Method for preparing vasopressin tannate
A technology of vasopressin and tannic acid, applied in the direction of oxytocin/vasopressin, chemical instruments and methods, specific peptides, etc., can solve the problems of complex preparation process and low yield, and achieve the effect of optimizing the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 (HPLC method to detect the purity of crude vasopressin raw material and purified intermediate solution)
[0032] Instrument: Waters2695 / 2489 high performance liquid chromatography
[0033] Separation column: Agilent XDB-C184.6×250mm, 5μm
[0034] Mobile phase: A is 0.1% TFA aqueous solution in volume percentage, B is 0.1% TFA-50% acetonitrile aqueous solution in volume percentage
[0035] Flow rate is 1.0mL / min, detection wavelength is 214nm, room temperature detection,
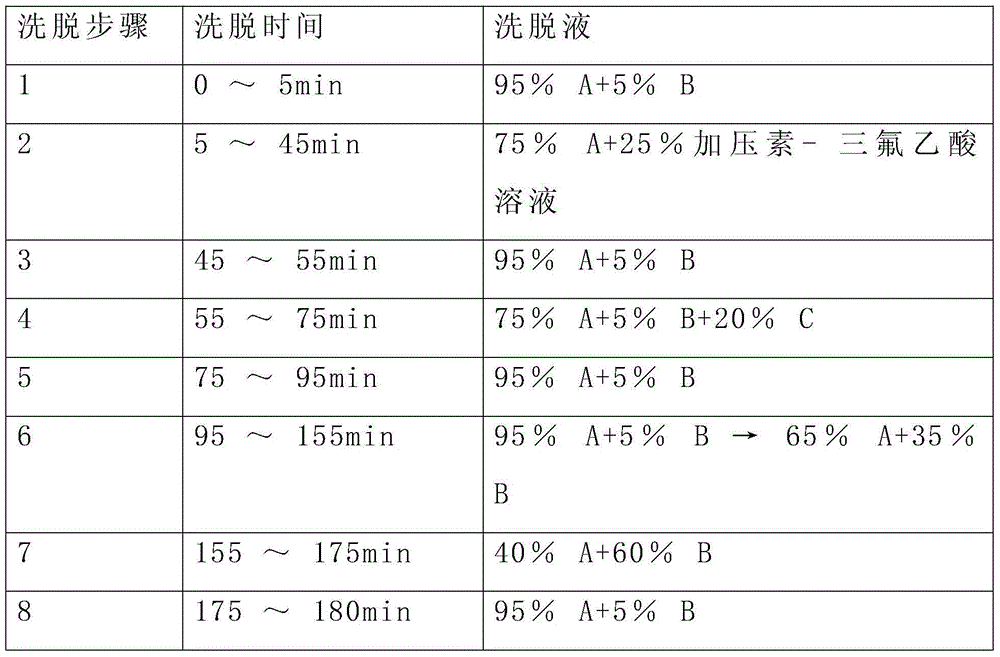
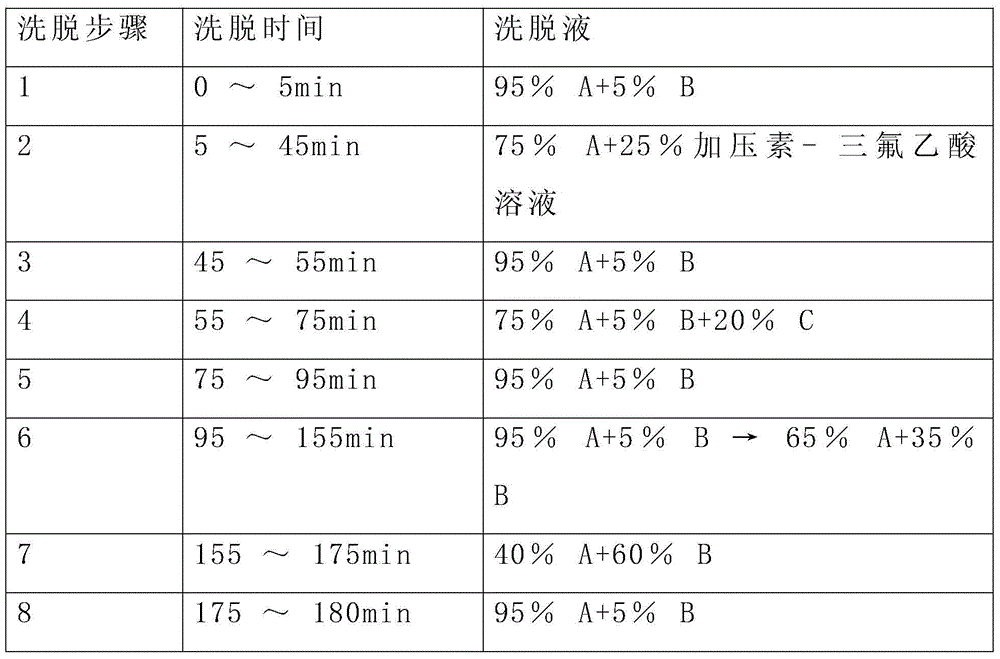
[0036] The elution gradient is shown in Table 1 below, and the percentages are volume percentages.
