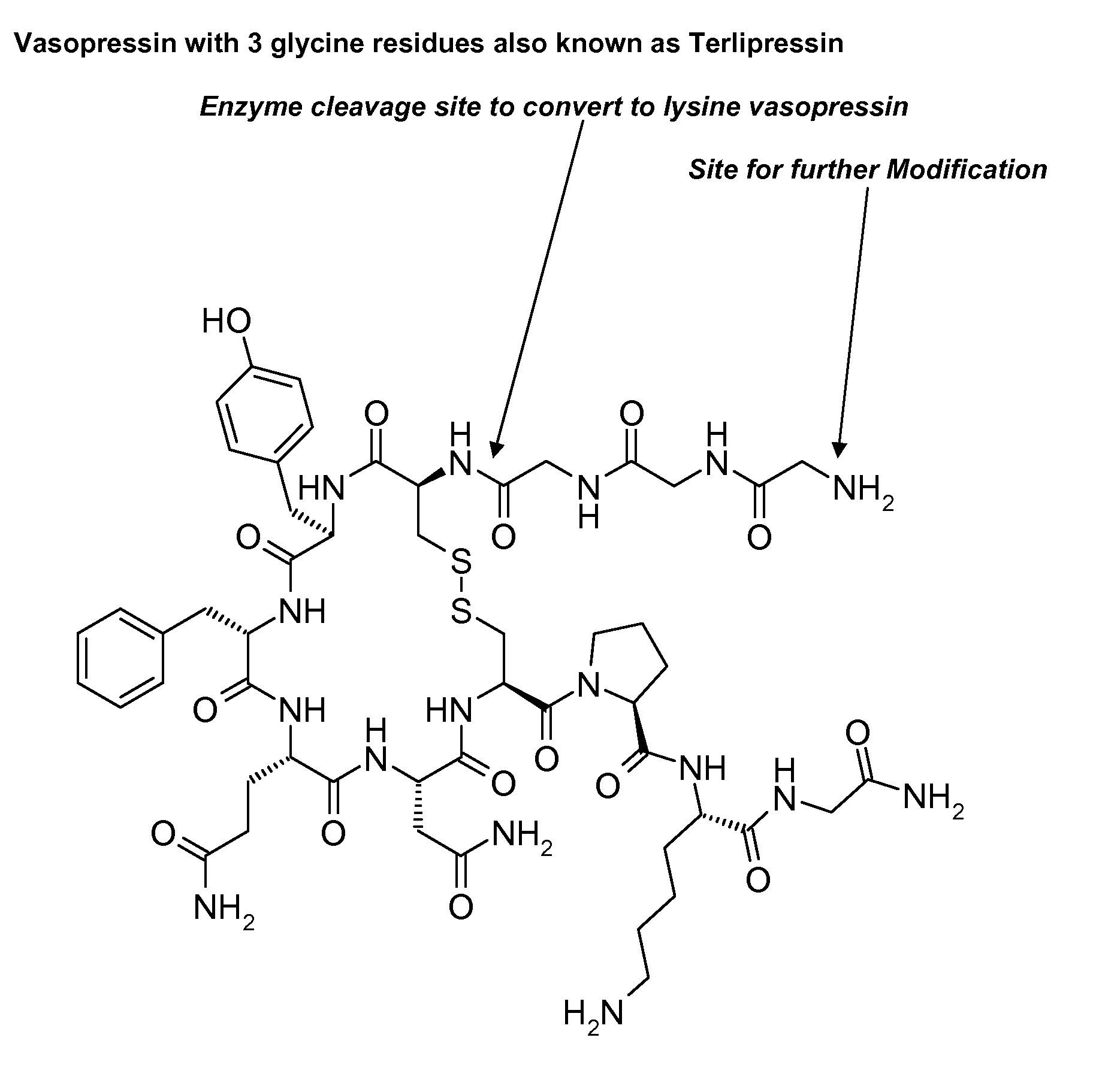
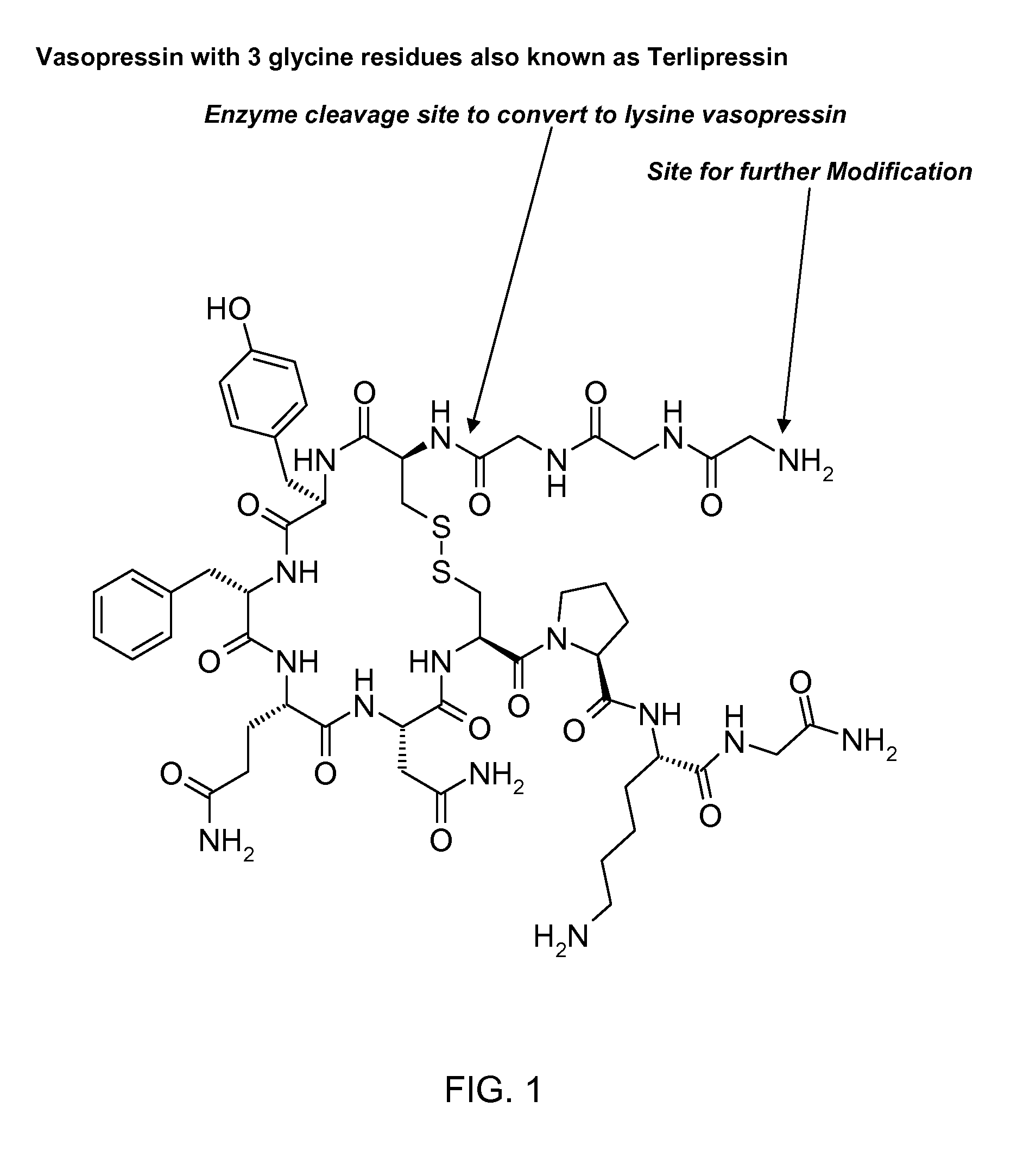
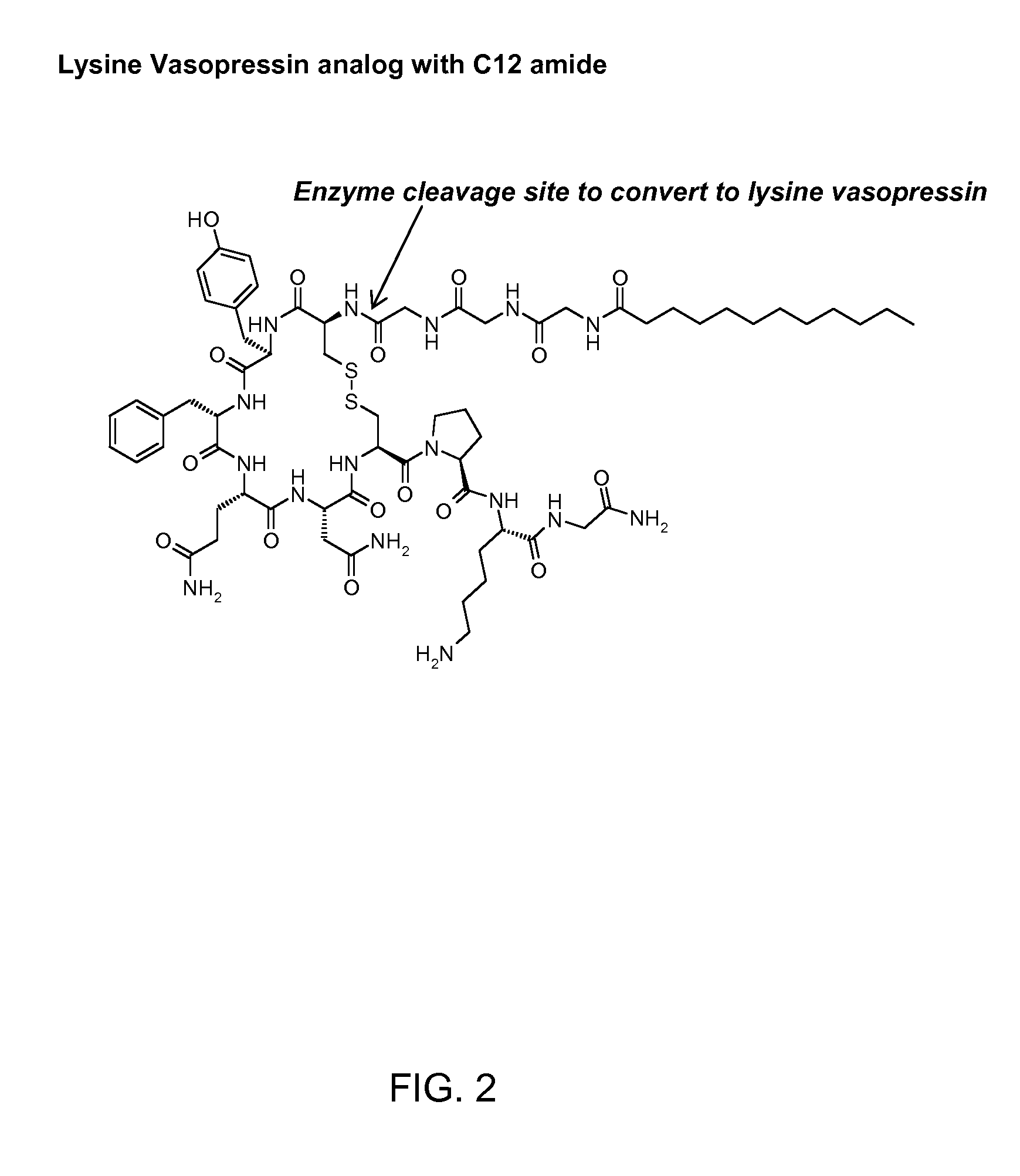
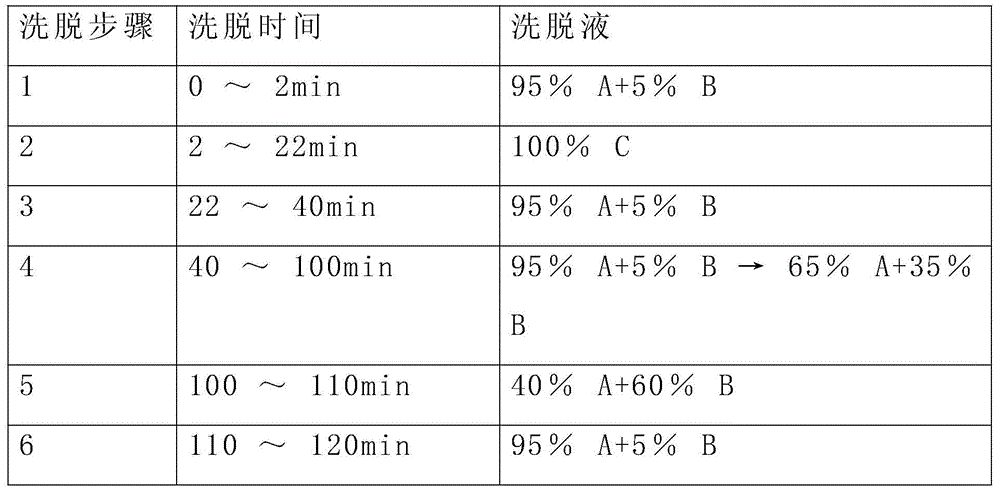
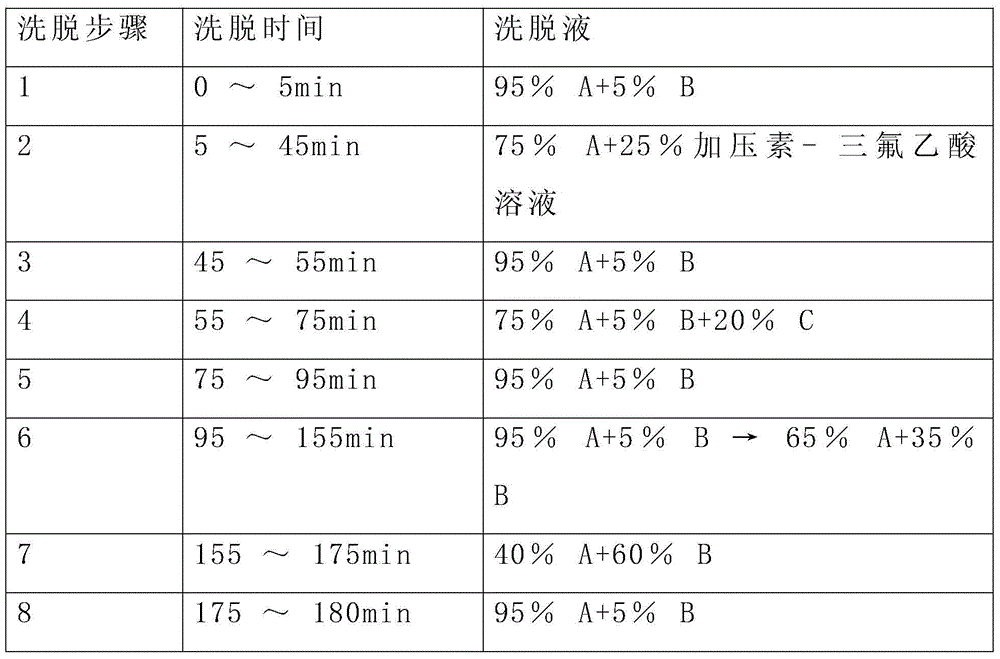
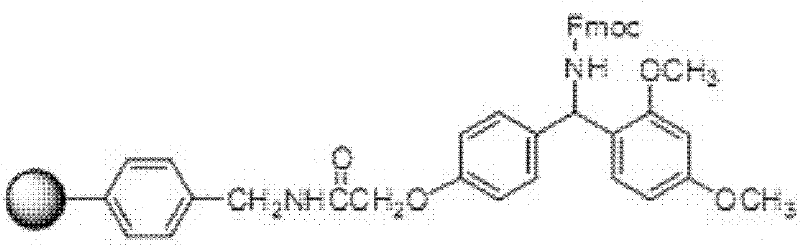
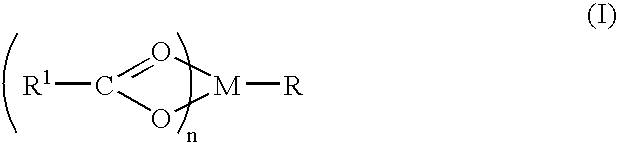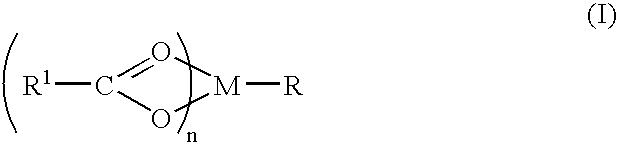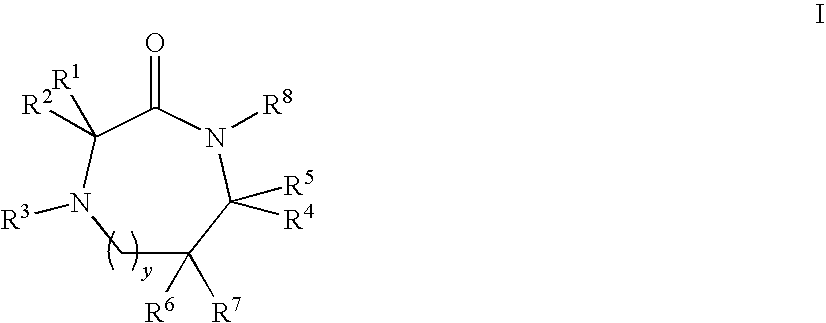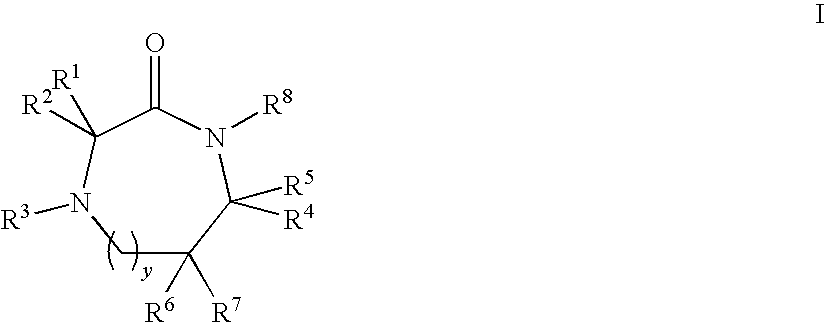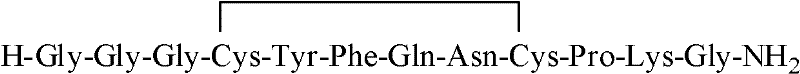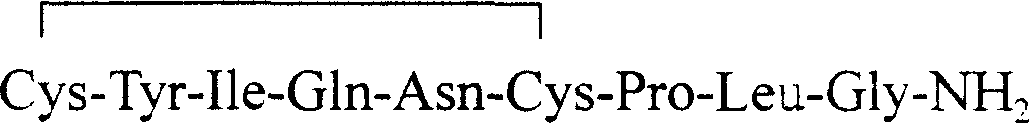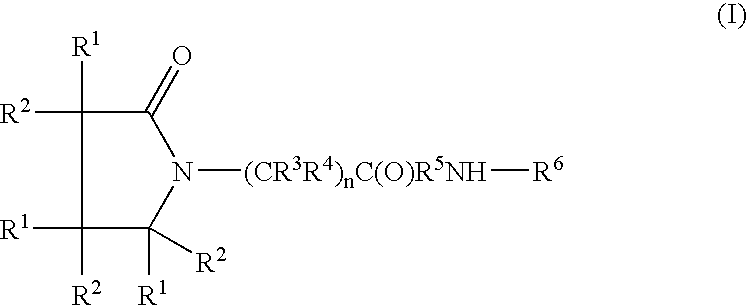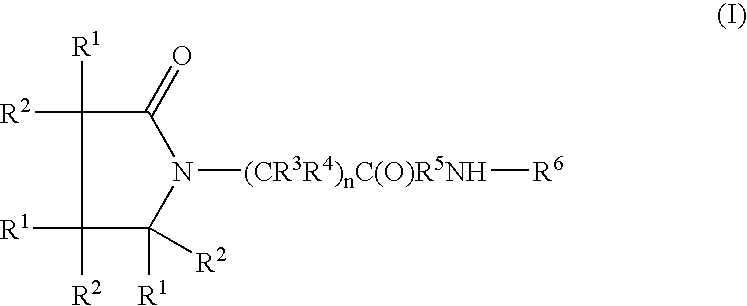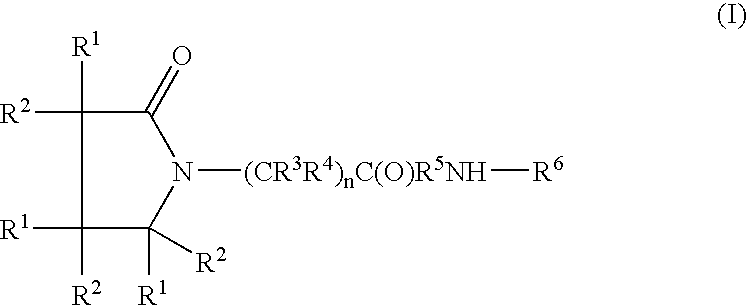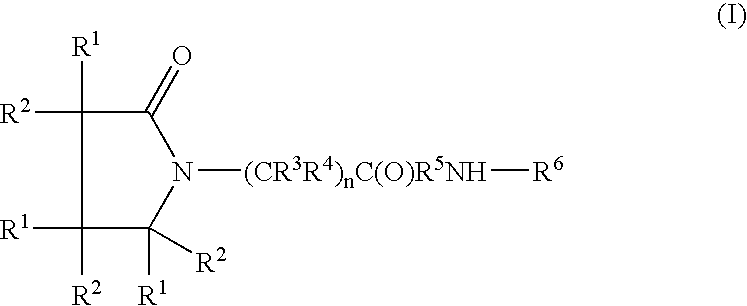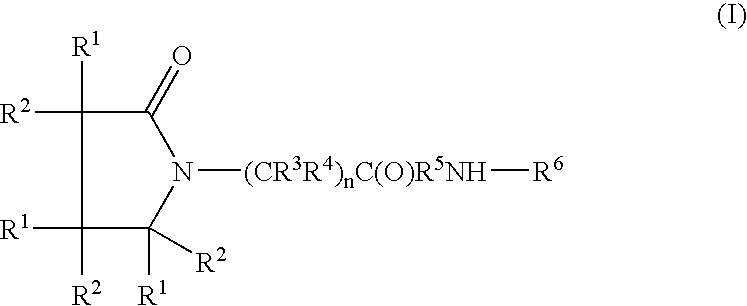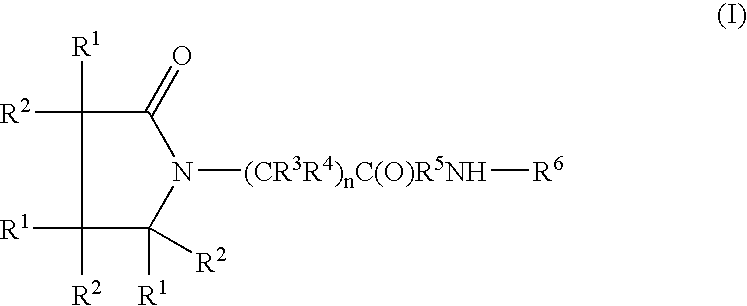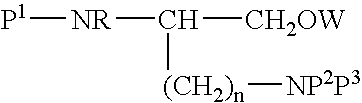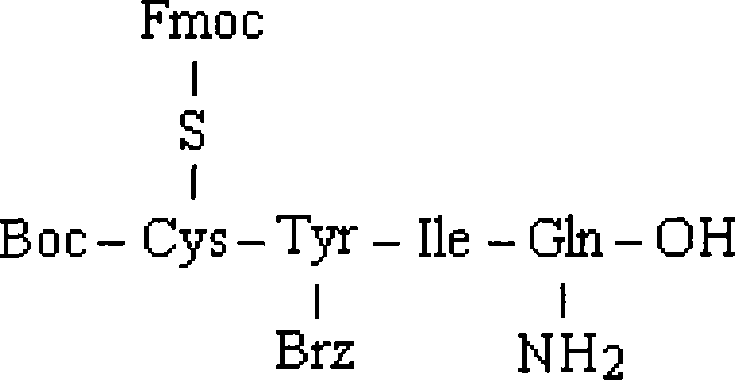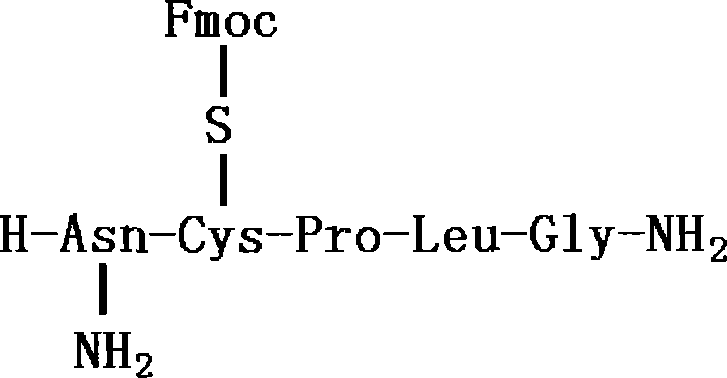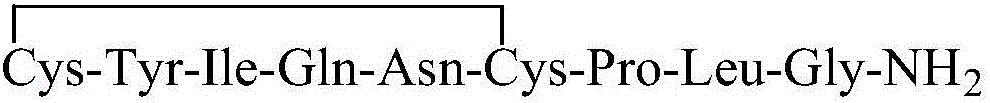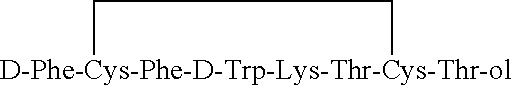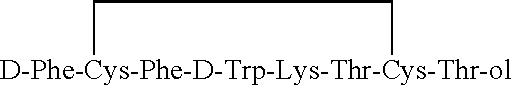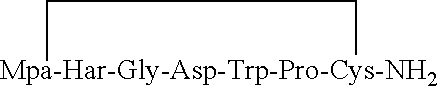Patents
Literature
271results about "Oxytocins/vasopressins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composition for long-acting peptide analogs
ActiveUS20090088387A1Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
The invention describes compositions of peptide analogs that are active in blood or cleavable in blood to release an active peptide. The peptide analogs have a general formula: A-(Cm)x-Peptide, wherein A is hydrophobic moiety or a metal binding moiety, e.g., a chemical group or moiety containing 1) an alkyl group having 6 to 36 carbon units, 2) a nitrilotriacetic acid