifying fresh pig brains taken as raw materials, wherein the extracting step comprises the steps of preparation of acetone powder, extraction of total lipids of tissues, folch distribution, virus removal and acid-hydrolysis virus inactivation; the purifying step comprises the steps of desalination, silica gel chromatography, reversed phase chromatography, concentration, acetone recrystallization and freeze-drying. The method has the beneficial effects that the adverse effect occurrence rate of an injection prepared from the monosialotetrahexosyl ganglioside sodium with the high purity is decreased by 47% according to the tracking statistics of 100 users after the virus removal and the acid-hydrolysis virus inactivation are carried out on the injection under a specific pH condition.
Owner:HUNAN SAILONG PHARMA
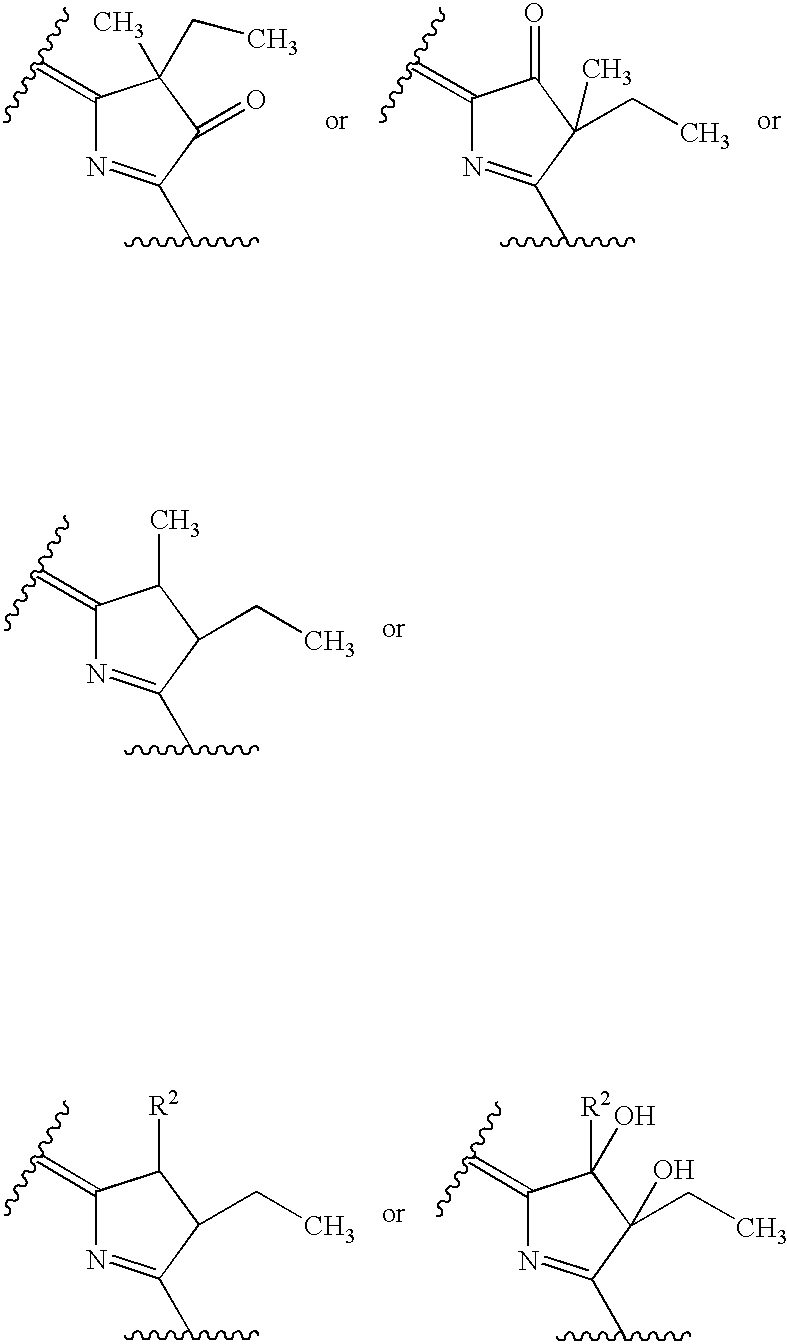
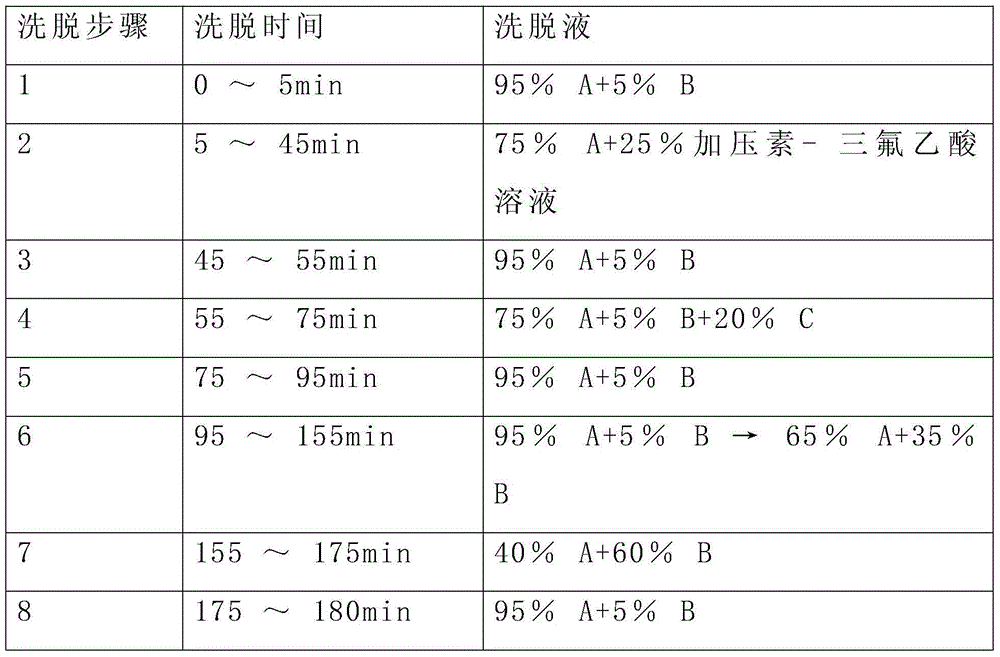
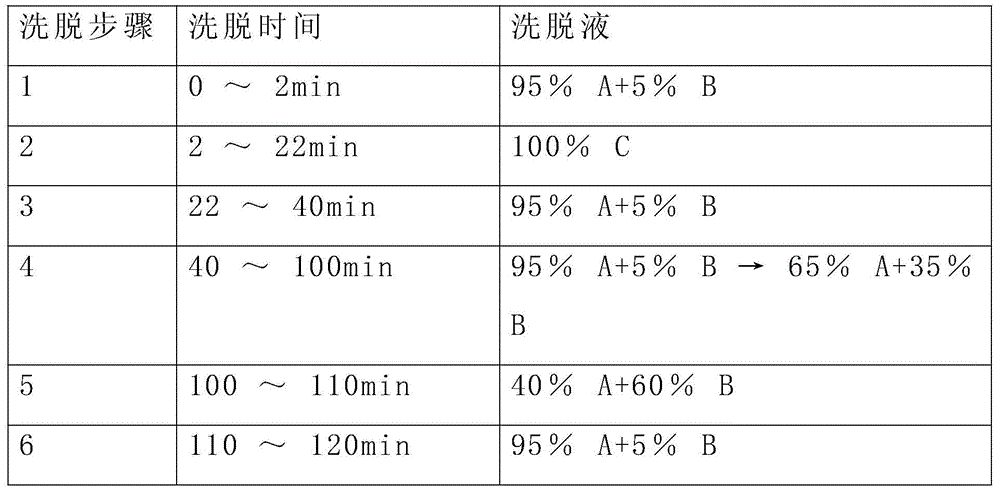
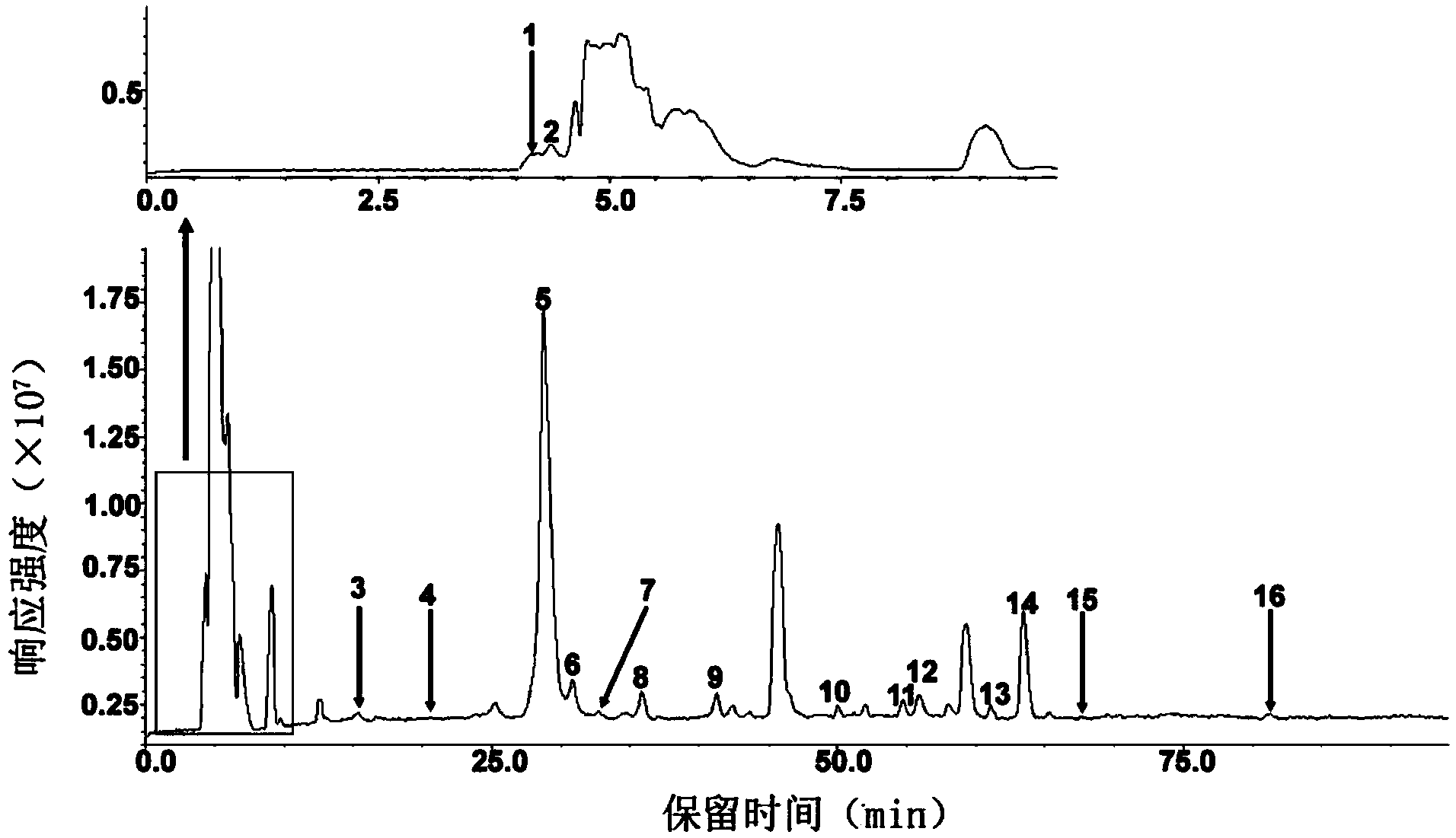
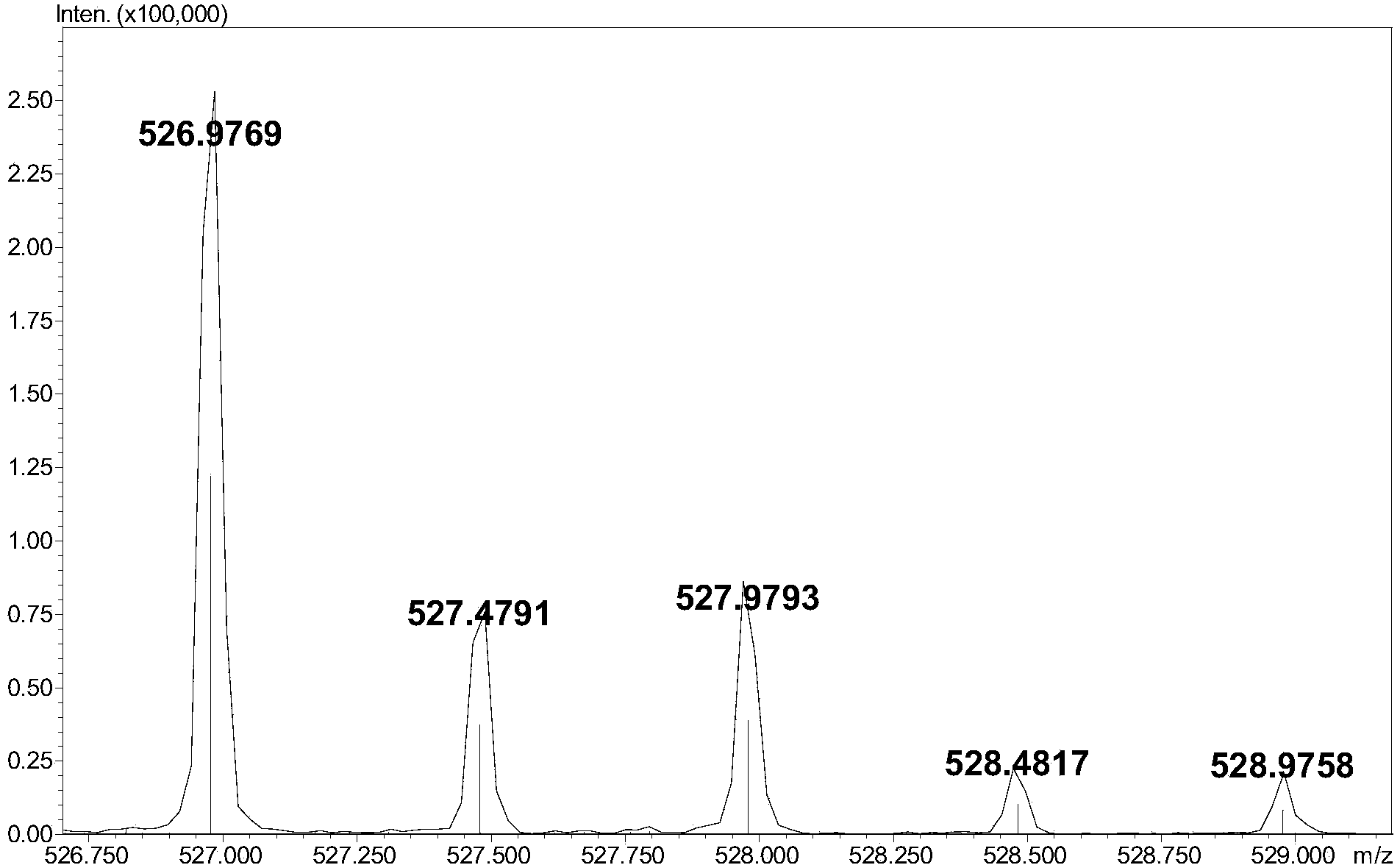
