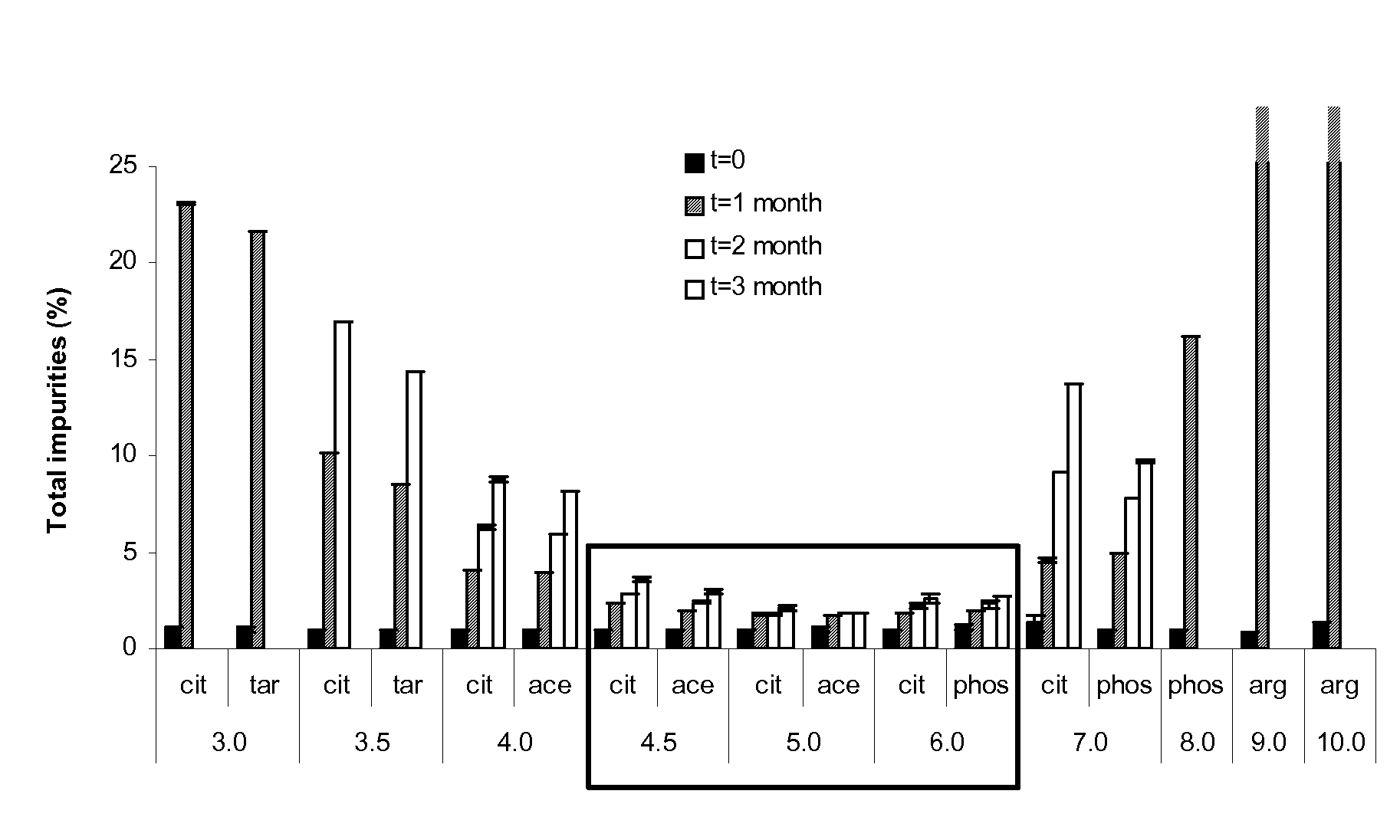
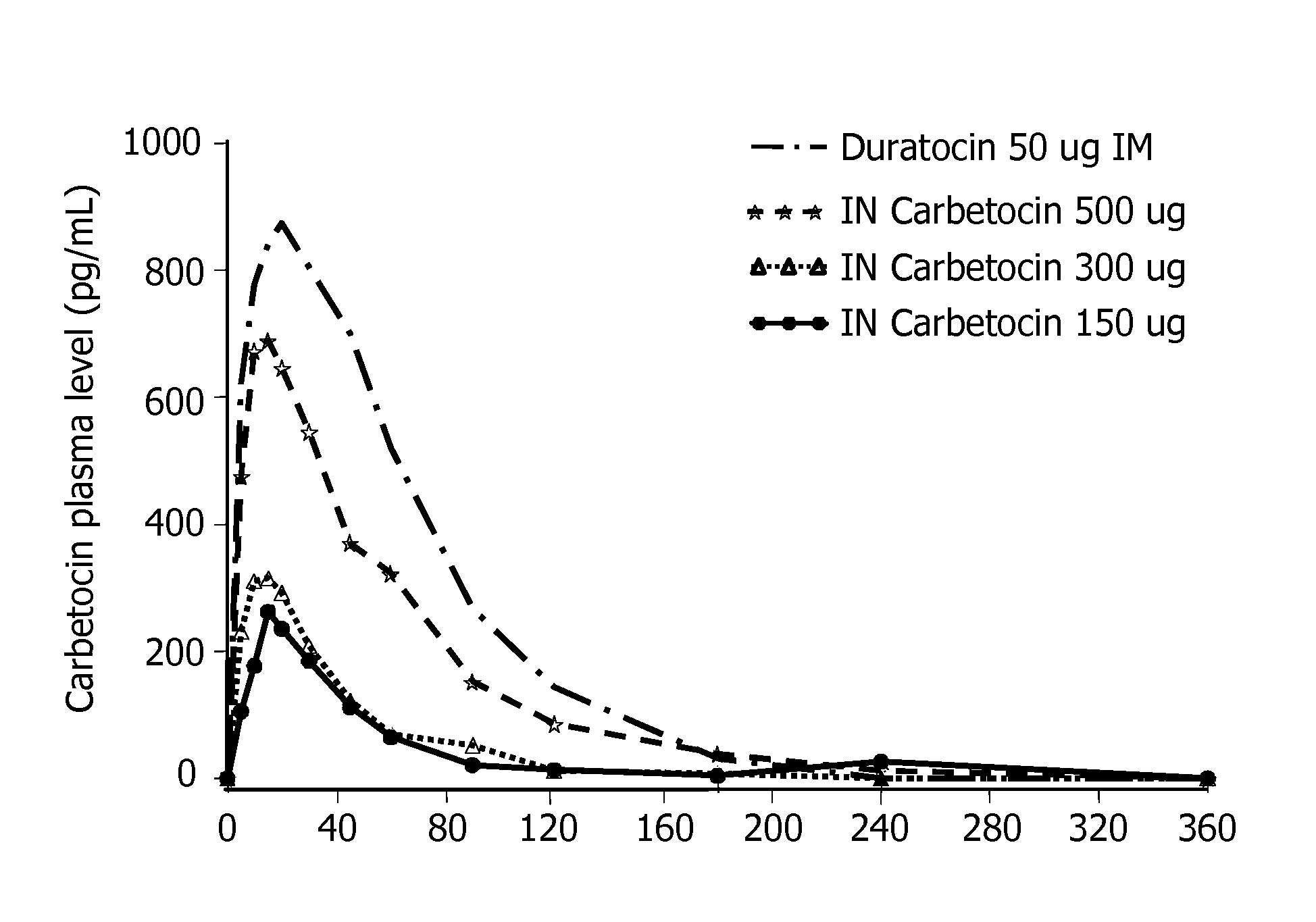
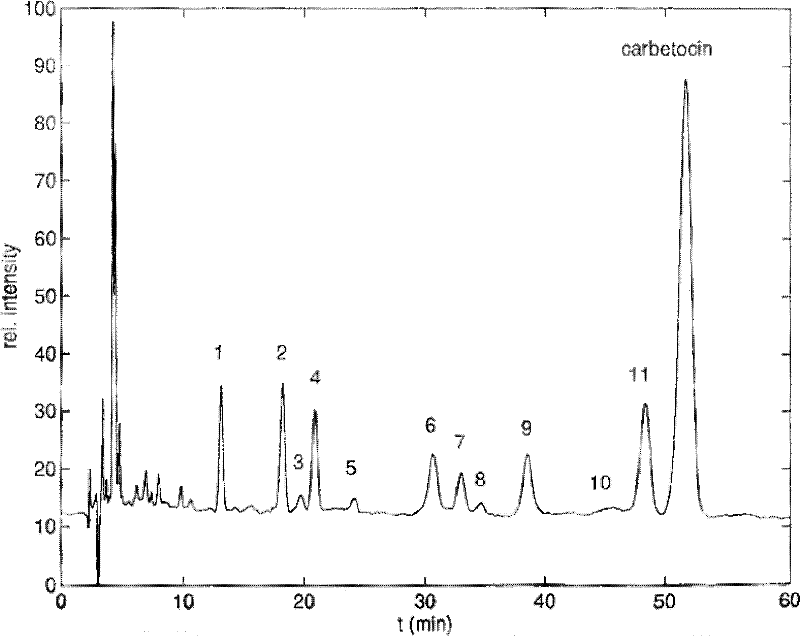
