Preparation method of benzaldehyde and its derivatives
A technology of benzaldehyde and its derivatives, which is applied in the field of preparation of benzaldehyde and its derivatives, can solve the problems that the conversion rate of toluene cannot be taken into account simultaneously and the selectivity of benzaldehyde can not be taken into account at the same time, and the separation of products is difficult, so as to achieve good application prospects and solve the difficulties of separation and purification , good recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] First, accurately weigh 0.004mmol of ferrous acetate, 0.04mmol of succinimide and 0.04mmol of calcium hydroxide, and add them into the Young's reaction tube. The volume of the Young's reaction tube in this example is 10mL, and the magnetic stir bar. Then, oxygen was introduced into the Young's reaction tube, and the volume of oxygen was 8 mL, so that the reaction was carried out under the condition of gaseous oxidant.
[0035]
[0036] Then accurately add 1.5 mL of anhydrous toluene into the Young's reaction tube with a syringe, and place the above-mentioned Young's reaction tube on a magnetic stirrer, and stir at 140° C. for 20 h.
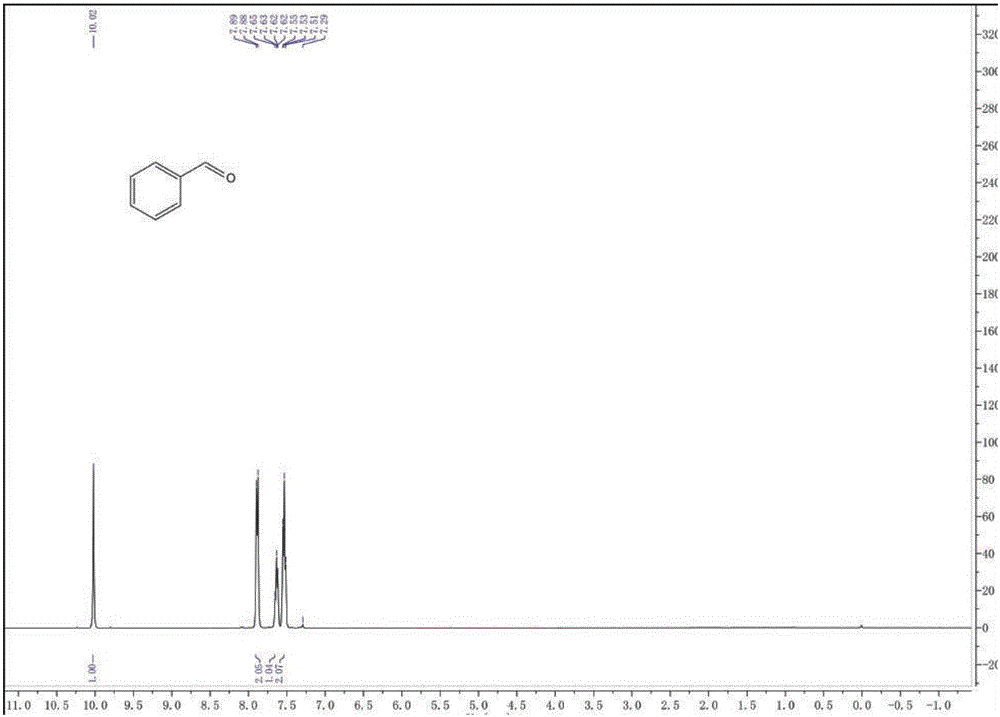
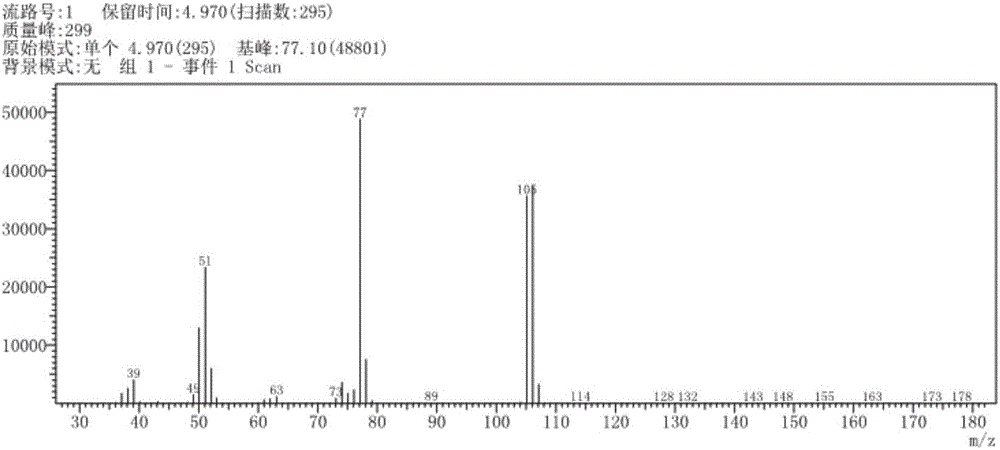
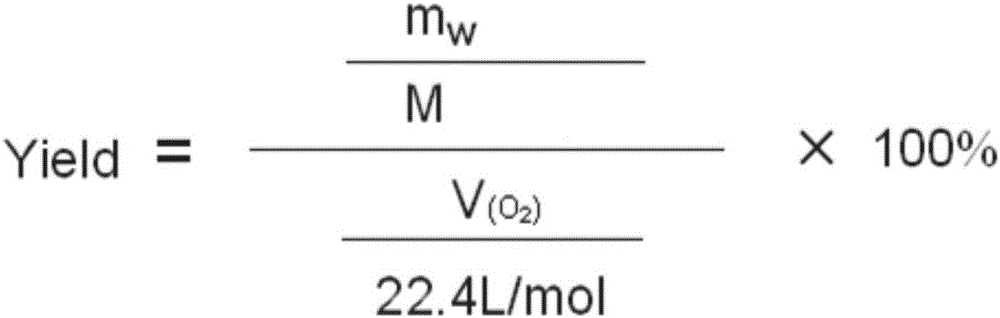
[0037] After the reaction, the pH of the reaction solution was adjusted to be acidic, and the reaction solution was post-treated to obtain 0.32 g of pure benzaldehyde with a yield of 78%. The productive rate of benzaldehyde here is the productive rate relative to oxygen, calculated by the following formula:
[0038] Among them, the mol...
Embodiment 2