group, 3) an imidodiacetic acid group, or 4) a moiety of formula (ZyHisw)p, wherein Z is any amino acid residue other than histidine, His is histidine, y is an integer from 0-6; w is an integer from 1-6; and p is an integer from 1-6; wherein if A has alkyl group with 6 to 36 carbon units x is greater than 0; and Cm is a cleavable moiety consisting of glycine or alanine or lysine or arginine or N-Arginine or N-lysine, wherein x is an integer between 0-6 and N may be any amino acid or none. The peptide analogs are complexed with polymeric carrier to provide enhanced half-life.
Owner:PHARMAIN CORP
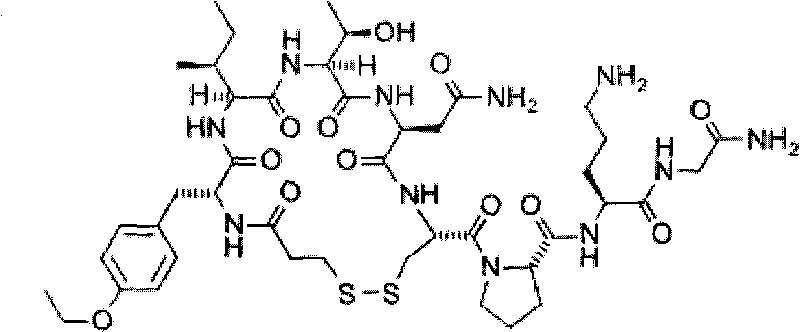
Heptapeptide oxytocin analogues
InactiveUS6143722AImprove stabilityAdequate potencyNervous disorderPeptide/protein ingredientsOxytocinAmide bonds
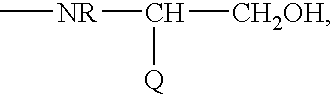
PCT No. PCT / SE97 / 01968 Sec. 371 Date Aug. 2, 1999 Sec. 102(e) Date Aug. 2, 1999 PCT Filed Nov. 21, 1997 PCT Pub. No. WO98 / 23636 PCT Pub. Date Jun. 4, 1998Heptapeptide analogues or pharmaceutically acceptable salts thereof consist of a hexapeptide moiety S and a C-terminal beta -aminoalcohol residue Z bound to the moiety S by an amide bond, wherein the beta -aminoalcohol Z is -NR-CH(Q)-CH2OH, Q is (CH2)n-NH-A is H or -C(=NH)NH2, and R is CH3 or C2H5, and the moiety S wherein H is a D-aromatic alpha -aminoacid and Y is an aliphatic alpha -aminoacid and have oxytocin antagonist activity. Also disclosed is: a method of their synthesis; pharmaceutical compositions containing these analogues; the synthesis of such compositions; a method of control of uterine contractions.