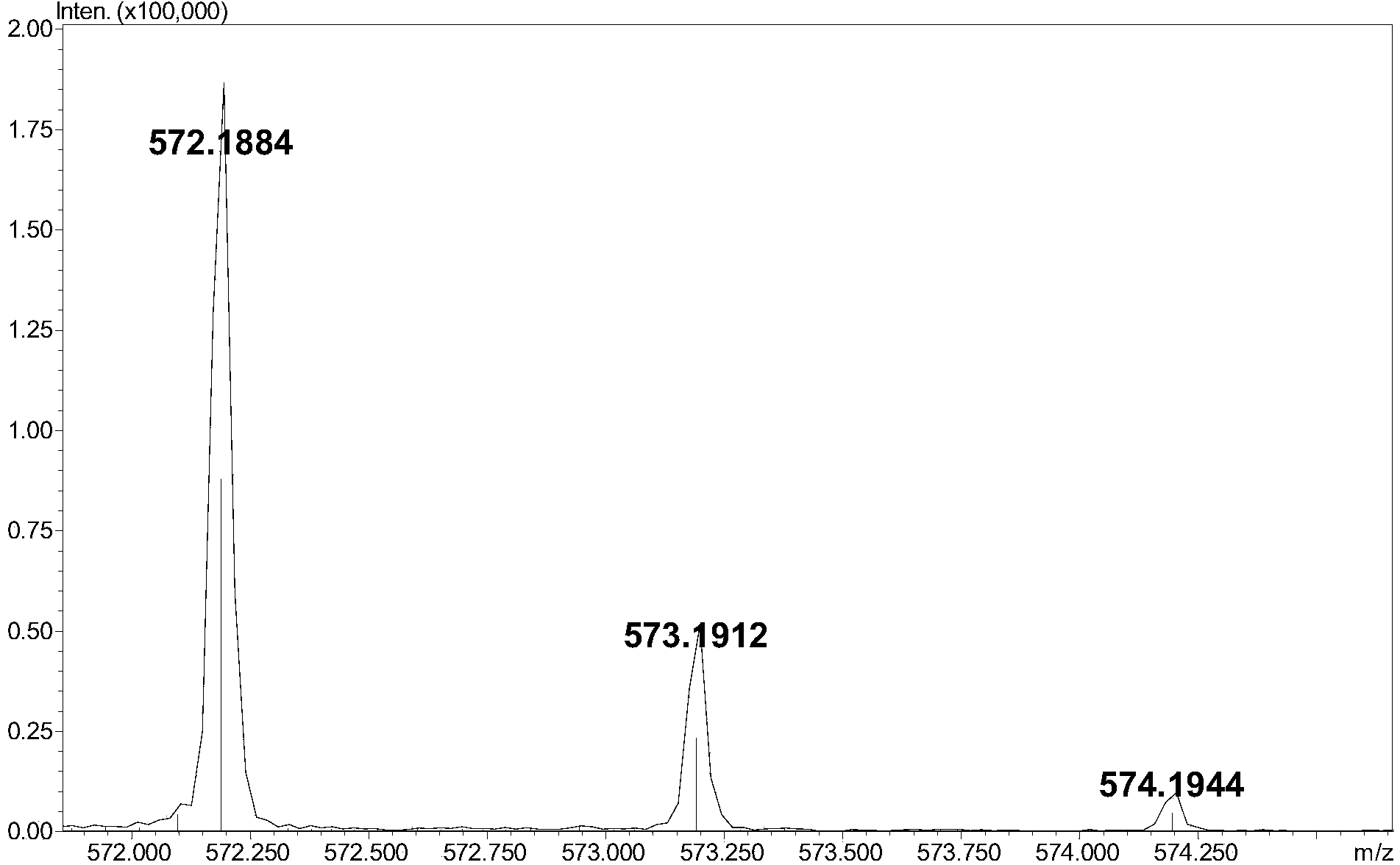
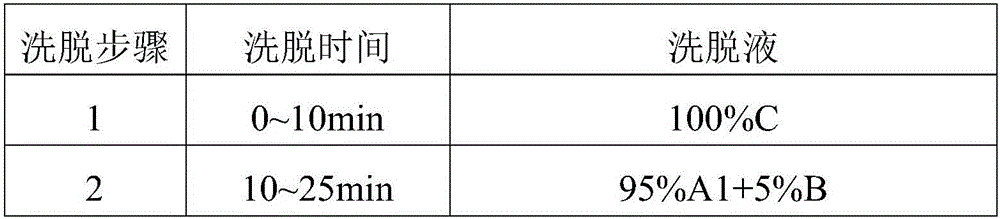
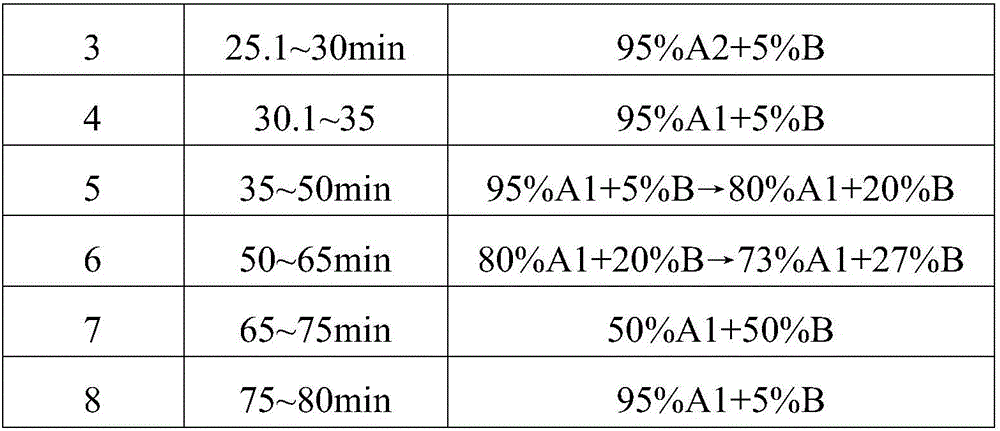
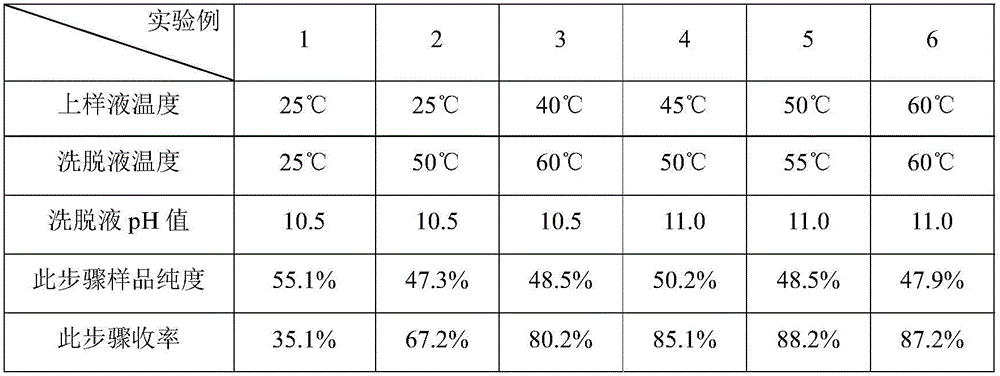
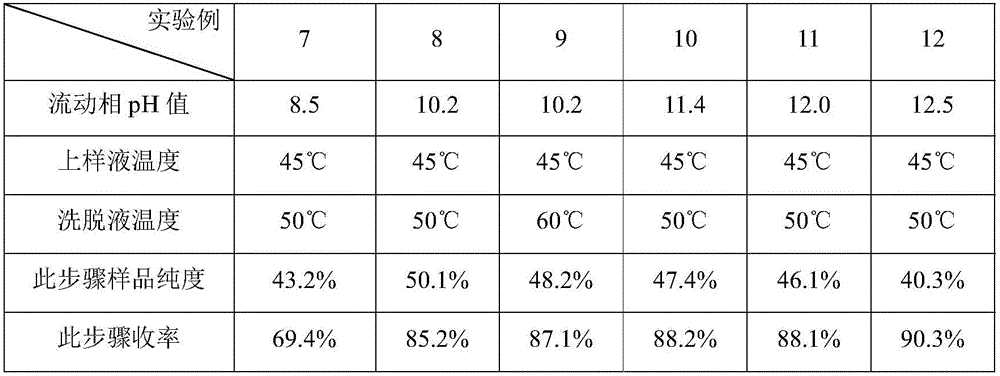
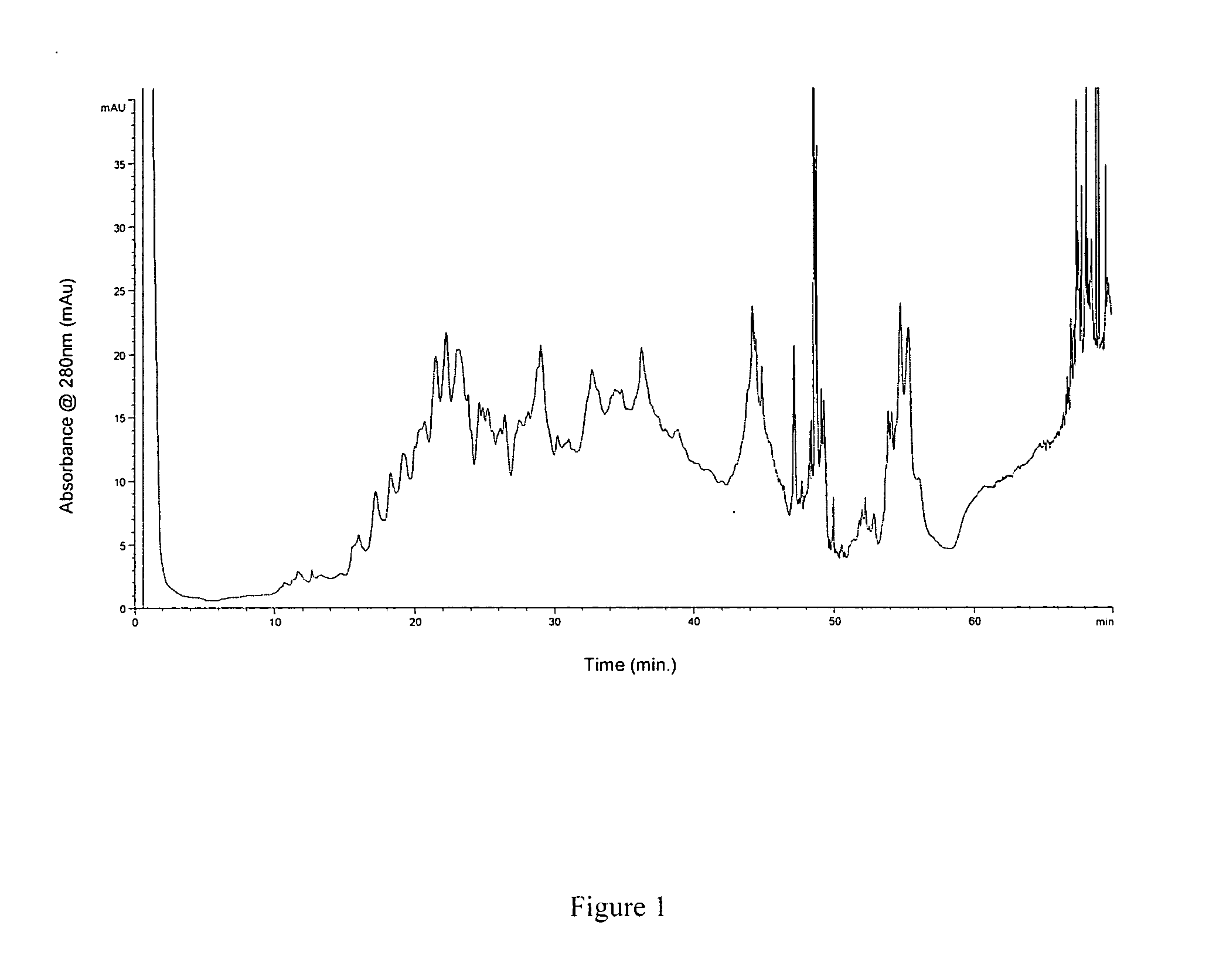
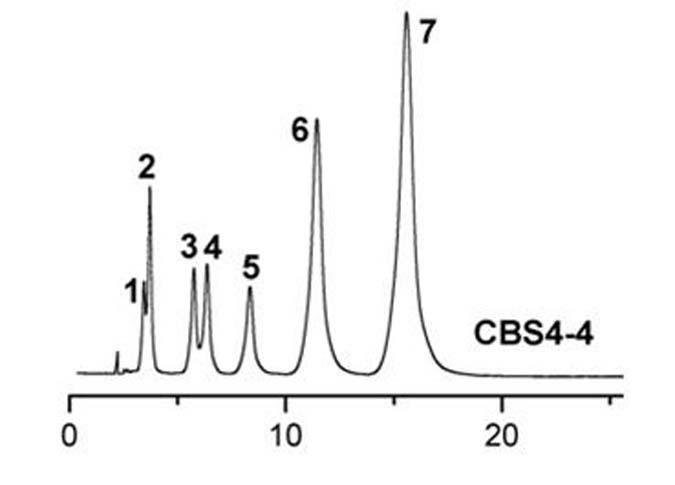
Preparation method of oxytocin [4-Glu]
ActiveCN106478780APrevent elutionSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDesalinationReversed-Phase Liquid Chromatography
