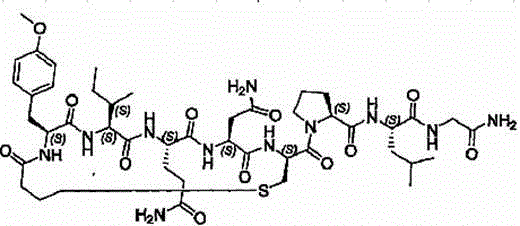
Synthesis process of carbetocin
A technology of carbetocin and synthesis process, which is applied in the field of chemical synthesis of polypeptide drugs, can solve the problems of difficult synthesis, high production cost, and expensive reagents, and achieve good cyclization effect, short process, and environmental protection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: a kind of Synthetic process of carbetocin , characterized in that it includes the following steps:
[0029] Step 1. Weigh 588.2g (synthesis scale: 1000mmol) of AM Resin (1.70mmol / g) into the reaction kettle, add about 5L of dichloromethane (DCM) to swell for about 20min. After the swelling time expires, remove the DCM;
[0030]Add the reaction solution that has been activated in an ice bath. This reaction solution consists of 647.6g Rink Amide AM-Linker, 194.6g 1-hydroxybenzotriazole 1-hydroxybenzotriazole (HoBt) ice bath for 2min, and then add 371mLN,N' -Diisopropylcarbodiimide DIC was obtained by activating it in the solution for 5 minutes. After 8 hours of reaction, a sample of ninhydrin was tested and it was negative, indicating that the coupling reaction was complete, and the reaction solution could be removed.
[0031] Add 5LDMF to wash 2 times, remove DMF, add 5L DBLK to deprotect twice, the time is 5min and 15min respectively, wash with 5LDMF onc...
Embodiment 2
[0040] Embodiment 2: a kind of Synthetic process of carbetocin , characterized in that it includes the following steps:
[0041] (1) Weigh 484.8g of AM Resin (synthesis scale 800mmol) (1.65mmol / g) into the reaction column, add 4LDCM to swell for about 20min. After the swelling time expires, remove the DCM, add the reaction solution that has been activated in an ice bath (690.7g Rink Amide-AM Linker, 207.4g HoBt in an ice bath for 2min, then add 238.0mL DIC to activate in the solution for 5min) and wait for 8 hours to react , sampling ninhydrin test, negative, indicating that the coupling reaction is complete, the reaction solution can be taken out, add 4LDMF to wash twice, after taking out DMF, add 4LDBLK (20% piperidine / DMF solution) to deprotect twice, time They are 5min and 15min respectively, wash once with 4LDMF between two deprotection times for about 1min, and drain. After deprotection, wash in the following order: DMF x 1 time, DMF x 1 time, MeOH x 1 time, DCM x 1 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com