Owner:FERRING BV
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
Solid-phase synthesis method of carbetocin
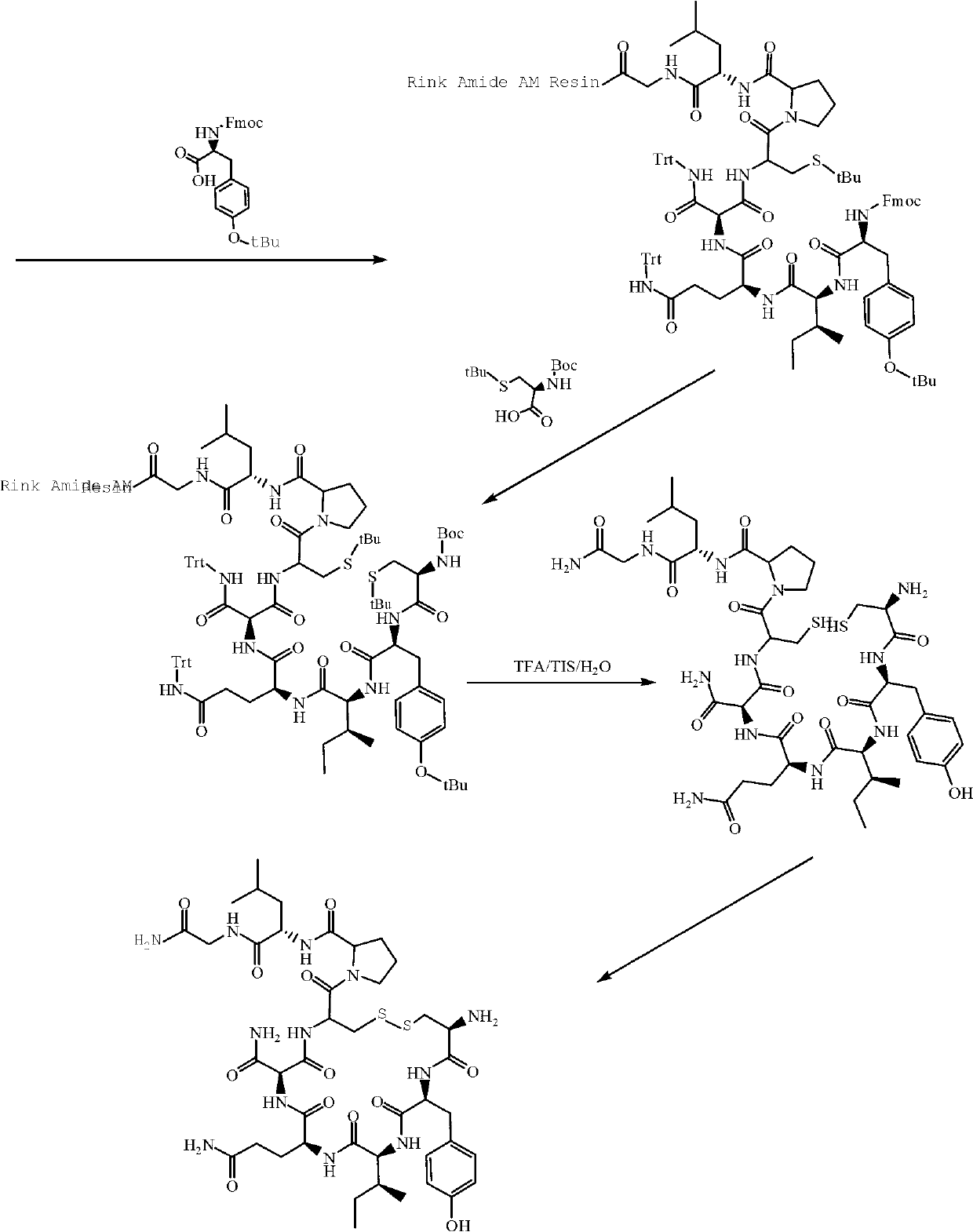
InactiveCN102796178ACheap and easy to getMild conditionsOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingCarbetocin
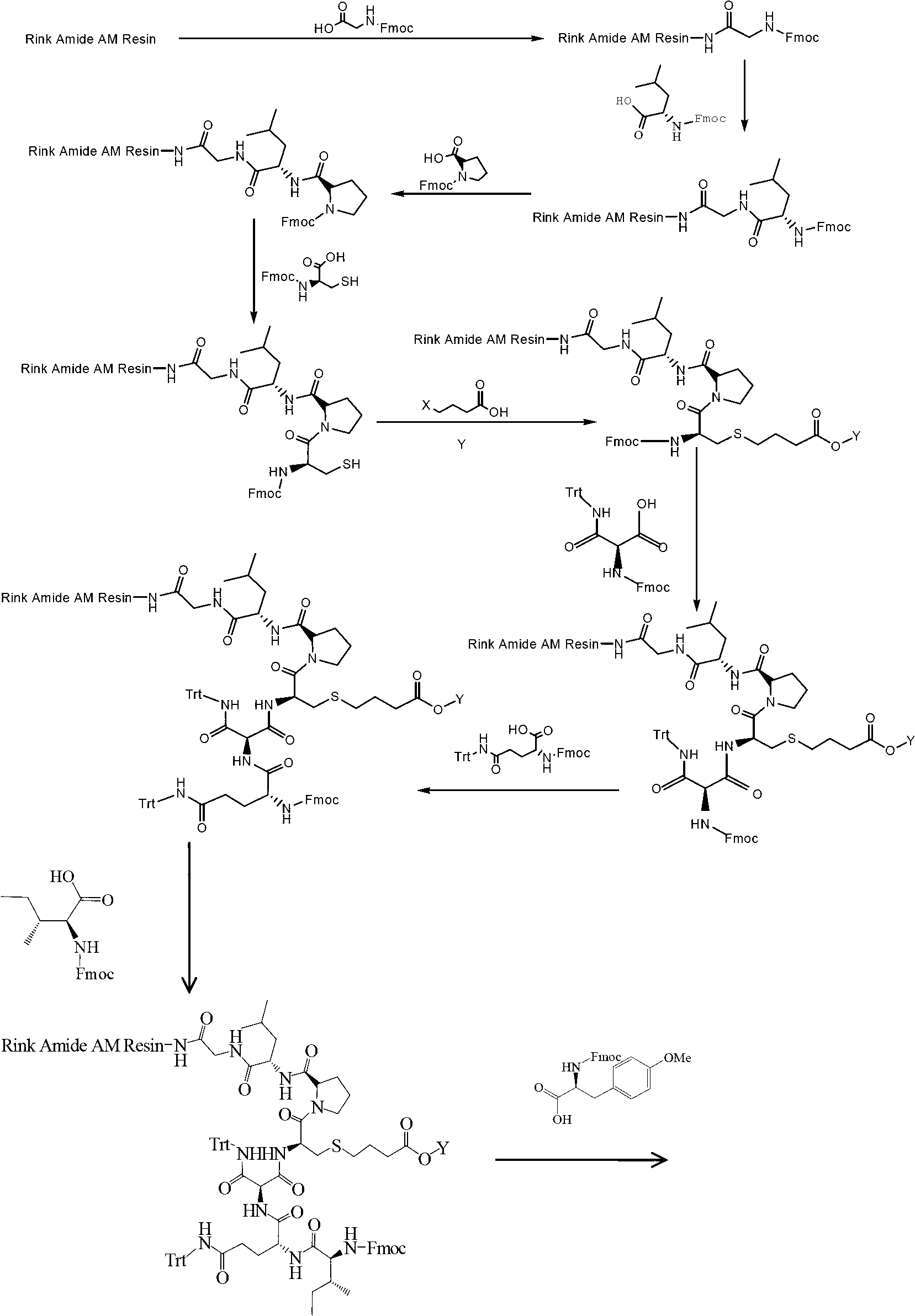
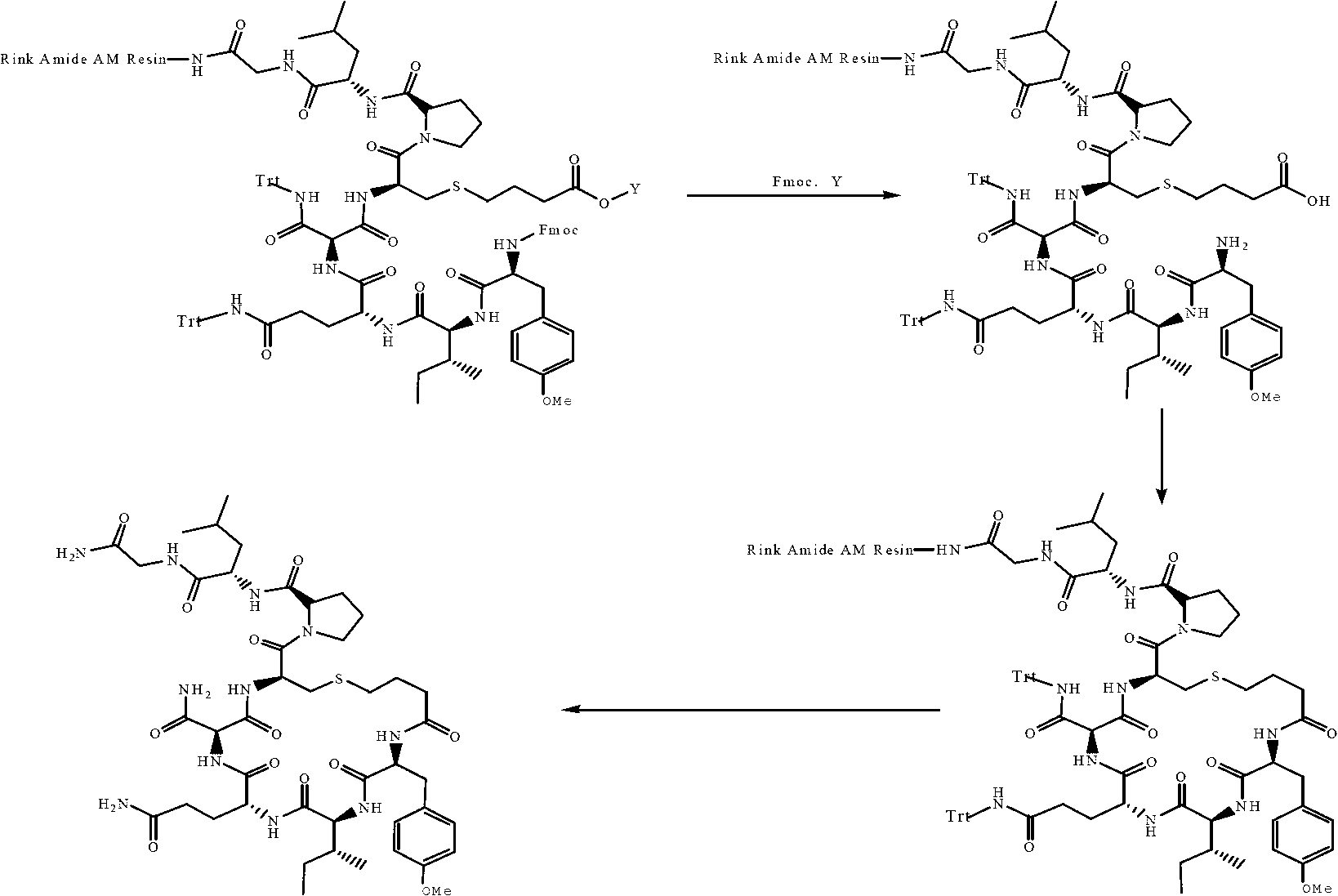
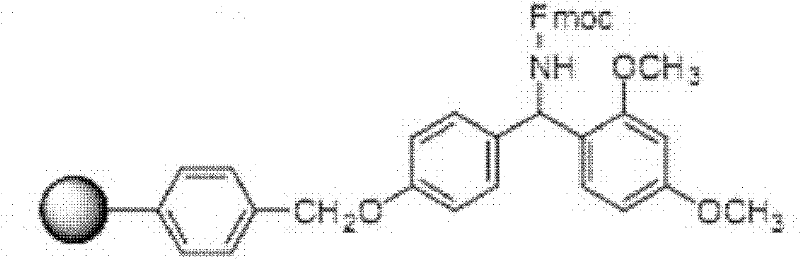
The invention provides a solid-phase synthesis method of carbetocin, and the method comprises the following steps of: sequentially coupling amino acid containing Fmoc (fluorenylmethoxycarbonyl) protecting group by Rink Amide AM Resin to obtain Fmoc-Cys(CH2CH2CH2COOCH2CH2CN)-Pro-Leu-Gly-Rink Amide AM Resin; then sequentially coupling the remaining Fmoc-amino acid, removing the protecting group, and cyclizing to obtain carbetocin-Amide AM Resin; and cracking, purifying and freeze-drying to obtain the carbetocin. The carbetocin is subjected to solid-phase cyclization synthesis by using a novel mercapto protecting group and a false solid-phase dilution principle. The solid-phase synthesis method is simple to operate, low in cost and environmentally-friendly, and meanwhile can be used for greatly improving the purity and the yield as a solid-phase cyclization technology is used at the same time.