The invention discloses a preparation method of oxytocin [4-Glu]. The preparation method comprises steps as follows: a precursor crude product solution of oxytocin [4-Glu] is sequentially subjected to reversed-phase cyclization, reversed-phase purification and reversed-phase desalination with a high-performance liquid reversed-phase chromatography method; filler of the high-performance liquid reversed-phase chromatography method is silicone C18; a precursor crude product of oxytocin [4-Glu] contains two free sulfydryls. The problems about cyclization, purification and desalination are solved once with an innovative reversed-phase adsorption method for cyclization, purification and desalination, the production process is optimized, and the method is suitable for industrial continuous production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
High molecular type reverse chromatography fixing phase and its preparing method
InactiveCN1987453AStable pore structureThe adsorption-desorption process is fastComponent separationOther chemical processesChemical structureChromatographic separation
The invention is especially related to fixed phase of reversed phase chromatography (FPRPC) in macromolecule type of a series of different chemical constitution, and preparation method. Possessing macromolecule micro beads in regular spherical porous type, the disclosed FPRPC in macromolecule type possesses basic property of FPRPC. Using the disclosed FPRPC to carry out verification of chromatographic separation indicates that when macromolecule micro beads in porous type acts as FPRPC, pore structure is steady; under condition of compression elution, micro beads does not break up; adsorption-desorption procedure is quick; comparing with chemical bond silicon ball fixed phase in same granularity, FPRPC possesses closed column performance and closed separation. The disclosed FPRPC is applicable to separating and purifying medical bulk drug, and natural drug as well as applicable to enriching and preparing trace quantity substance in biologic and samples.