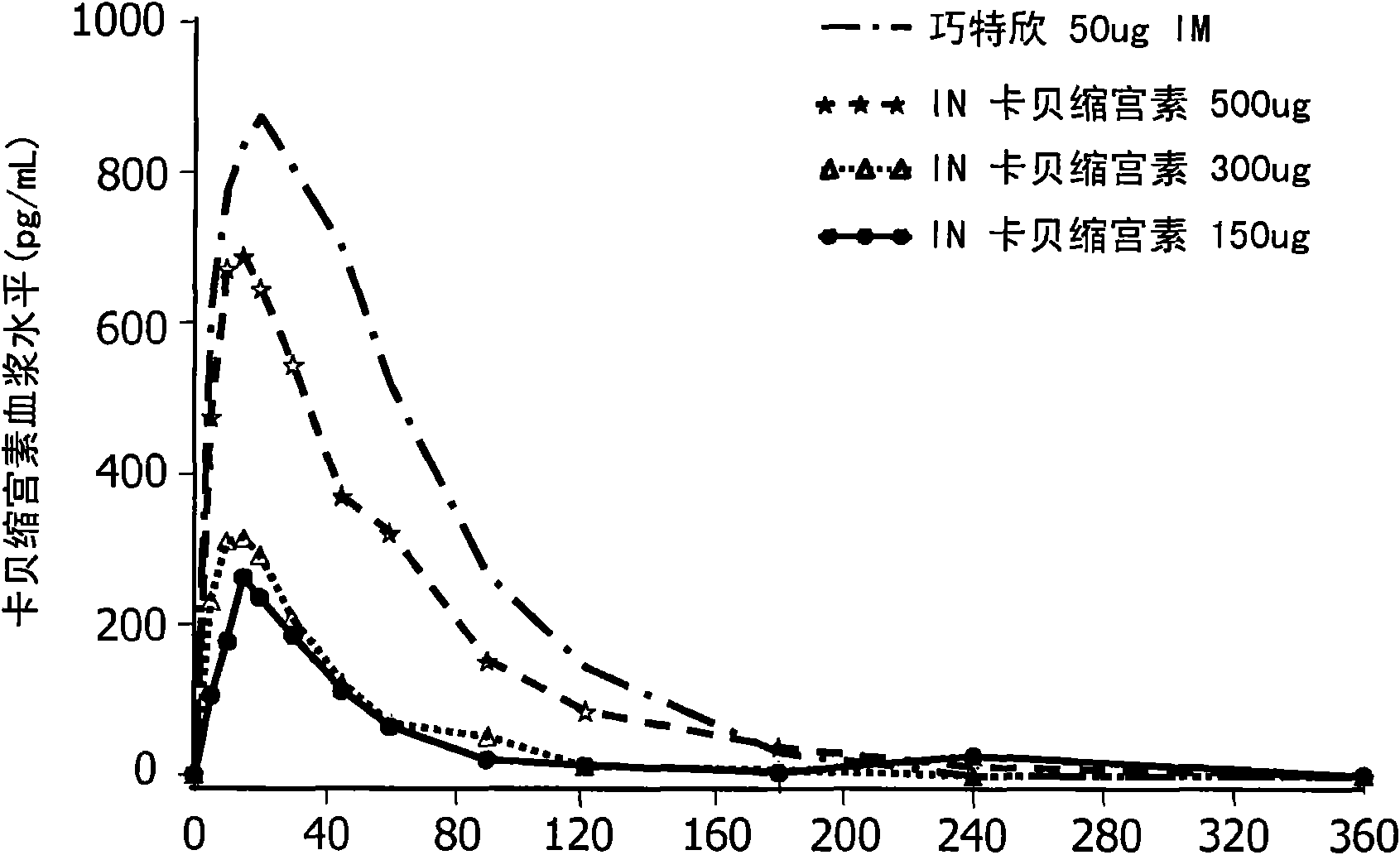
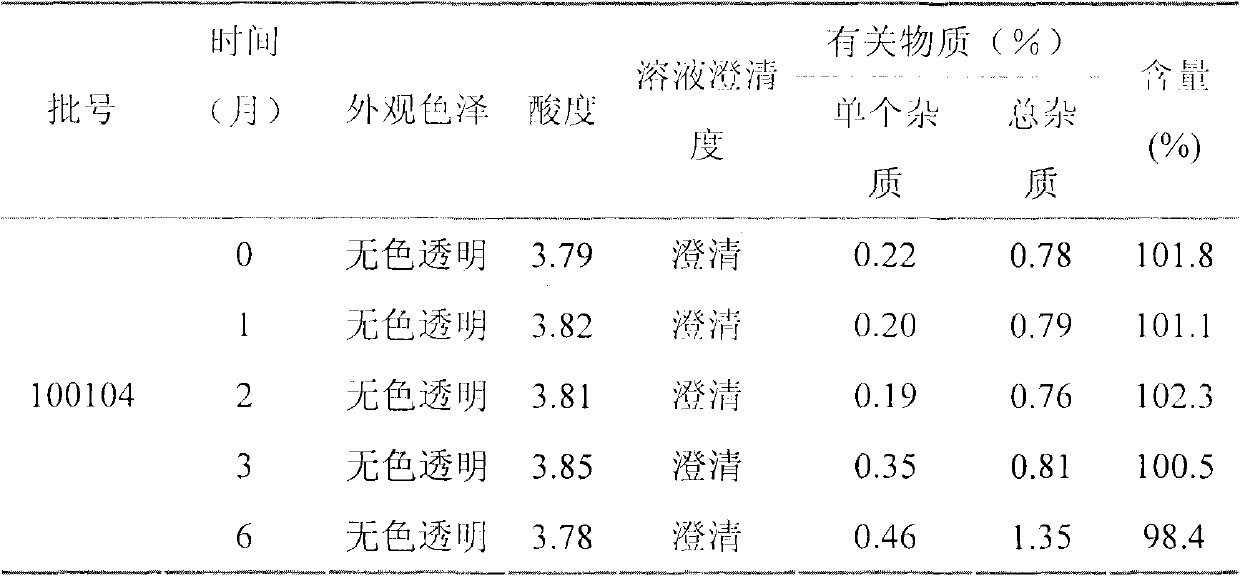
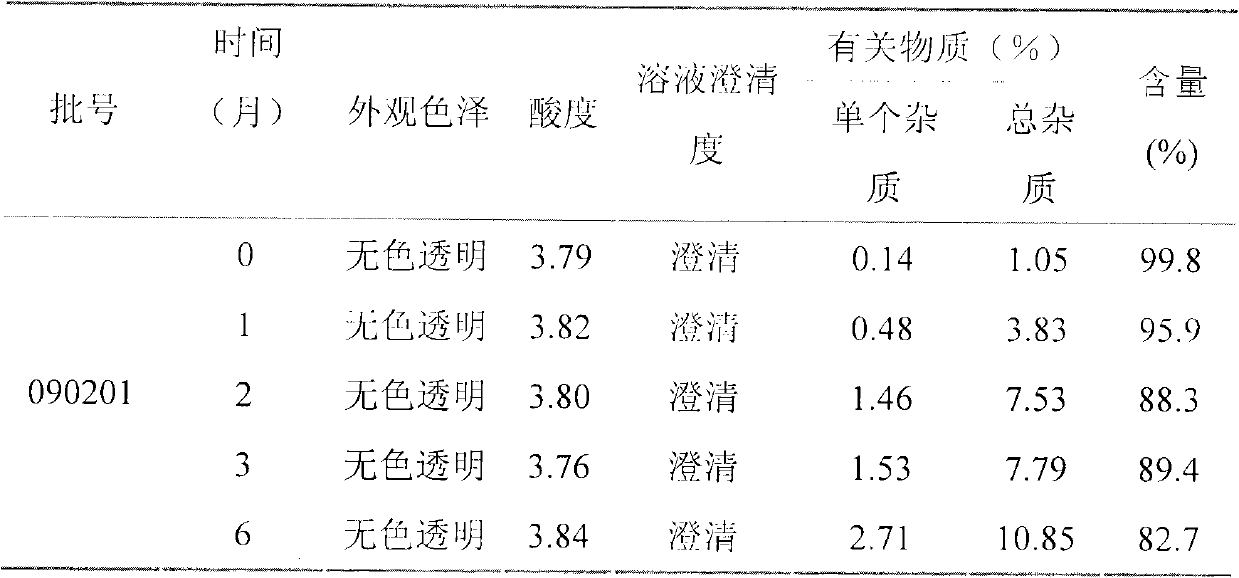
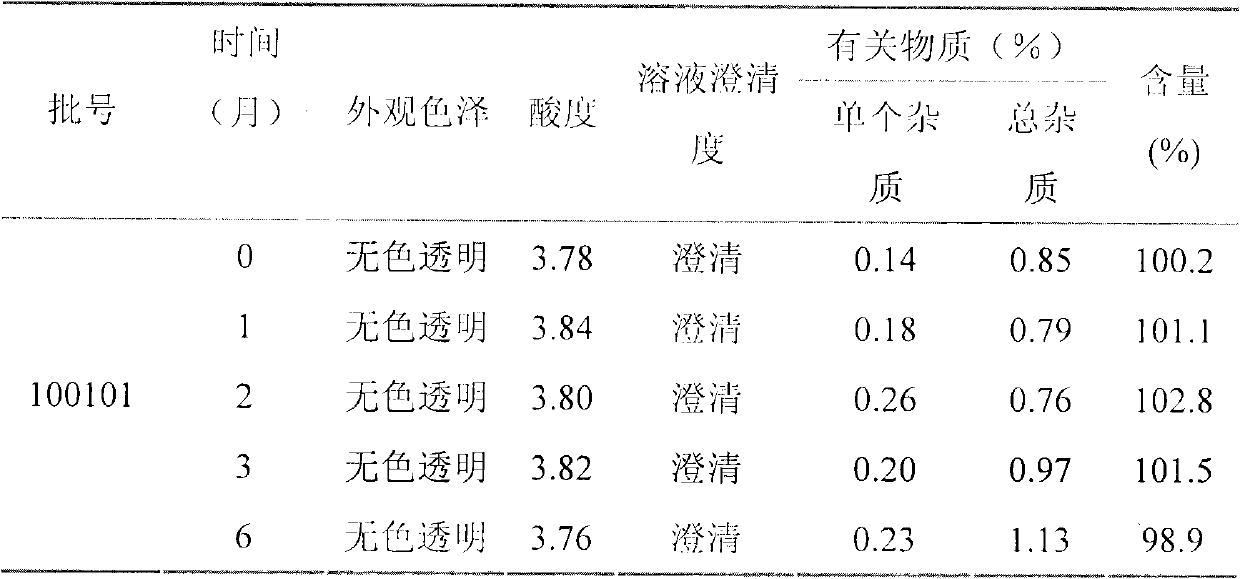