Owner:WUXI KAILI PHARMA
Composition for long-acting peptide analogs
ActiveUS7960336B2Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
Owner:PHARMAIN CORP
Method for improving stability of polypeptide active pharmaceutical ingredients
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Method for synthesizing terlipressin by solid-phase oxidization and cyclization
ActiveCN101693738AReduce dosageHigh purityOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingTerlipressin
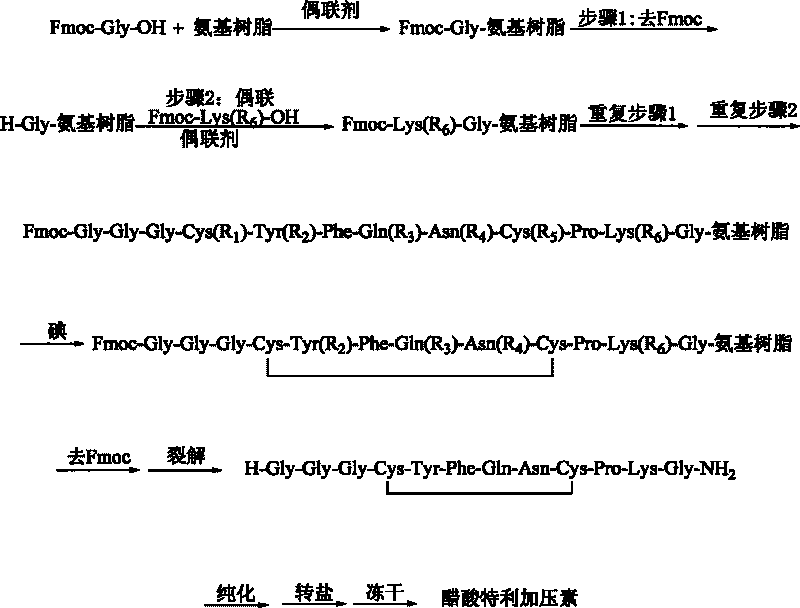
The invention discloses a method for synthesizing terlipressin by solid-phase oxidization and cyclization. The method comprises the following steps: (1) obtaining Fmoc-Gly-amino resins by using Fmoc-Gly-OH and amino resins; (2) coupling the Fmoc-Gly-amino resins one by one to obtain linear terlipressin-amino resins; (3) synthesizing the linear terlipressin-amino resins into terlipressin-amino resins by adopting iodine solid-phase oxidized cyclization; (4) cracking and cutting the terlipressin-amino resins to obtain raw peptides of the terlipressin; and (5) carrying out purification, salt conversion and freeze-drying on the raw peptides to obtain terlipressin acetate. The invention has the key innovations that solid-phase oxidization reduces a large amount of waste liquid brought by liquid-phase oxidization, accelerates the reaction time, belongs to process greening reformation and greatly improves the total yield.
Owner:HYBIO PHARMA
Linear and cyclic melanocortin receptor-specific peptidomimetics
ActiveUS8114844B2Oxytocins/vasopressinsCyclic peptide ingredientsMelanocortin 1 receptorMelanocortin receptor
Linear and cyclic peptidomimetics which bind to one or more melanocortin receptors are provided, which peptidomimetics include at least one ring-constrained amino acid surrogate of formula I:where R1, R2, R3, R4, R5, R6, R7, R8, and y are as defined in the specification, together with methods for synthesizing ring-constrained amino acid surrogates of formula I and peptidomimetics incorporating the same, and methods of use of peptidomimetics in the treatment of various diseases, syndromes and conditions.
Owner:PALATIN TECH INC
Synthesis process of carbetocin
InactiveCN104592362AHigh purityReduced operating requirementsOxytocins/vasopressinsPeptide preparation methodsSide chainCarbetocin
The invention provides a synthesis process of carbetocin. The synthesis process comprises the following steps: performing a coupling reaction on Fmoc-Gly-OH with Rink Amide-AM Resin obtained in the first step to obtain Fmoc-Gly-Rink Amide-AM Resin; performing deprotection (20% piperidine) with DBLK to obtain H-Gly-Rink Amide-AM RFesin, and orderly completing the coupling of the H-Gly-Rink Amide-AM Resin with Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ile-OH, Fmoc-Tyr(Me)-OH and tetrachlorobutyric acid until carbetocin linear peptide resin is synthesized; mixing a cracking agent with the carbetocin linear peptide resin obtained in the fourth step to have a cracking reaction, thereby removing the Rink Amide-AM Resin and side chain protecting groups; cyclizing the carbetocin linear crude peptide into a carbetocin crude product, and separating and purifying the carbetocin crude product to obtain the carbetocin. The synthesis process has the advantages that the polymerization side reaction is prevented, the process route is greatly simplified, the production cost is reduced and the synthesis efficiency is improved; in addition, the purity of the finished product is high; in short, the synthesis process is convenient for large-scale production, and meanwhile, advantageous for environmental protection, and has remarkable economic and social benefits.
Owner:苏州天马医药集团天吉生物制药有限公司
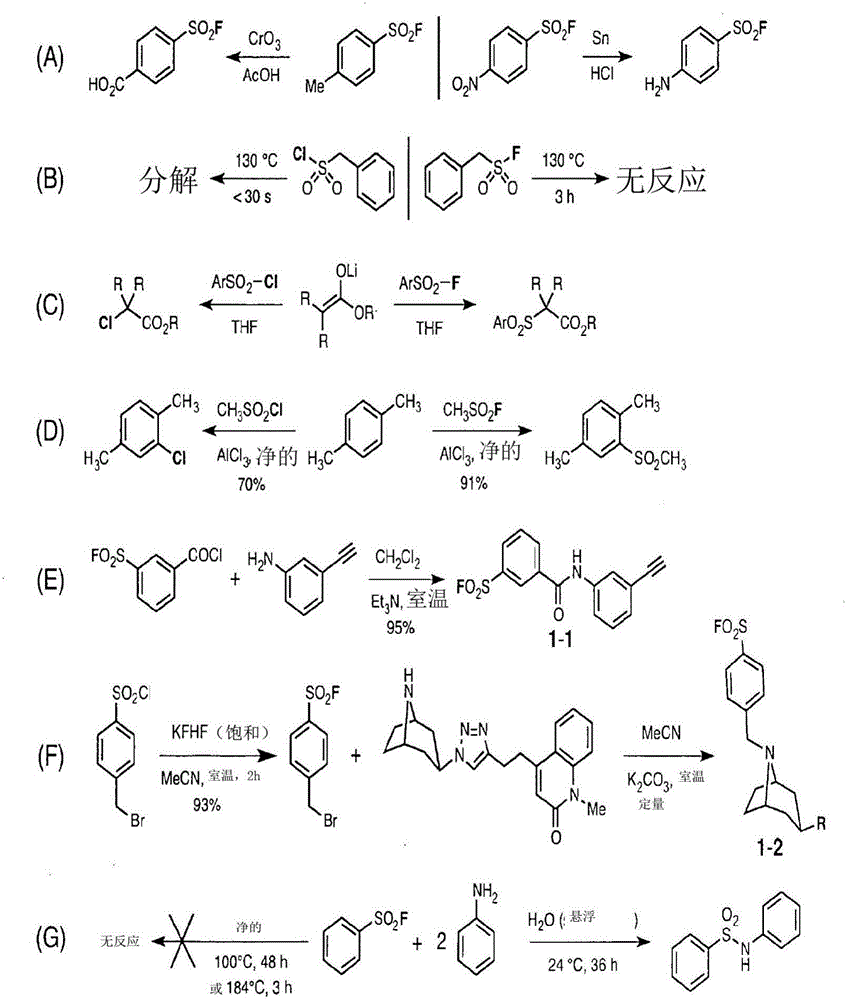
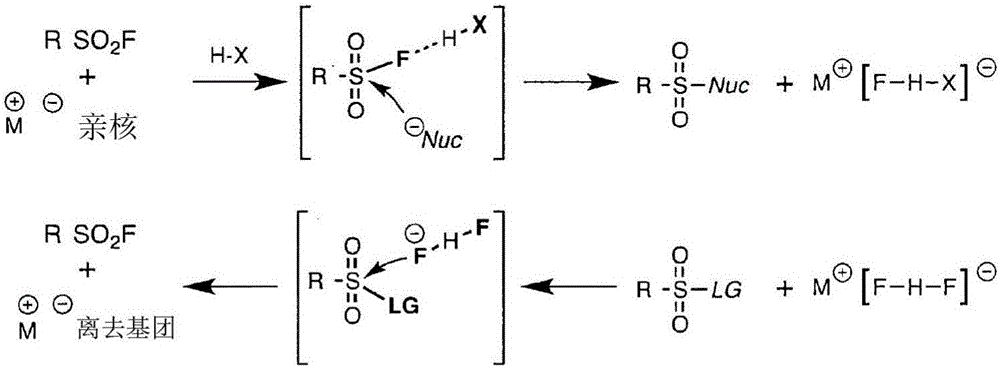
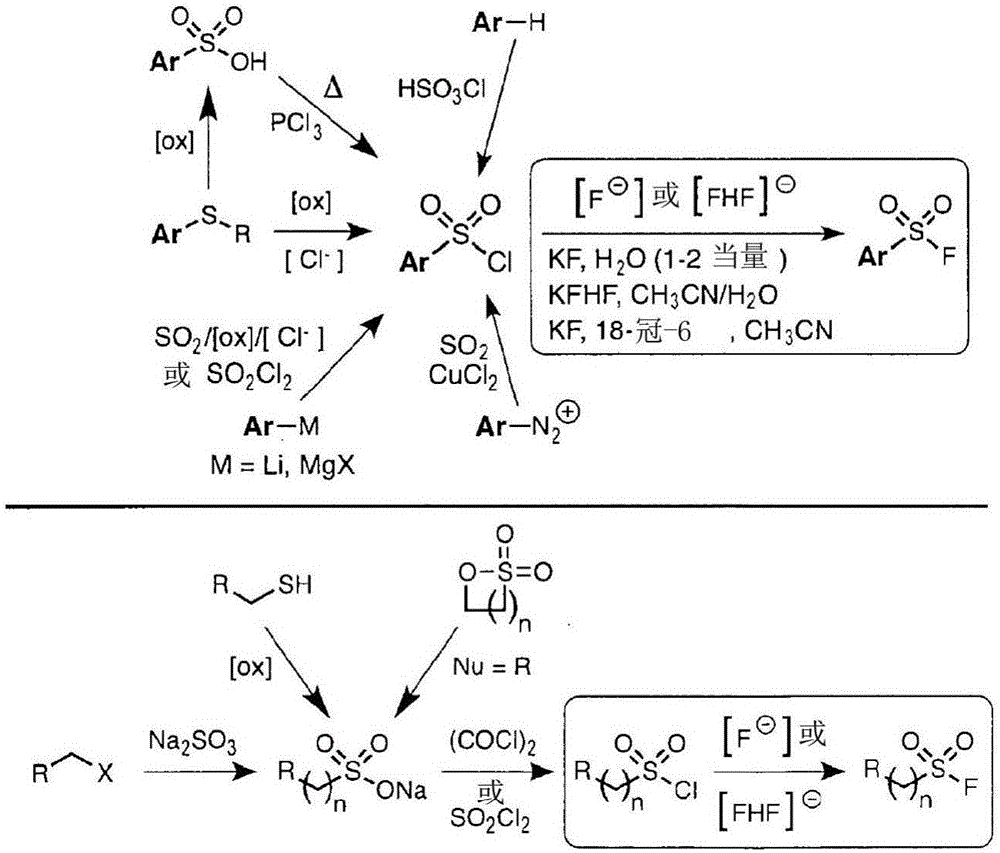
Sulfur(vi) fluoride compounds and methods for the preparation thereof
This application describes a compound represented by Formula (I): (I) wherein: Y is a biologically active organic core group comprising one or more of an aryl group, a heteroaryl aryl group, a nonaromatic hydrocarbyl group, and a nonaromatic heterocyclic group, to which Z is covalently bonded; n is 1, 2, 3, 4 or 5; m is 1 or 2; Z is O, NR, or N; X1 is a covalent bond or -CH2CH2-, X2 is O or NR; and R comprises H or a substituted or unsubstituted group selected from an aryl group, a heteroaryl aryl group, a nonaromatic hydrocarbyl group, and a nonaromatic heterocyclic group. Methods of preparing the compounds, methods of using the compounds, and pharmaceutical compositions comprising the compounds are described as well.
Owner:THE SCRIPPS RES INST
Linear and Cyclic Melanocortin Receptor-Specific Peptidomimetics
Linear and cyclic peptidomimetics which bind to one or more melanocortin receptors are provided, which peptidomimetics include at least one ring-constrained amino acid surrogate of formula I:where R1, R2, R3, R4, R5, R6, R7, R8, and y are as defined in the specification, together with methods for synthesizing ring-constrained amino acid surrogates of formula I and peptidomimetics incorporating the same, and methods of use of peptidomimetics in the treatment of various diseases, syndromes and conditions.
Owner:PALATIN TECH INC
Method for preparing vasopressin tannate
InactiveCN104672308AAvoid the problem of excessive volume and diluted concentrationSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDivinylbenzeneDesalination
The invention discloses a method for preparing vasopressin tannate. The preparation method of the vasopressin comprises the following step: sequentially carrying out reverse-phase purification and reverse-phase desalination on a vasopressin crude product solution by a high performance liquid chromatography, so as to obtain the vasopressin tannate, wherein packing of the high performance liquid chromatography, is a polystyrene-divinylbenzene (PS-DVB) copolymer. By combination of reverse-phase purification and reverse-phase desalination, the latest application of the polymer packing polystyrene-divinylbenzene is designed; and the vasopressin tannate can be prepared on a large scale.