Owner:刘开禄
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

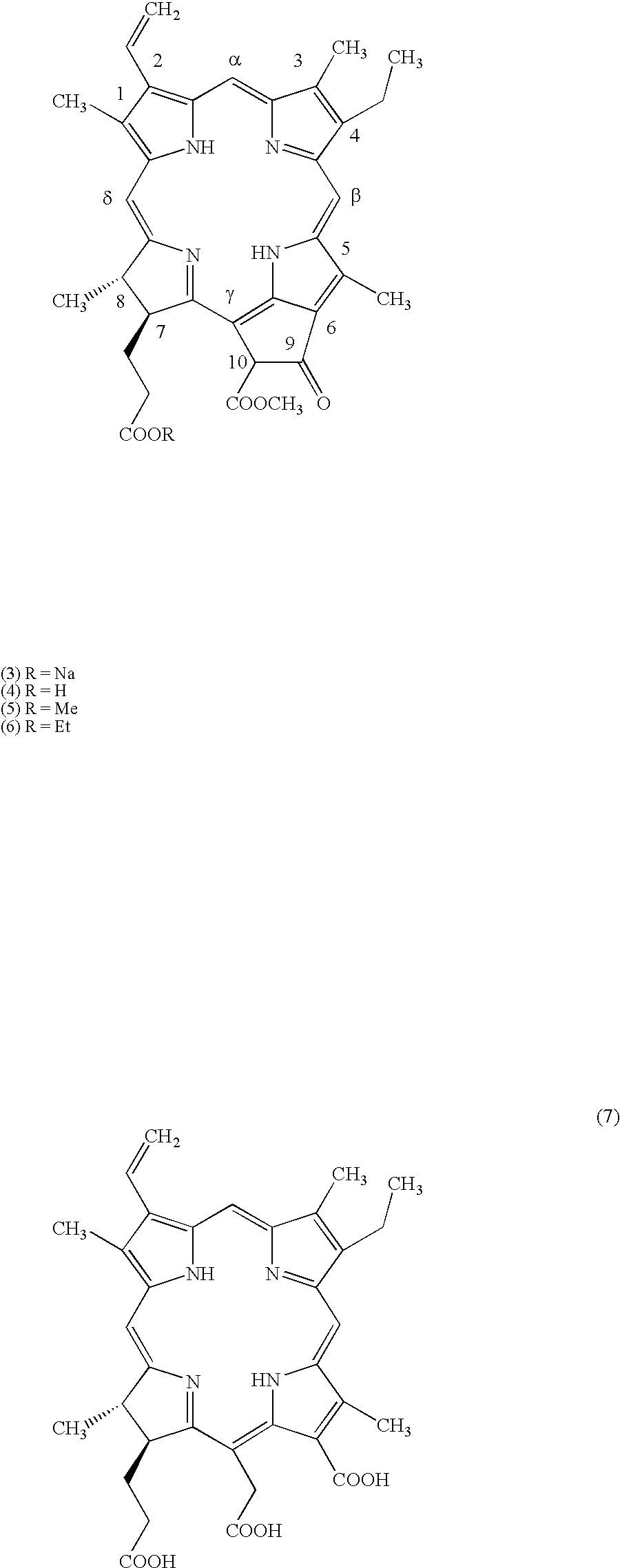
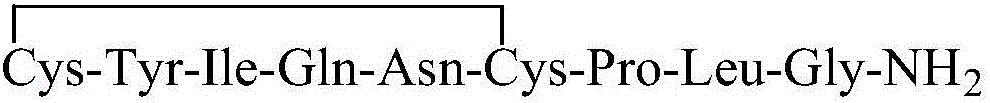
![Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/d54fbd7f-5418-40ef-adb0-35962201b317/111226101255.PNG)
![Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/d54fbd7f-5418-40ef-adb0-35962201b317/111226101259.PNG)
![Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof Alanine substituted calix[4]arene bonded silica stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/d54fbd7f-5418-40ef-adb0-35962201b317/111226101302.PNG)
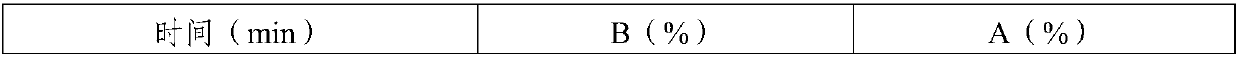
![Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/47b6254f-70f5-46e7-8a3a-26ac303b70af/110107155701.PNG)
![Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/47b6254f-70f5-46e7-8a3a-26ac303b70af/110107155704.PNG)
![Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof Tetraoxacalix[2]arene[2]triazine bonded silica stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/47b6254f-70f5-46e7-8a3a-26ac303b70af/110107155708.PNG)
![Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/7784f176-6e0a-4163-b191-efb64a834204/110107164122.PNG)
![Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/7784f176-6e0a-4163-b191-efb64a834204/110107164126.PNG)
![Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof Tetraazacalix [2] arene [2] triazine bonded silica gel stationary phase and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/7784f176-6e0a-4163-b191-efb64a834204/110107164132.PNG)
![Preparation method of oxytocin [4-Glu] Preparation method of oxytocin [4-Glu]](https://images-eureka.patsnap.com/patent_img/5d8ada9d-0395-42b2-b082-aeeb775b9a3b/BDA0001201957660000011.png)
![Preparation method of oxytocin [4-Glu] Preparation method of oxytocin [4-Glu]](https://images-eureka.patsnap.com/patent_img/5d8ada9d-0395-42b2-b082-aeeb775b9a3b/FDA0001201957650000012.png)
![Preparation method of oxytocin [4-Glu] Preparation method of oxytocin [4-Glu]](https://images-eureka.patsnap.com/patent_img/5d8ada9d-0395-42b2-b082-aeeb775b9a3b/FDA0001201957650000021.png)