[0037] Table 1 Mobile phase elution ladder
[0038] Elution step Elution time eluent 1 0~5min 95%A+5%B 2 5~25min 95%A+5%B→50%A+50%B 3 25~30min 100%B 4 30~35min 95%A+5%B
Embodiment 2
[0039] Embodiment 2 (75mm inner diameter L&L4003 preparative column packing)
[0040] Using Load&Lock dynamic axial compression and static locking technology, the filler is styrene-divinylbenzene copolymer (reversed phase packing Agilent PLRP-S), the pore size is 10nm, the particle size is 10μm, and the column packing density is 0.33g / mL. The bed pressure is 650psi, Varian chromatographic packing system is used, 370g dry powder filler, 2L methanol is stirred and homogenized, poured into a 75mm inner diameter L&L4003 preparative column, the compression ratio is 3:1, the carrier gas is N2, and the carrier gas pressure is adjusted so that the oil pressure gauge pressure 2000psi, dynamic axial compression to a column bed height of 26cm, used as a preparative column for reverse-phase purification and reverse-phase desalting schemes.
Embodiment 3
[0041] Embodiment 3 (reverse phase purification of vasopressin crude product raw material)
[0042] Instrument: Varian SD-1 high pressure liquid phase preparation system
[0043] Chromatographic column: self-packed preparative column Load&Lock400375×260mm in Example 2, PLRP-S10μm10nm
[0044] Mobile phase: A is 0.1% trifluoroacetic acid aqueous solution by volume, B is 0.1% trifluoroacetic acid acetonitrile solution by volume, and the crude vasopressin is dissolved in 5% acetonitrile aqueous solution as solvent to prepare a 10g / L solution , after filtration and clarification, the sample liquid is mobile phase C, and its HPLC purity measured by the method of Example 1 is 88.13%, and the retention time is 14.40min.
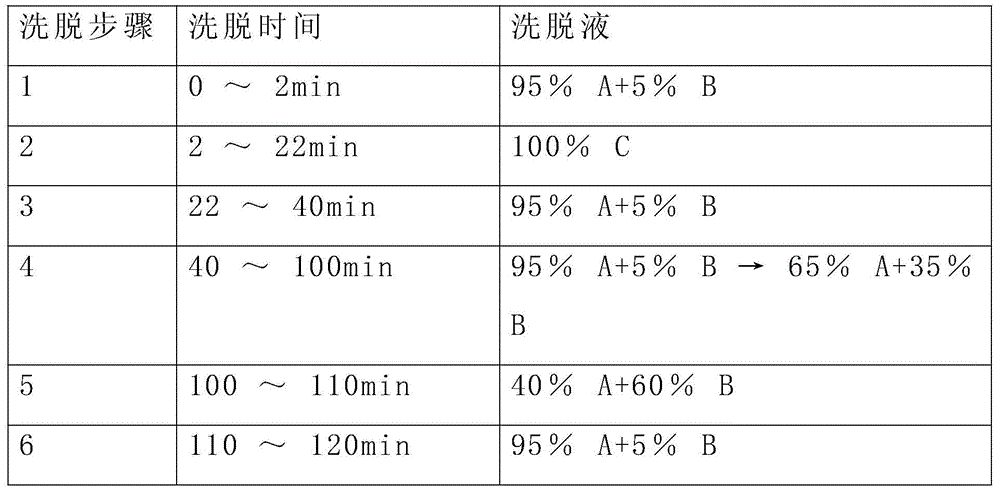
[0045] The reverse-phase purification conditions of this example are as follows: flow rate 200mL / min, detection at 220nm, purification and elution gradient as shown in the table below, and the main peak of the target with a retention time of 48-54min is collected a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com