Owner:QINGDAO KANGYUAN PHARMA
Preparation method of Terlipressin
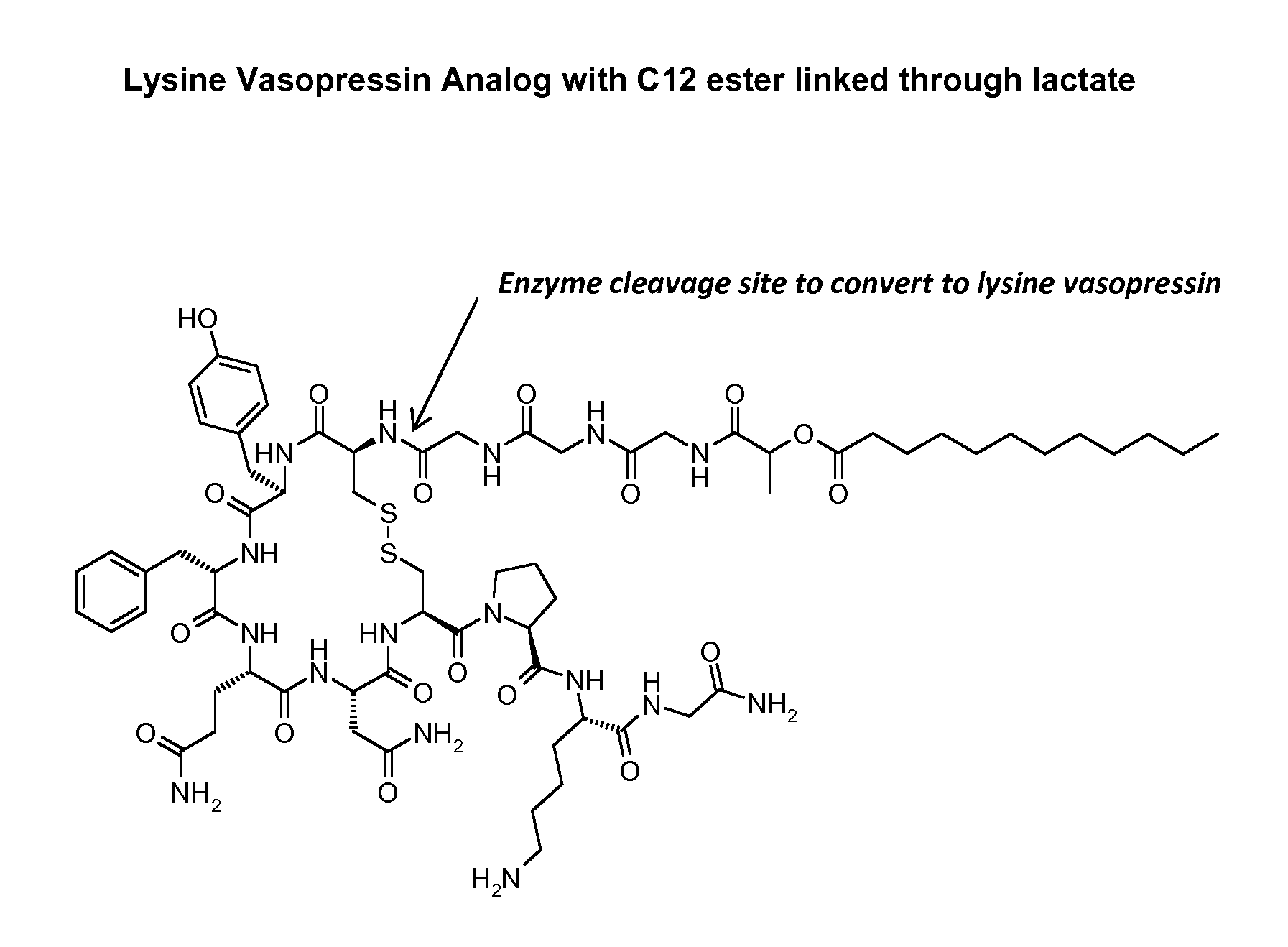
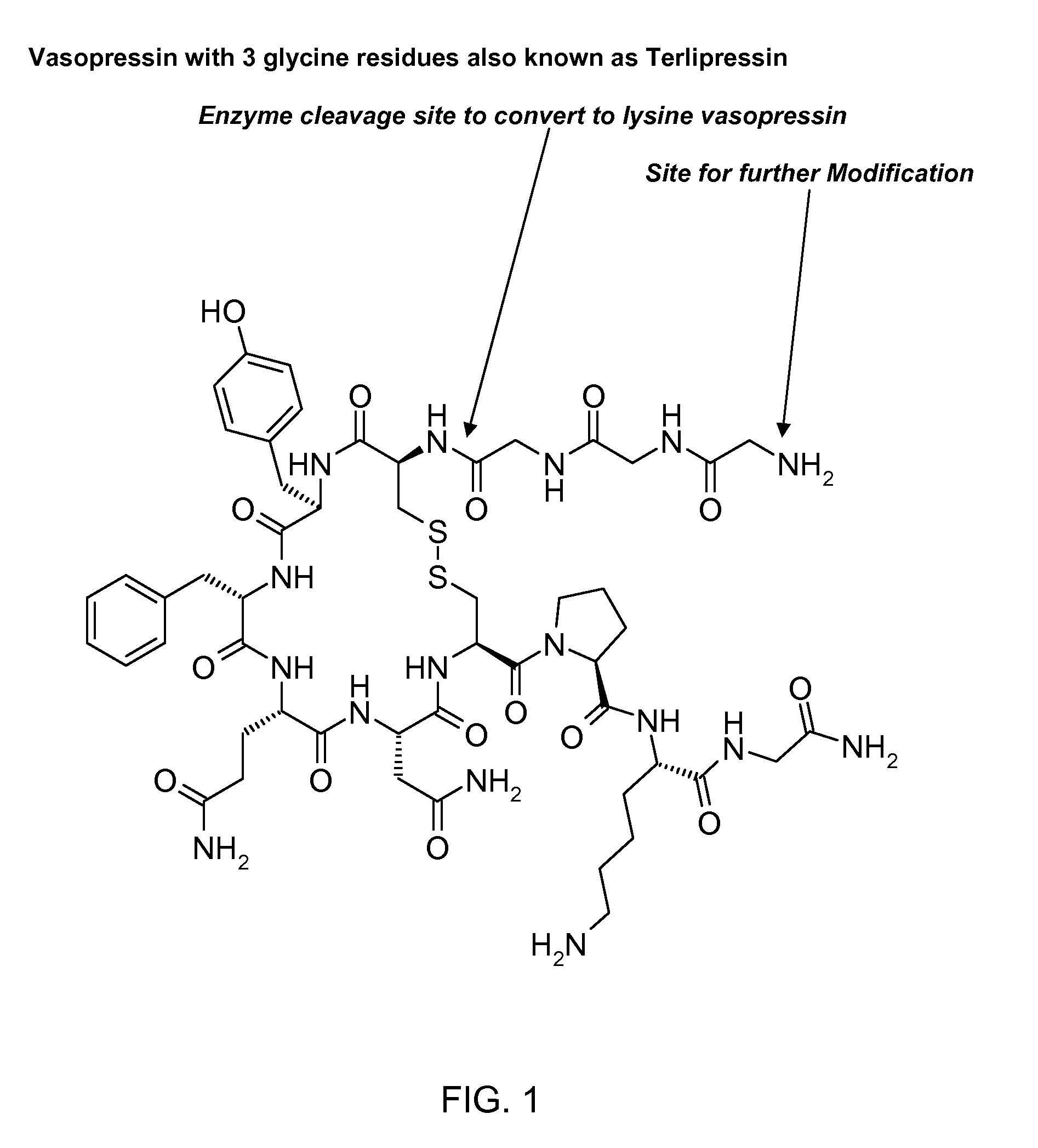
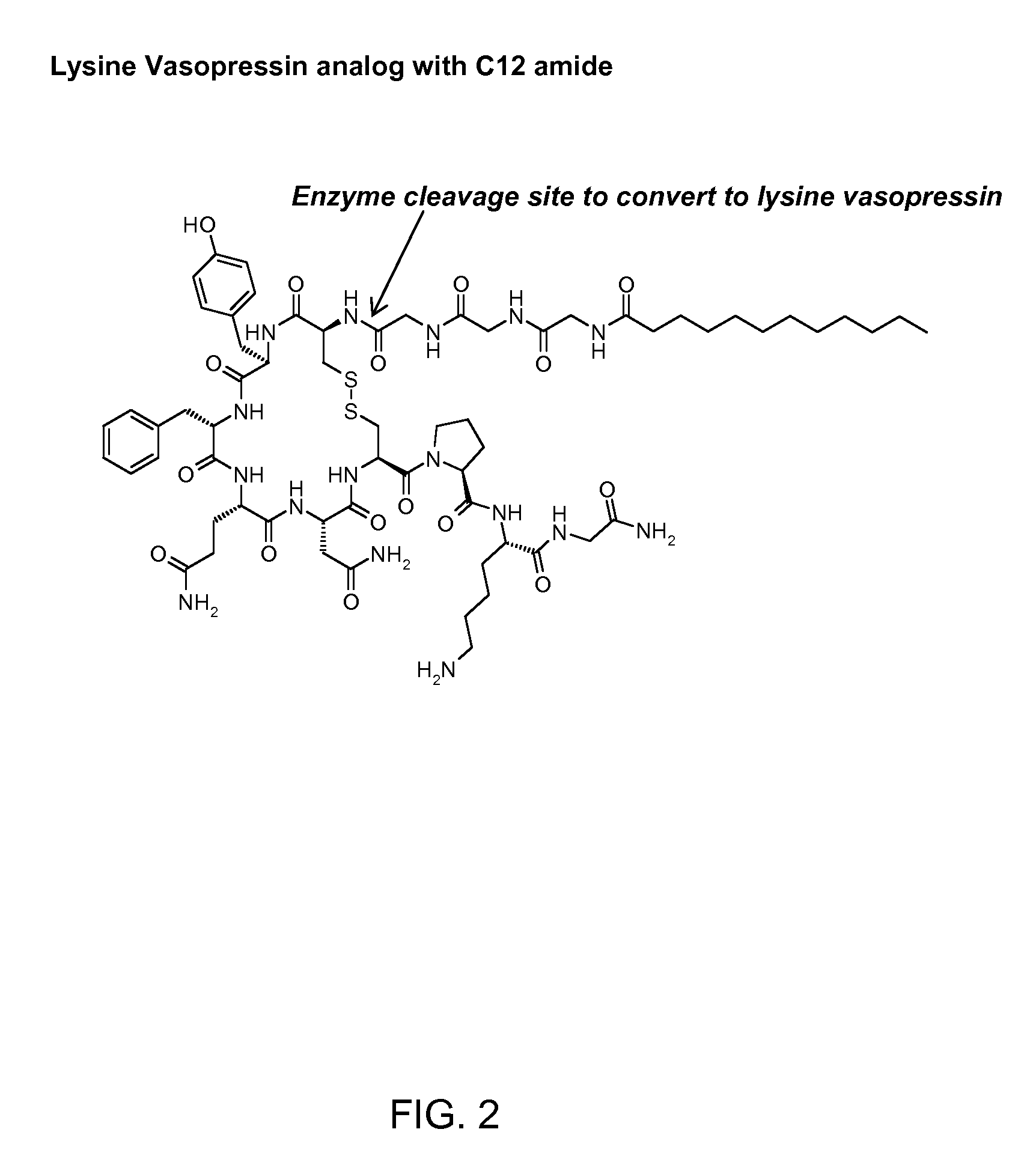
InactiveCN102408471AHigh yieldShorten the production cycleOxytocins/vasopressinsPeptide preparation methodsSynthesis methodsGly-Gly-Gly
The invention belongs to the technical field of preparation methods of polypeptide drugs and particularly relates to a preparation method of Terlipressin. The method uses protected amino acid fragment Gly-Gly-Gly as a raw material to avoid the generation of impurities. The method comprises the following steps: preparing Terlipressin linear peptide resin by adopting a solid phase polypeptide method, performing acidolysis to obtain crude Terlipressin linear peptide, oxidizing to obtain crude Terlipressin and purifying to obtain pure Terlipressin, wherein the solid phase polypeptide method is as follows: amino resin is connected in the Fmoc-protected amino acid of the corresponding fragments in the following sequence in turn through a solid phase coupling synthesis method to prepare the Terlipressin linear peptide resin, the sequence is R1-X-Cys(R2)-Tyr(tBu)-Phe-Gln(R3)-Asn(R3)-Cys(R2)-Pro-Lys(Boc)-Gly-resin, the fragment X is Gly-Gly-Gly, R1 is R4 or H, R2 is Trt or Acm, R3 is Trt or H, R4 is Fmoc or Boc, and the fragment X is grafted only by performing the solid phase coupling synthesis reaction once. The method uses the new raw material, thus the product yield can be increased and the production cycle is greatly shortened.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Preparing process for synthesizing oxytocin from solid-phase polypeptide
ActiveCN1990501AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsProduction rateRink amide resin
The invention discloses a method for preparing oxytocin through solid phase polypeptide synthesis, comprising following steps: taking Rink Amide resin (comprising Rink Amide MBHA resin, Rink Amide Am resin) as raw material, taking amino acid protected by Fmoc, TBTU or HBTU / HOBt as condensing agent, making up amino acid in sequence; adding peptide cutting agent for peptide cutting, adding for precipitation and getting reduced coarse product; adding basic matter, feeding air for oxidation or oxiding with H2O2 with pH being 7.5- 10.0, getting oxidized coarse product; separating and purifying by using C18 or C 8 column and getting final product. The method is characterized by low production cost, simple process, little pollution, high production rate and convenience for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
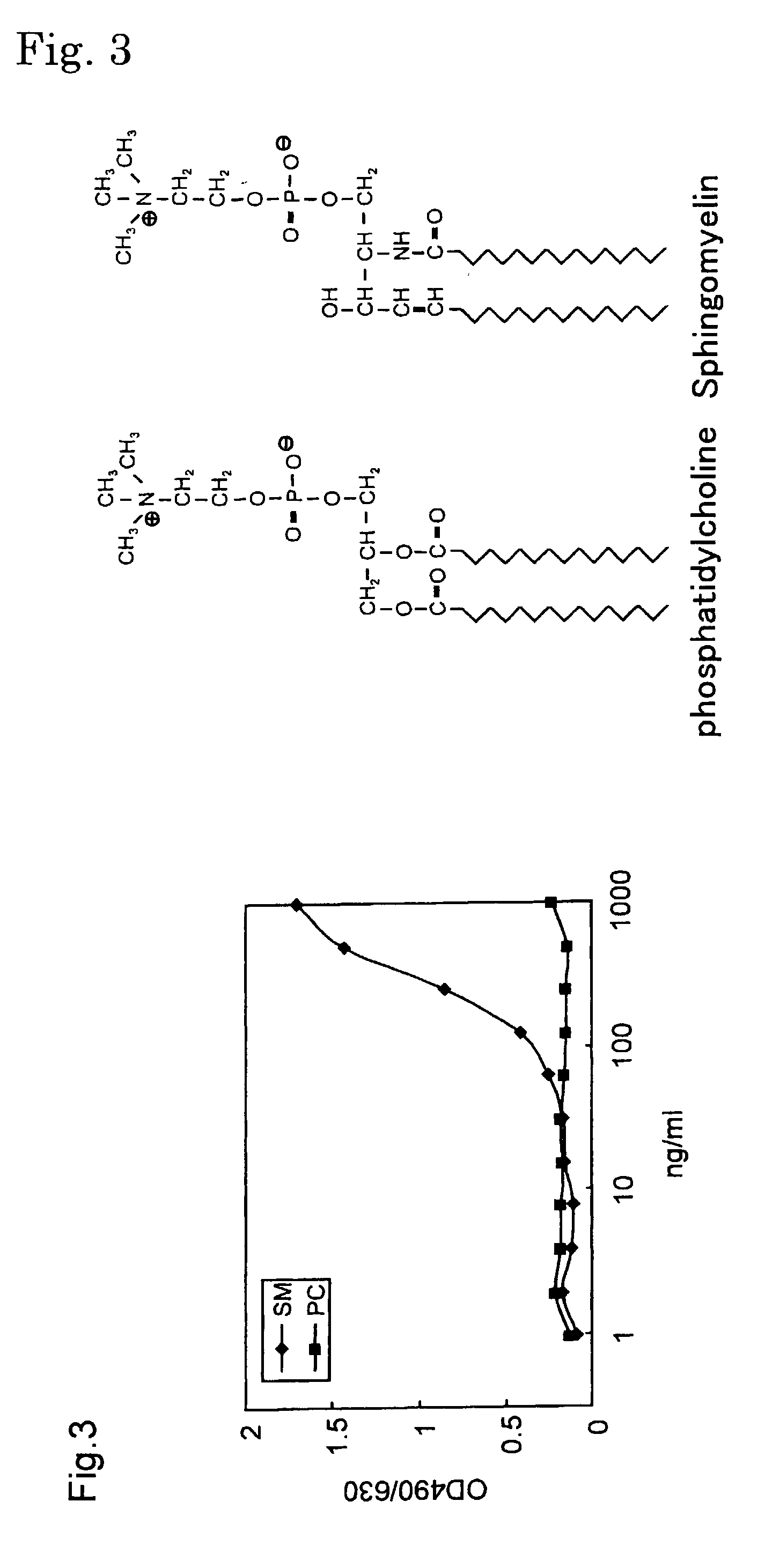
Sphingomyelin detecting probe
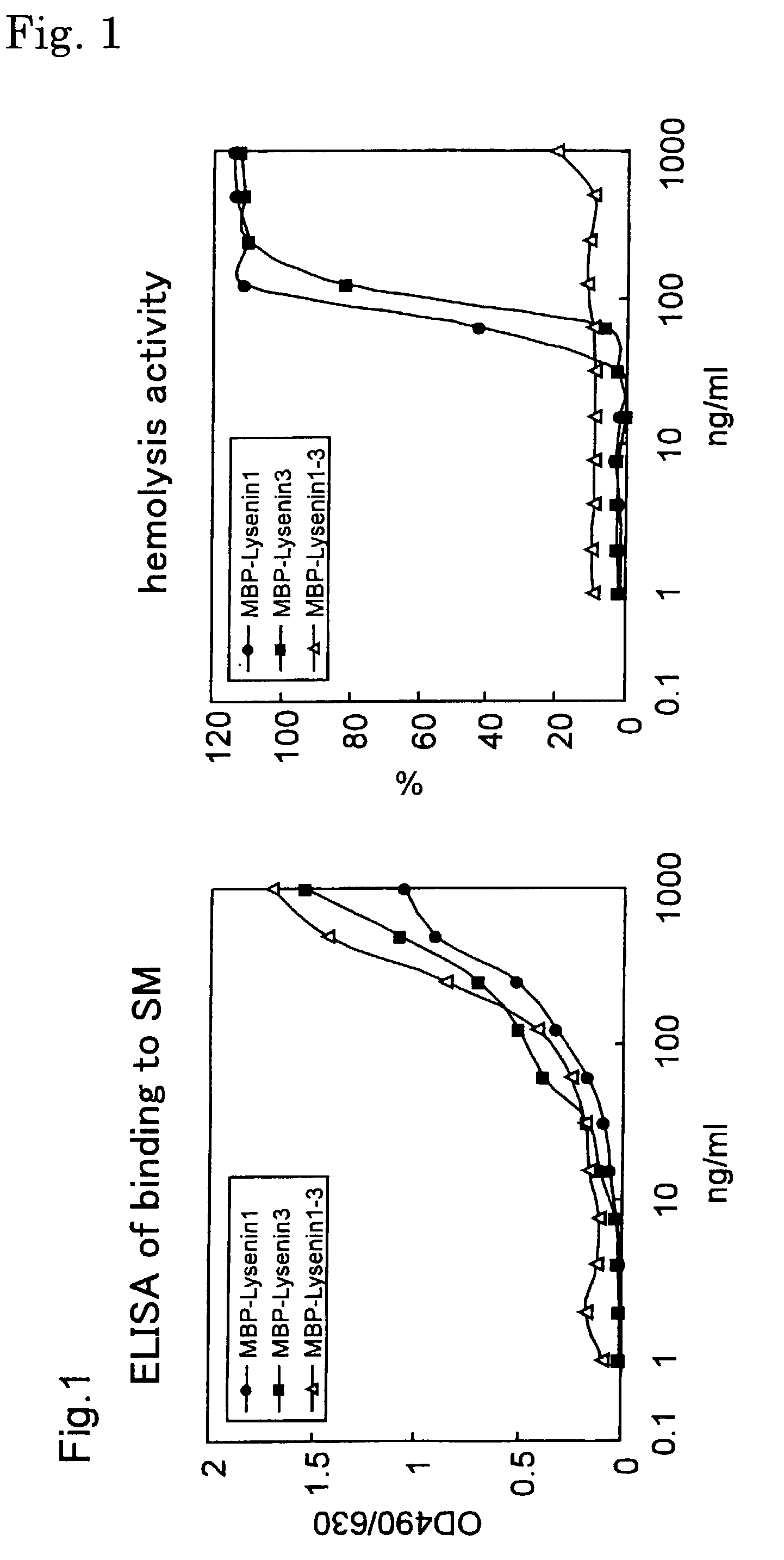