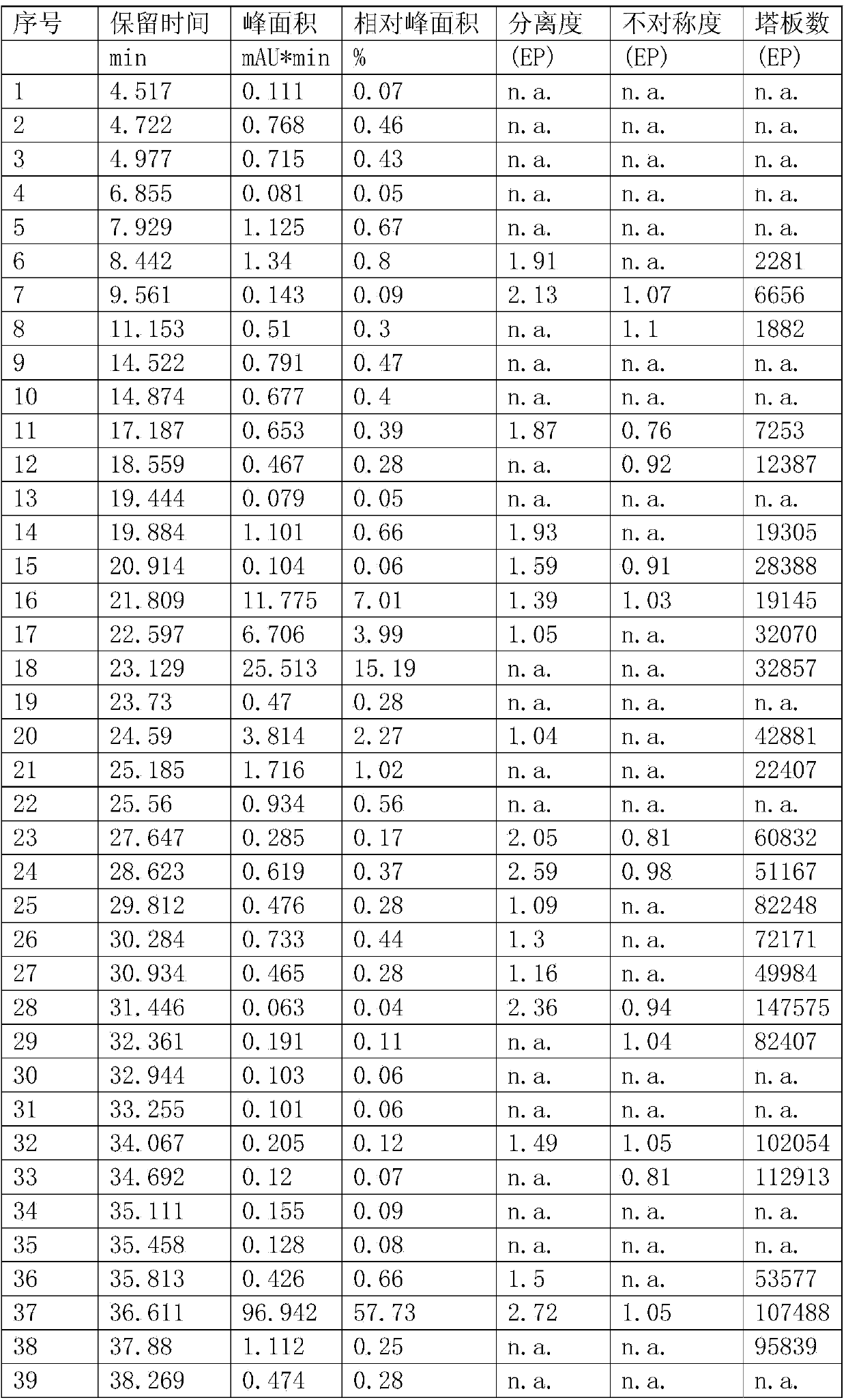
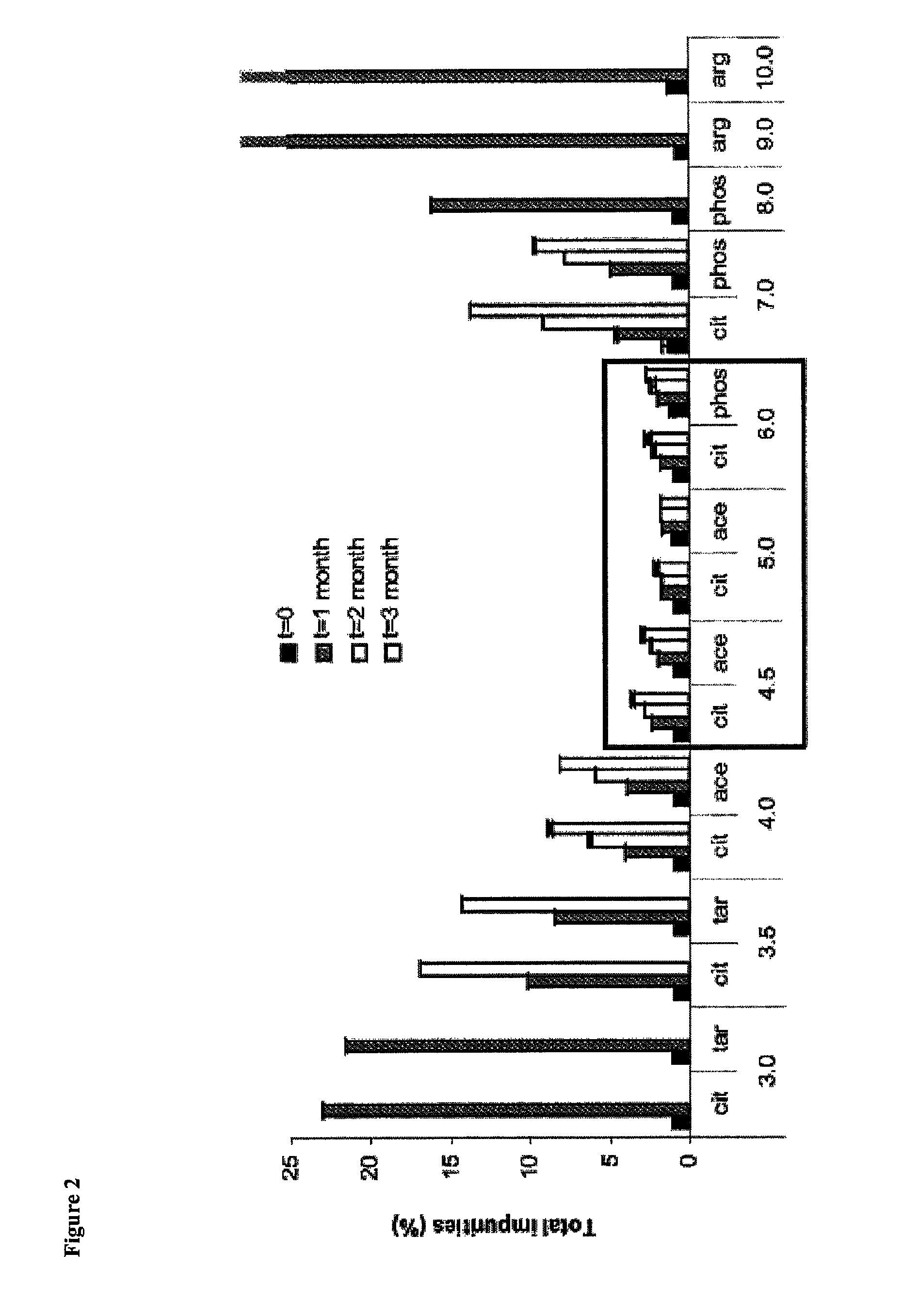
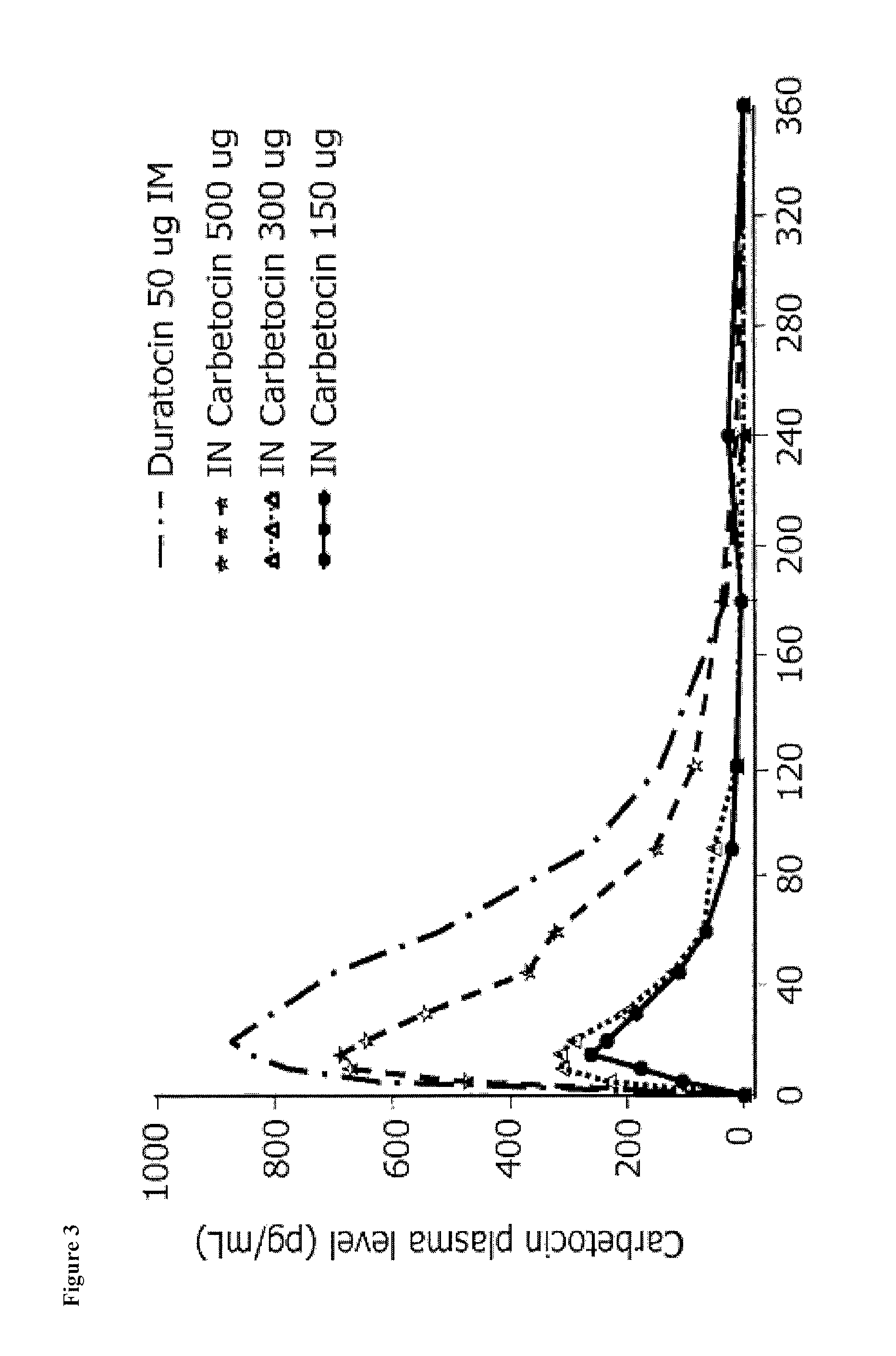