[0043] First, accurately weigh 0.004mmol of ferrous chloride, 0.03mmol of N-hydroxysuccinimide and 0.04mmol of sodium carbonate, and add them into Young's reaction tube. The volume of Young's reaction tube in this example is 10mL, and the into the magnetic stir bar. Then, oxygen was introduced into the Young's reaction tube, and the volume of oxygen was 8 mL, so that the reaction was carried out under the condition of gaseous oxidant. Then accurately add 1.5 mL of anhydrous p-chlorotoluene into the Young's reaction tube with a syringe, and place the above-mentioned Young's reaction tube on a magnetic stirrer, and stir at 120° C. for 18 h.
[0044] After the reaction, the pH of the reaction solution was adjusted to be acidic, and the reaction solution was post-treated to obtain 0.38 g of pure chlorobenzaldehyde, which could be obtained by the yield calculation formula in Example 1, and the yield was 74%.
Embodiment 3
[0046] First, accurately weigh 0.01mmol of copper nitrate, 0.03mmol of N-bromosuccinimide and 0.03mmol of sodium acetate, and add them into Young's reaction tube. The volume of Young's reaction tube in this example is 10mL, and it has been placed in Magnetic stir bar. Then, oxygen was introduced into the Young's reaction tube, and the volume of oxygen was 8 mL, so that the reaction was carried out under the condition of gaseous oxidant. Then accurately add 1.5 mL of anhydrous p-bromotoluene into the Young's reaction tube with a syringe, and place the above-mentioned Young's reaction tube on a magnetic stirrer, and stir at 140° C. for 19 h.
[0047] After the reaction, the pH of the reaction solution was adjusted to be acidic, and the reaction solution was post-treated to obtain 0.45 g of pure bromobenzaldehyde, which could be obtained by the yield calculation formula in Example 1, and the yield was 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com