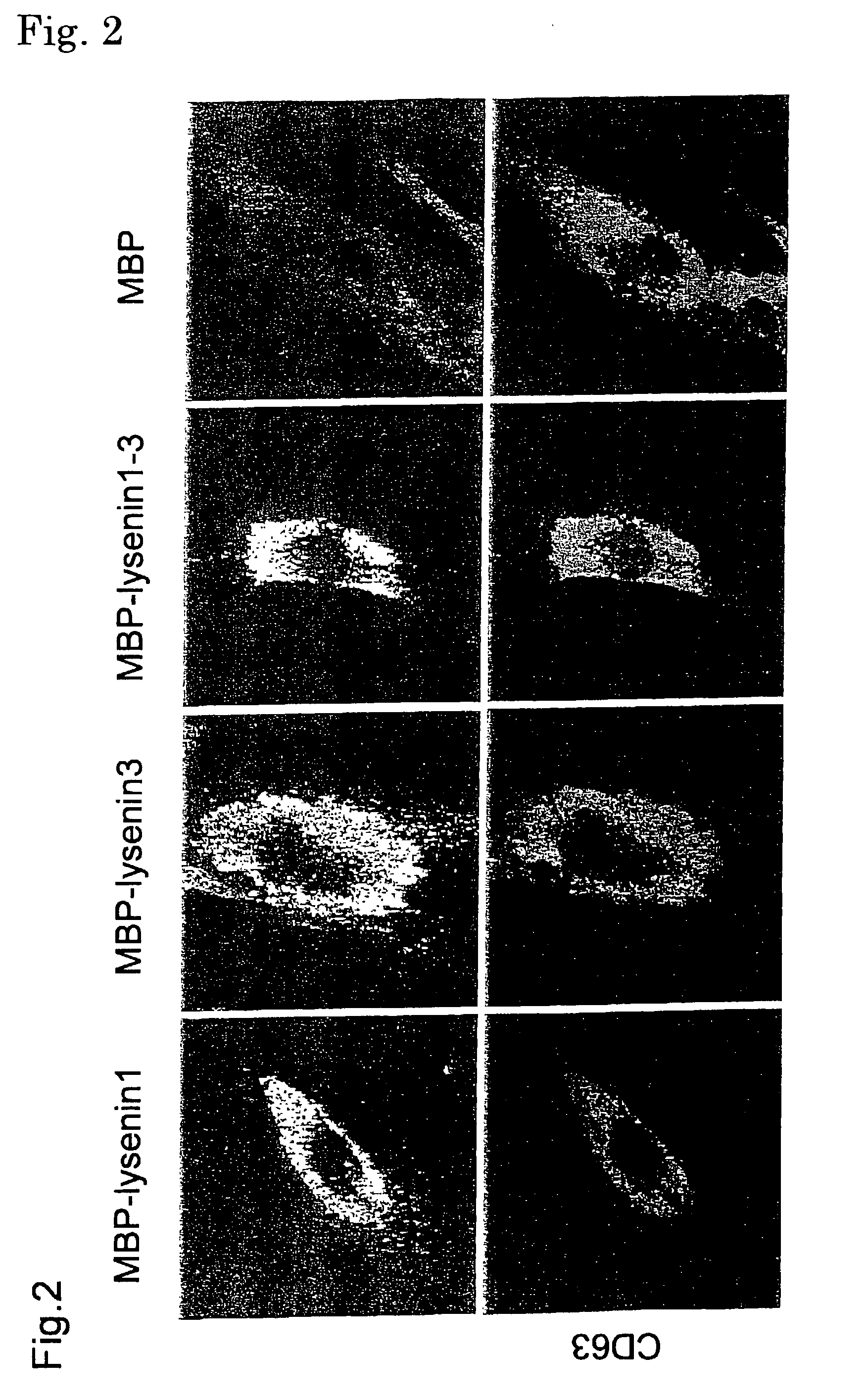
An object of the present invention is to provide a protein useful as a sphingomyelin detecting probe, which specifically recognizes sphingomyelin and has low cytotoxicity. The present invention provides a protein which has an amino acid sequence having, as the amino acid sequence from the 1st to the 48th amino acid, the amino acid sequence from the 1st to the 48th amino acid in Lysenin 1, and as the amino acid sequence from the 49th to the 298th amino acid, the amino acid sequence from the 51st to the 300th amino acid in Lysenin 3; and a protein which is obtained by deleting N terminal and / or C terminal from earthworm toxins Lysenin 1 or 3, and which specifically recognizes sphingomyelin.
Owner:RIKEN
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
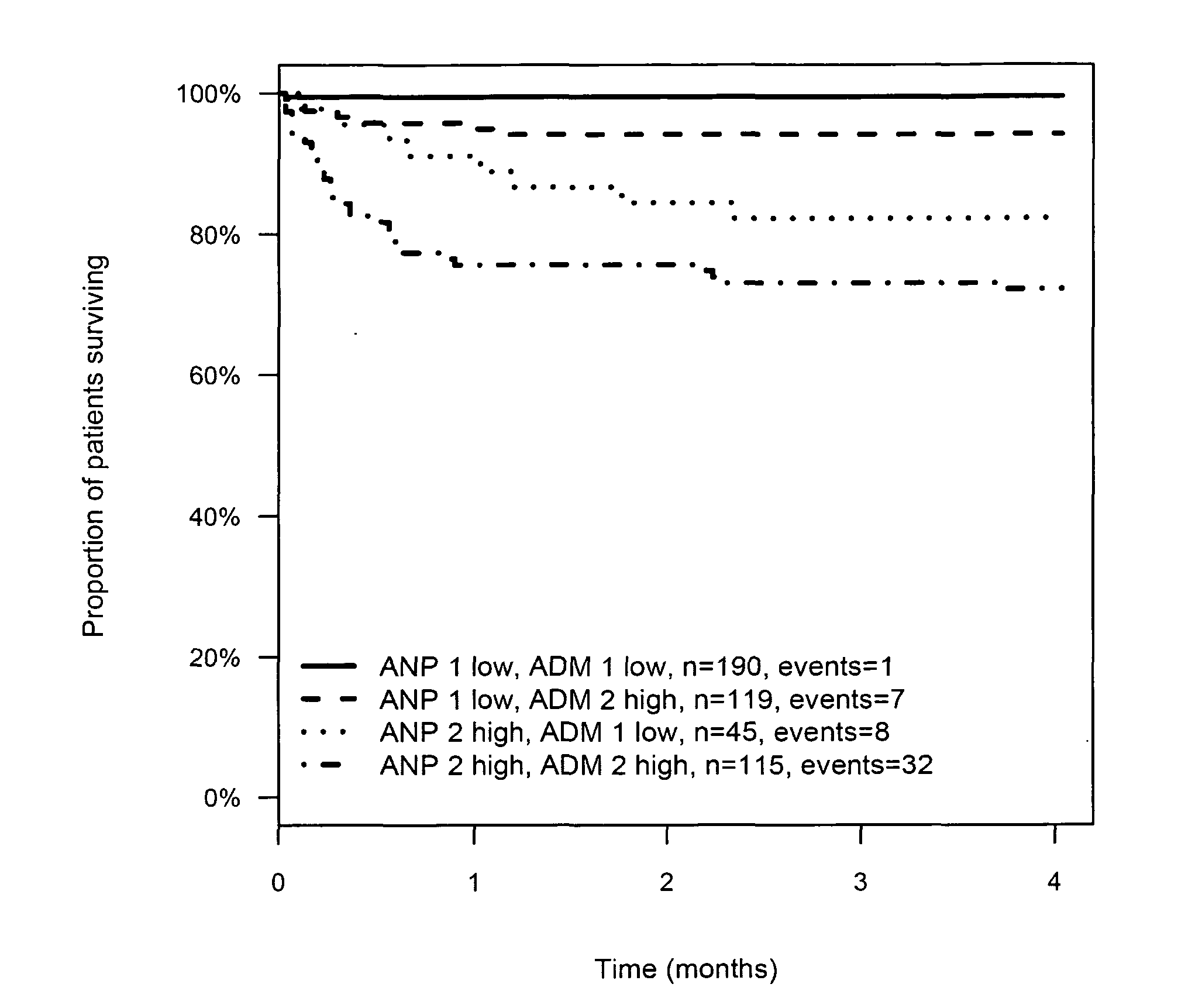
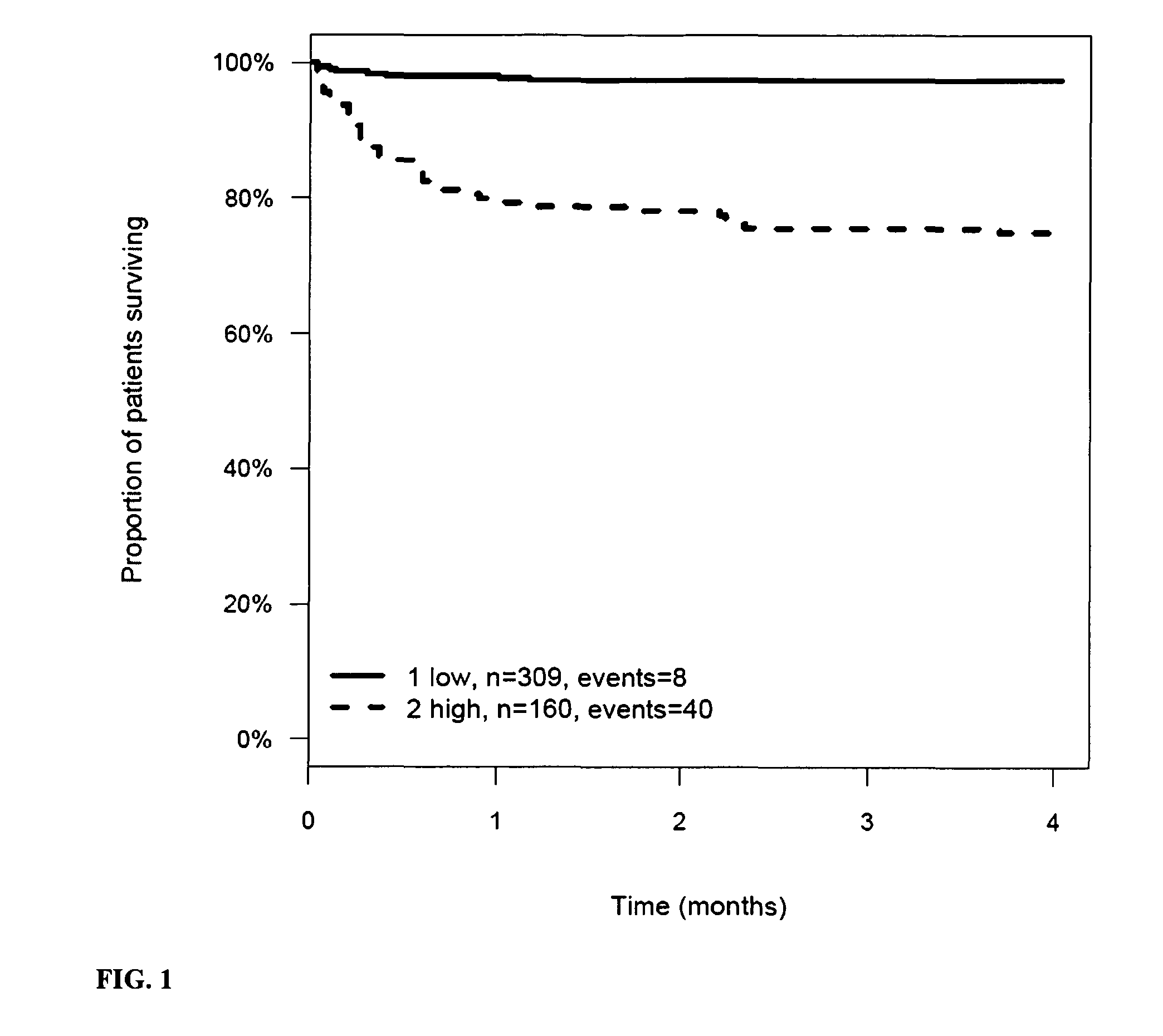
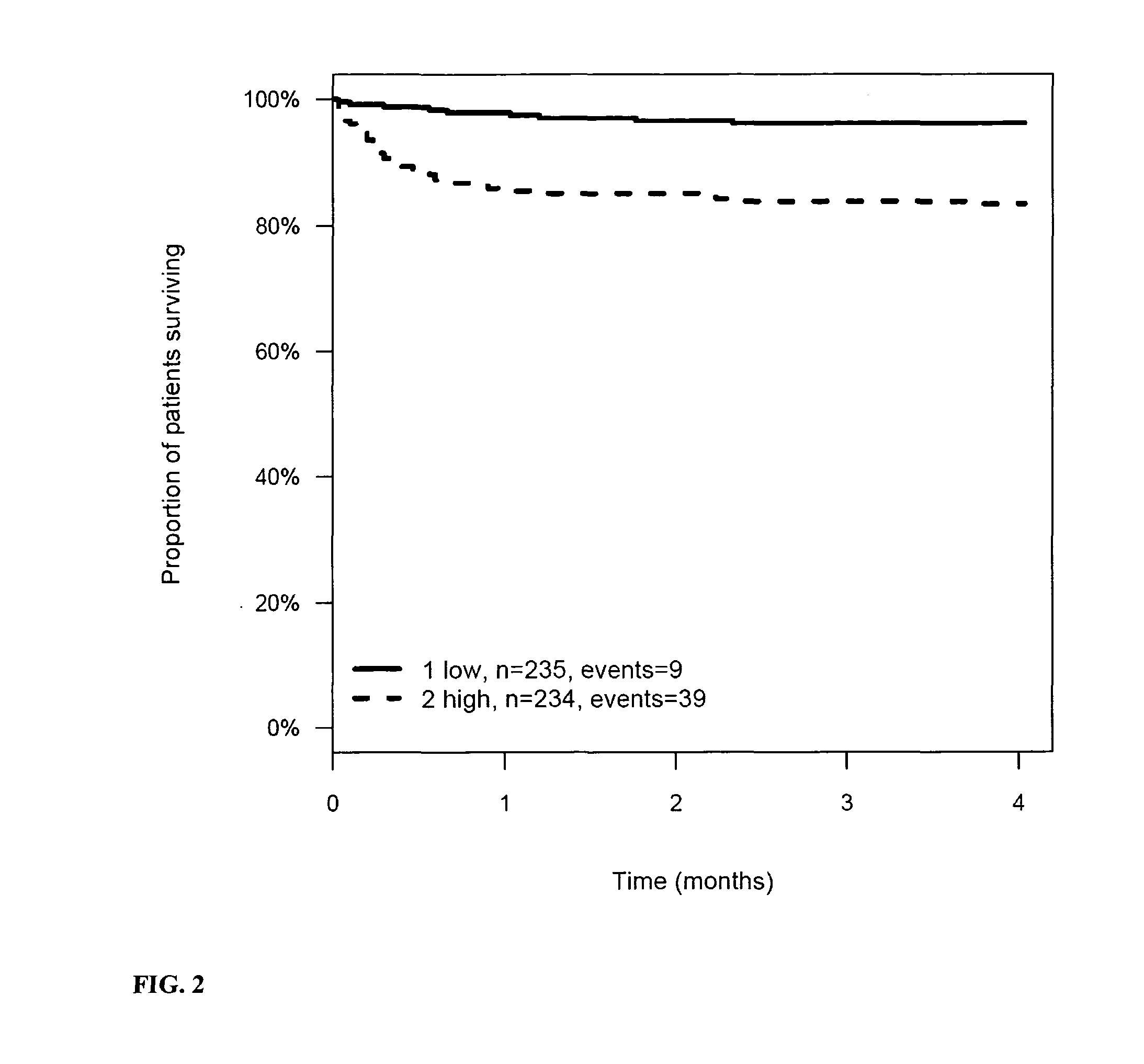
Prognosis and risk assessment in stroke patients by determining the level of marker peptides
InactiveUS20110263821A1Increase probabilityChemiluminescene/bioluminescenceLibrary screeningCalcitoninCancer risk assessment
The present invention relates to a method for prognosis of an outcome or assessing the risk of a patient having suffered a stroke or a transient ischemic attack, comprising the determination of the level of at least one marker peptide in said sample said marker peptide selected from the group comprising ANP, AVP, ADM, ET-1, troponin, CRP, calcitonin and hGH or fragments thereof or its precursor or fragments thereof and attributing the level of said at least one marker peptides its precursor or fragments thereof with the prognosis of an outcome or assessing the risk for said patient.
Owner:BRAHMS GMBH
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formula where R1 is H, C1-C4 alkyl and OH; R2 in is H, C1-C4 alkyl and OH; R3 is H and C1-C4 alkyl; R4 is H and C1-C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2-C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Method for synthesizing atosiban by solid phase
ActiveCN101696236AReduce poisonReduce pollutionOxytocins/vasopressinsPeptide preparation methodsRink amide resinTrifluoroacetic acid
The invention relates to a method for synthesizing atosiban by a solid phase, which comprises the following steps: 1) de-Fmoc-protecting Rink Amide resin serving as a carrier to obtain H2N-Rink Amide resin; 2) connecting carboxyl of Fmoc-Gly-OH and amino of the resin by using HOBT and DIPCI as condensation reagents to obtain Fmoc-Gly (resin); 3) solid-phase synthesizing sequence residue amino acid in turn by adopting Fmoc strategy; 4) performing solid-phase cyclization by using iodine; 5) then cutting the sequence residue amino acid by using pyrolysis reagents (trifluoroacetic acid / thioanisole / 1, 2-dimercaptoethane / water), and settling the sequence residue amino acid by diethyl ether to obtain raw atosiban peptide; and 6) treating the raw product through HPLC preparation and separation to obtain a pure atosiban product.