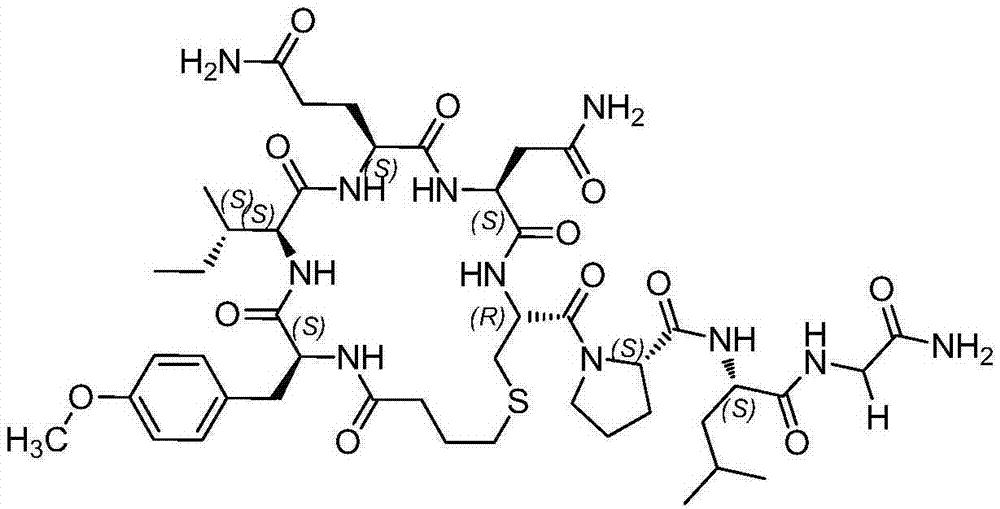
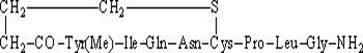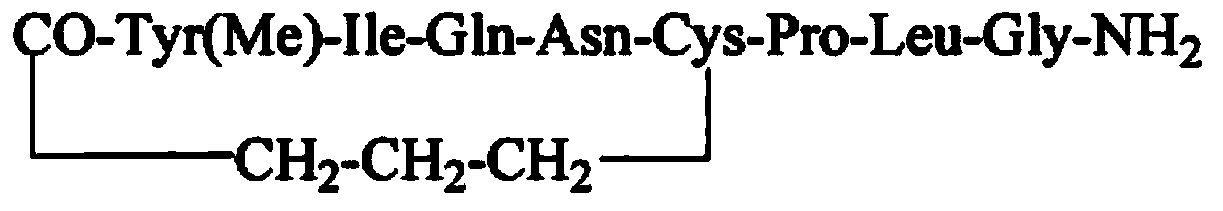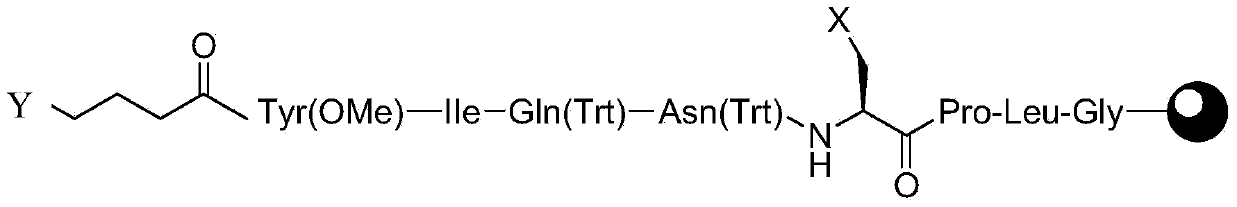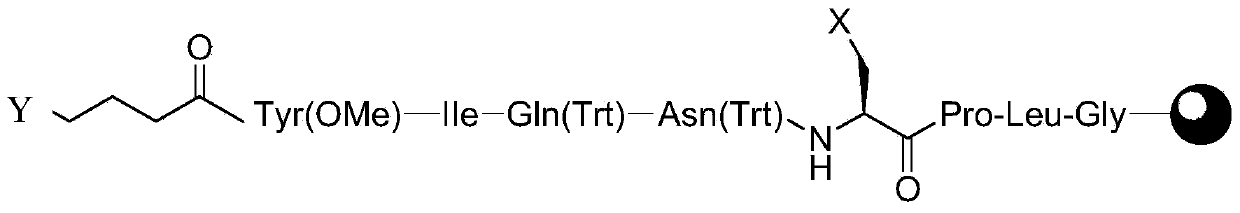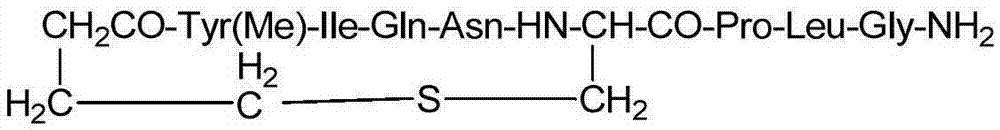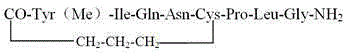