Owner:HAINAN ZHONGHE PHARM CO LTD
Purification method of carbetocin
InactiveCN102977192AEasy to operateObvious cost advantageOxytocins/vasopressinsPeptide preparation methodsIon exchangeCombinatorial chemistry
The invention discloses a purification method of carbetocin. The method comprises the following steps in sequence: purifying and synthesizing rough titanium through reversed-phase ion-pair chromatography, and removing an ion-pair agent through ion exchange resin, so as to obtain fine carbetocin peptide with purity of 99.5%. According to the purification method of carbetocin, the reversed-phase ion-pair chromatography is adopted, so that the purification yield and the purity of the carbetocin can be greatly improved.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Method for preparing octreotide and octreotide acetate
ActiveCN103965291AAvoid the problem of excessive volume and diluted concentrationSuitable for continuous productionOxytocins/vasopressinsAngiotensinsOctreotide acetateDesalination
The invention discloses a method for preparing octreotide and octreotide acetate. The method for preparing octreotide comprises the following steps: sequentially performing reverse phase purification and reverse phase desalination on an octreotide crude product solution by using a high performance liquid reversion phase chromatography method, wherein the packing of the high performance liquid reversion phase chromatography method is a poly styrene-divinyl benzene (PS-DVB) copolymer. The method integrates reverse phase purification and reverse phase desalination, novel application of the copolymer packing, namely, the styrene-divinyl benzene, is designed, and octreotide and octreotide acetate can be prepared in a large scale.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
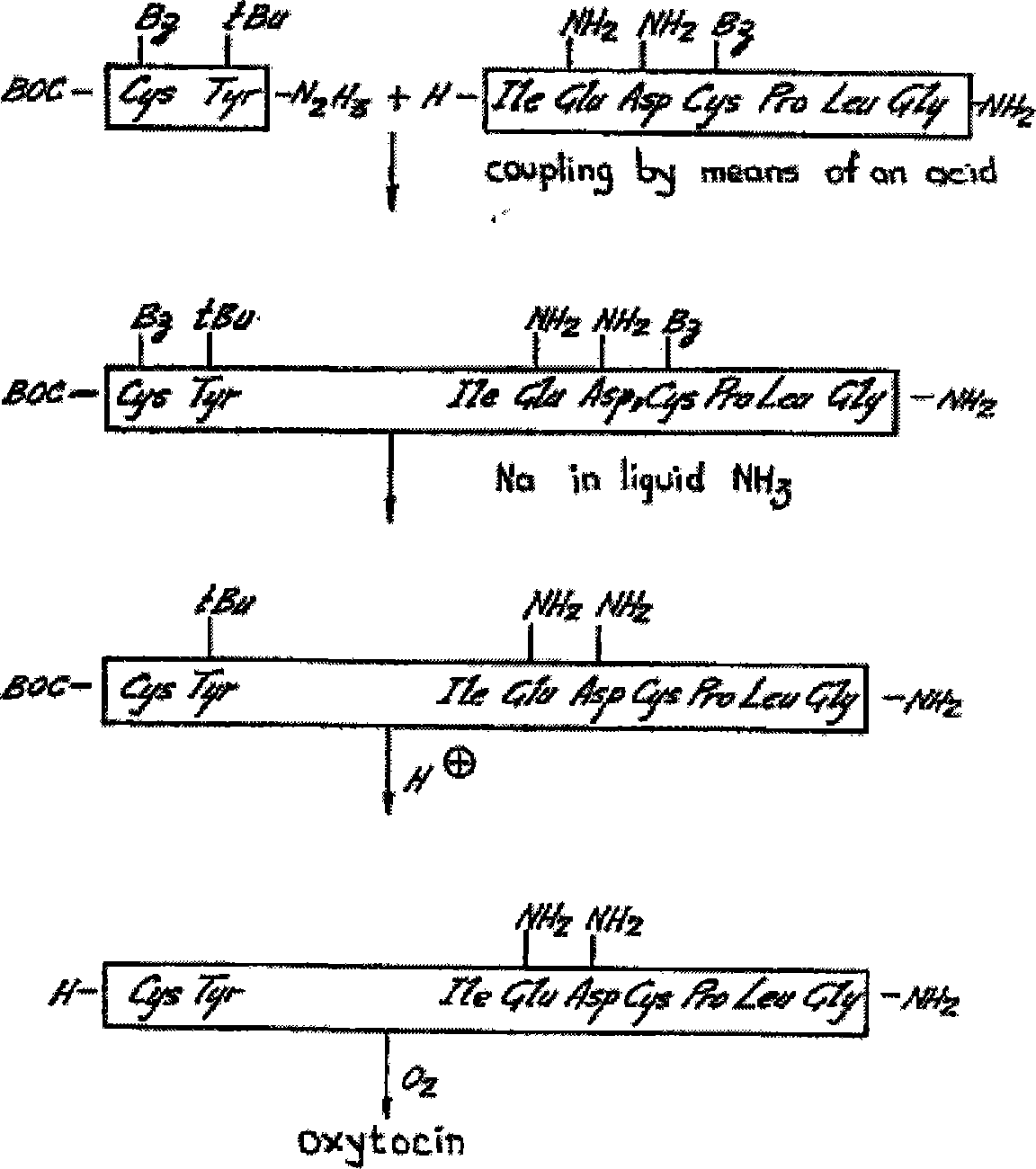
Intermediates and methods for making heptapeptide oxytocin analogues
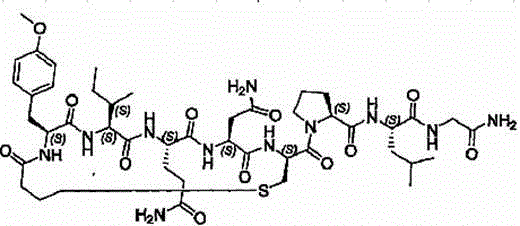
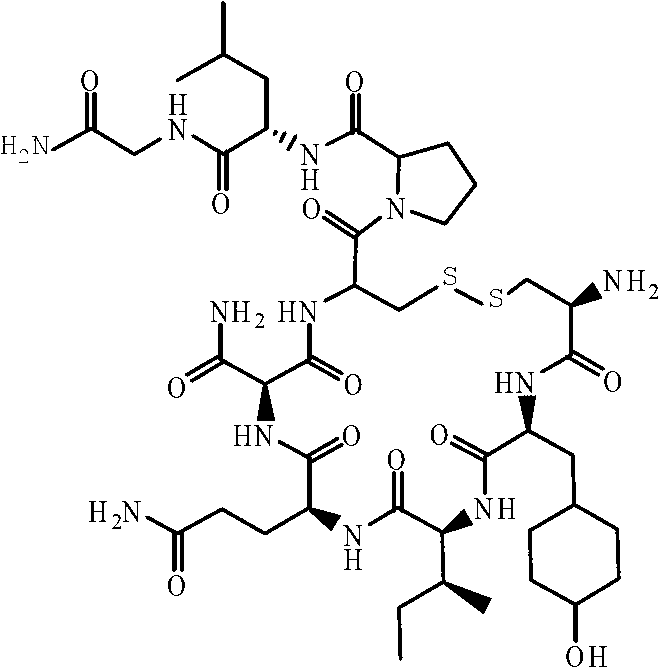
InactiveUS7091314B2Efficient and economical productionOxytocins/vasopressinsHormone peptidesAlcoholOxytocin
More efficient and / or economical methods for synthesizing heptapeptide alcohol analogs of oxytocin are provided along with novel intermediates which are useful in synthesizing such oxytocin analogs. Efficient and economical methods for synthesizing intermediates useful in synthesizing these oxytocin analogs are also provided.