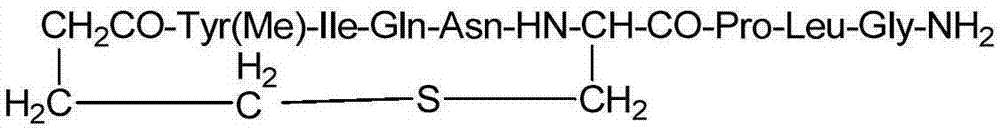Patents
Literature
74 results about "Carbetocin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
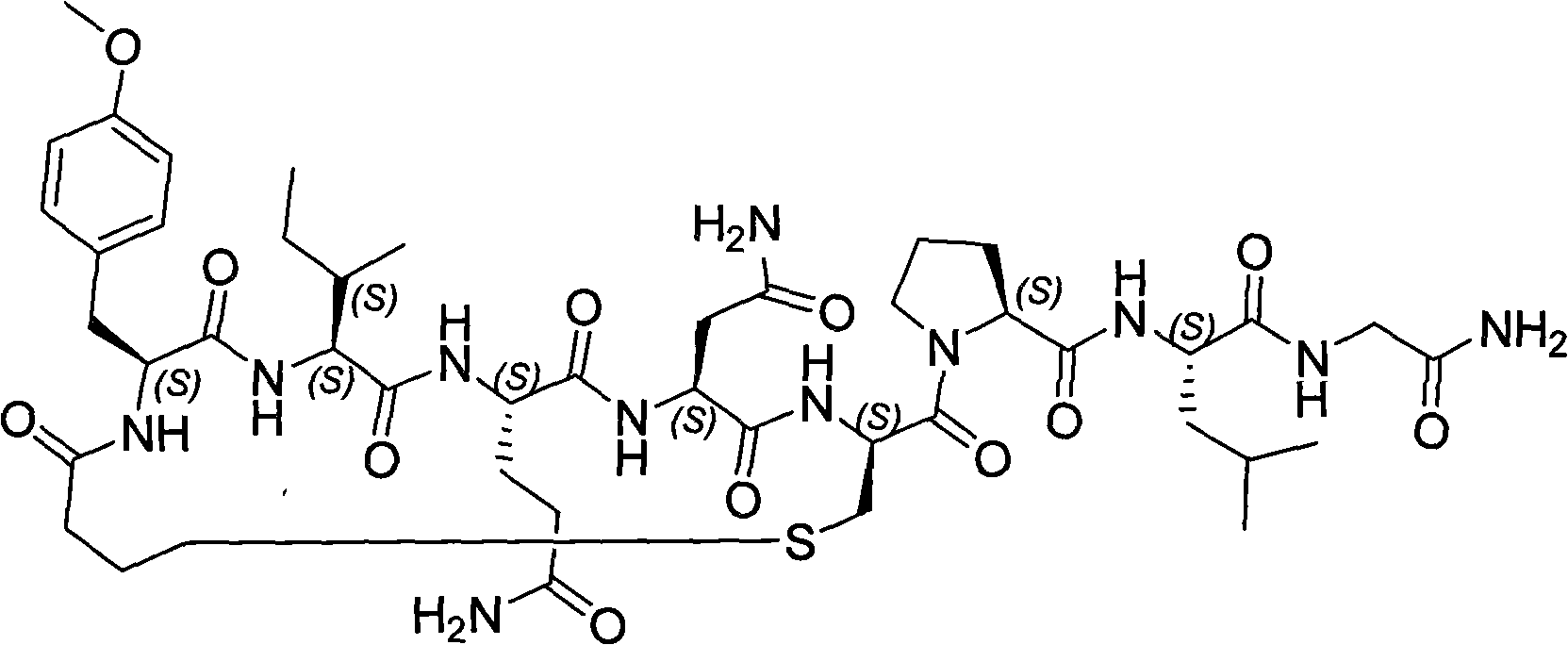
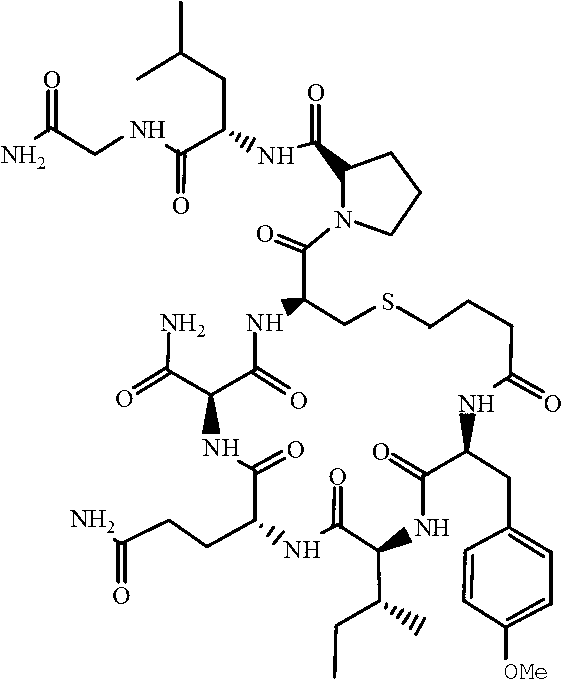
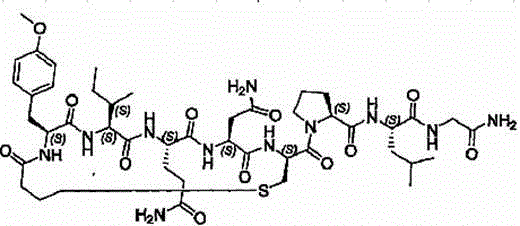