Owner:FERRING BV
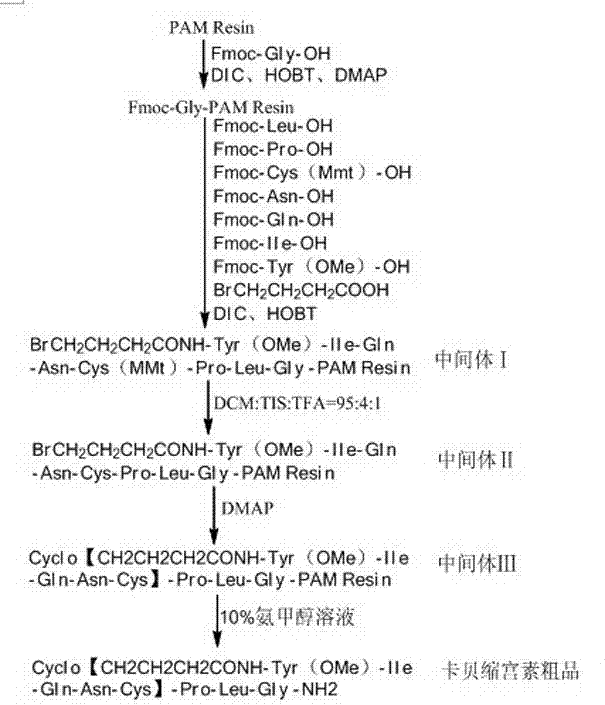
Method for preparing carbetocin
ActiveCN104262464AReduce usageLow costOxytocins/vasopressinsPeptide preparation methodsHigh concentrationFreeze-drying
The invention relates to a solid-phase synthesis method of carbetocin, which comprises the following steps: reacting a PAM (polyacrylamide) resin and Fmoc-Gly-OH to obtain an Fmoc-Gly-PAM resin; sequentially connecting amino acid with Fmoc protective group by solid-phase synthesis to obtain a BrCH2CH2CH2CONH-Tyr(OMe)-Ile-Gln-Asn-Cys(Mmt)-Pro-Leu-Gly-PAM resin; removing the Mmt protective group in the Cys; carrying out cyclization reaction by using DMAP (dimethylaminopyridine) as a cyclization reagent to obtain Cyclo[CH2CH2CH2CO-Tyr(OMe)-Ile-Gln-Asn-Cys]-Pro-Leu-Gly-PAM; carrying out ammonolysis on the cyclization product with an ammonia methanol solution to obtain a carbetocin crude product; and purifying and freeze-drying to obtain the carbetocin. The total yield is up to higher than 45%. Compared with the conventional preparation method, the method provided by the invention avoids using abundant high-concentration strongly-corrosive acid and flammable and explosive aether, has the advantages of high yield, low cost, mild reaction conditions and small environmental pollution, and is beneficial to industrialized production.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
Preparation method of terlipressin
InactiveCN103254295AImprove the coupling effectIncrease reaction rateOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingCombinatorial chemistry
The invention discloses a preparation method of terlipressin. The preparation method comprises the following steps of: deprotecting RinkAmideAM resin by using a deprotection reagent so as to remove a Fmoc protection group; coupling amino acids orderly with the deprotected RinkAmideAM resin as the starting raw material, the Fmoc protected amino acids as monomers and HOBt / DIC as a condensating agent, thus obtaining terlipressin peptide resin; before coupling the second to twelfth amino acids, depriving the Fmoc protection group of the last coupled product by using the deprotection reagent orderly; cracking the terlipressin peptide resin and then adding diethyl ether for settling the terlipressin peptide resin, thereby obtaining reduced terlipressin crude peptide; cyclizing the reduced terlipressin crude peptide to obtain oxidized terlipressin crude peptide; purifying the oxidized terlipressin crude peptide, converting the crude peptide into a salt, and then condensating and freeze-drying the oxidized terlipressin crude peptide, thus obtaining the terlipressin. The yield of the terlipressin prepared by the preparation method provided by the invention is 50% or above.
Owner:QINGDAO GUODA BIOLOGICAL PHARMA
Method for preparing oxytocin
ActiveCN101235081AHigh activityHigh chemical purityOxytocins/vasopressinsPeptide preparation methodsReaction temperatureOxytocin preparation
The invention discloses an oxytocin preparation method which comprises respectively synthesizing intermediate a and intermediate b, using diphenyl azidophopshate as condensing agent, in the presence of DMF, DPPA, dioxane, piperidine, TFA and DCM, to react the intermediates a, b, controlling the reaction temperature at -20-40DEG C and reaction time of 10-40h, feeding air, after the oxidization, drying in vacuum to remove the solvent in the reaction system to obtain oxytocin. The invention has complete connected peptide chain, simple purification, simple process, high yield, high optical activity and chemical purity of target product oxytocin.
Owner:YANCHENG KAILI PHARMA
Preparation method for oxytocin deamidation impurity
ActiveCN106749539APrevent elutionSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDesalinationReversed-Phase Liquid Chromatography
The invention discloses a preparation method for an oxytocin deamidation impurity. The preparation method comprises the following step of: successively subjecting a solution of a crude oxytocin deamidation impurity precursor to reversed-phase cyclization, reversed-phase purification and reversed-phase desalination by adopting high-performance liquid-phase reversed-phase chromatography, wherein a filling material for the high-performance liquid-phase reversed-phase chromatography is silica gel C18; and the crude oxytocin deamidation impurity precursor contains two free sulfhydryl groups. The preparation method provided by the invention innovatively adopts a reversed-phase adsorption method for cyclization, purification and desalination, solves the problems of cyclization, purification and desalination once for all, optimizes the production process, and is applicable to continuous industrial production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Process for production of cyclic peptides
Owner:TOVI AVI +4
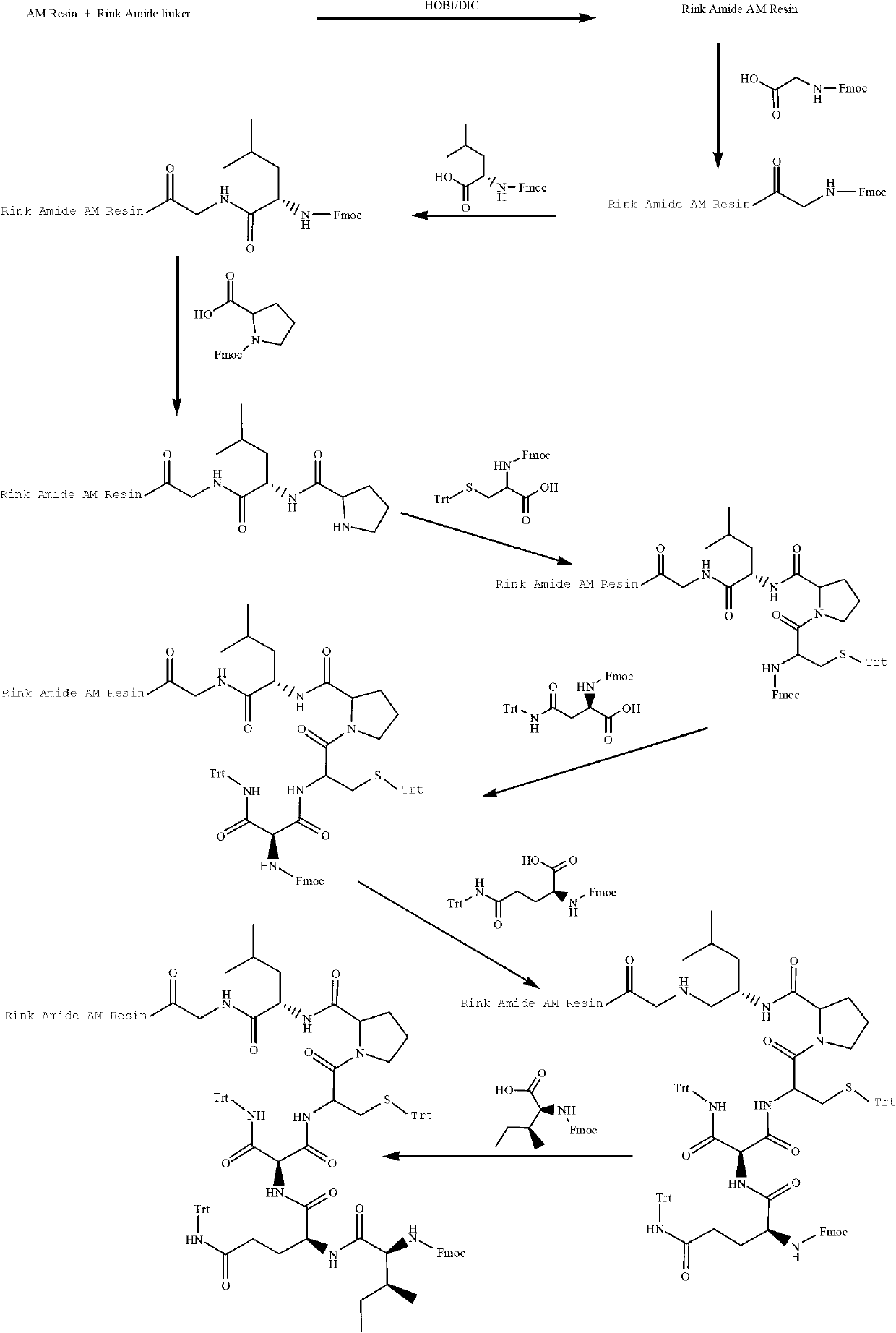
Solid-phase synthetic method of oxytocin
ActiveCN102850441AReduce pollutionThe synthesis process is simpleOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingCombinatorial chemistry
The invention provides a solid-phase synthetic method of oxytocin, which comprises the following steps: (I) preparing Rink Amide AM Resin by using AM Resin and Rink Amide Linker as raw materials; (II) orderly coupling Fmoc protecting group-containing amino acids to the Rink Amide AM Resin prepared in the step (I) through a solid-phase synthetic method, removing the protecting group, cracking to obtain linear oxytocin; (III) oxidizing the linear oxytocin, performing purification and freeze drying to obtain the oxytocin. The synthetic process of the invention is simple, low in cost, high in yield, easy for purification, less in environmental pollution, and suitable for industrial production.
Owner:YANCHENG KAILI PHARMA
Process for production of cyclic peptides
Owner:TOVI AVI +4
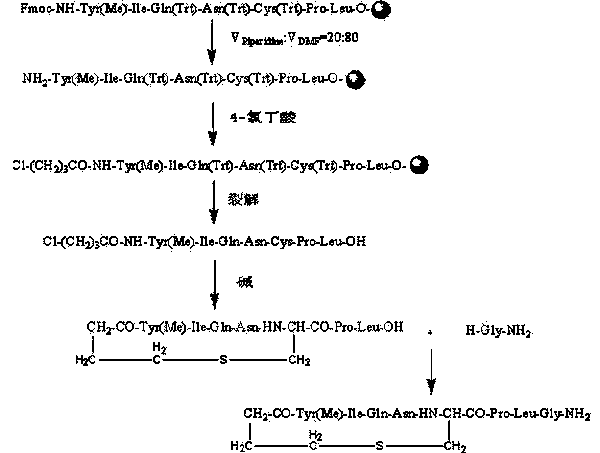
Carbetocin synthesis method
InactiveCN103992390AHigh purityHigh yieldOxytocins/vasopressinsPeptide preparation methodsSynthesis methodsFreeze-drying
The invention relates to a carbetocin synthesis method. The carbetocin synthesis method solves the problem that the prior art has a high cost, low purity and more impurities. The carbetocin synthesis method comprises the following steps of orderly coupling seven amino acids to wang resin, wherein the coupled amino acids orderly comprise Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ile-OH and Fmoc-Tyr(Me)-OH, removing a protective group Fmoc, connecting 4-chlorobutyric acid, carrying out cracking to obtain an acylated carbetocin seven-peptide linear peptide, carrying out liquid cyclizing under alkaline conditions to obtain a carbetocin seven-peptide crude cyclopeptide, carrying out purification and freeze-drying on the crude cyclopeptide to obtain a carbetocin seven-peptide fine cyclopeptide, and coupling the fragment of the carbetocin seven-peptide fine cyclopeptide subjected to purification and freeze-drying and H-Gly-NH2.HCl to obtain carbetocin. The invention provides the carbetocin synthesis method having a low cost and a high yield and suitable for large-scale production.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com