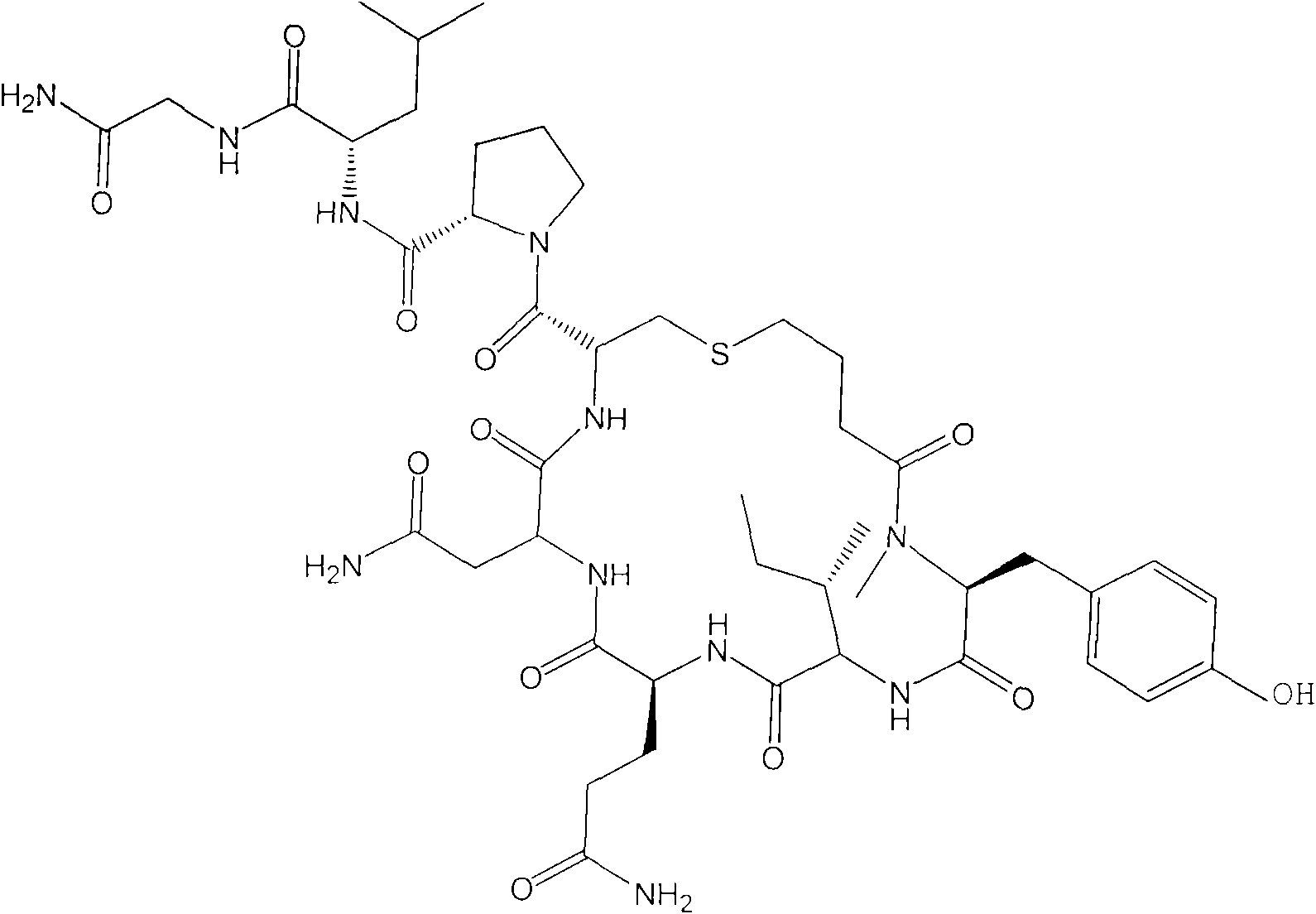
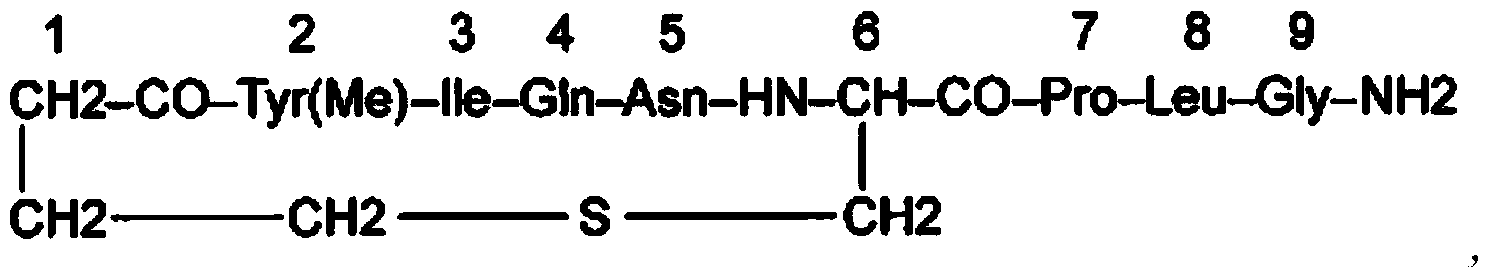
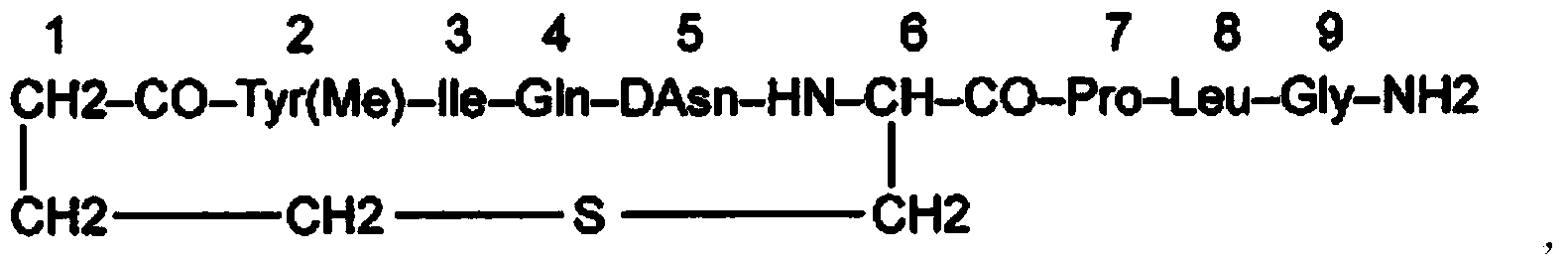
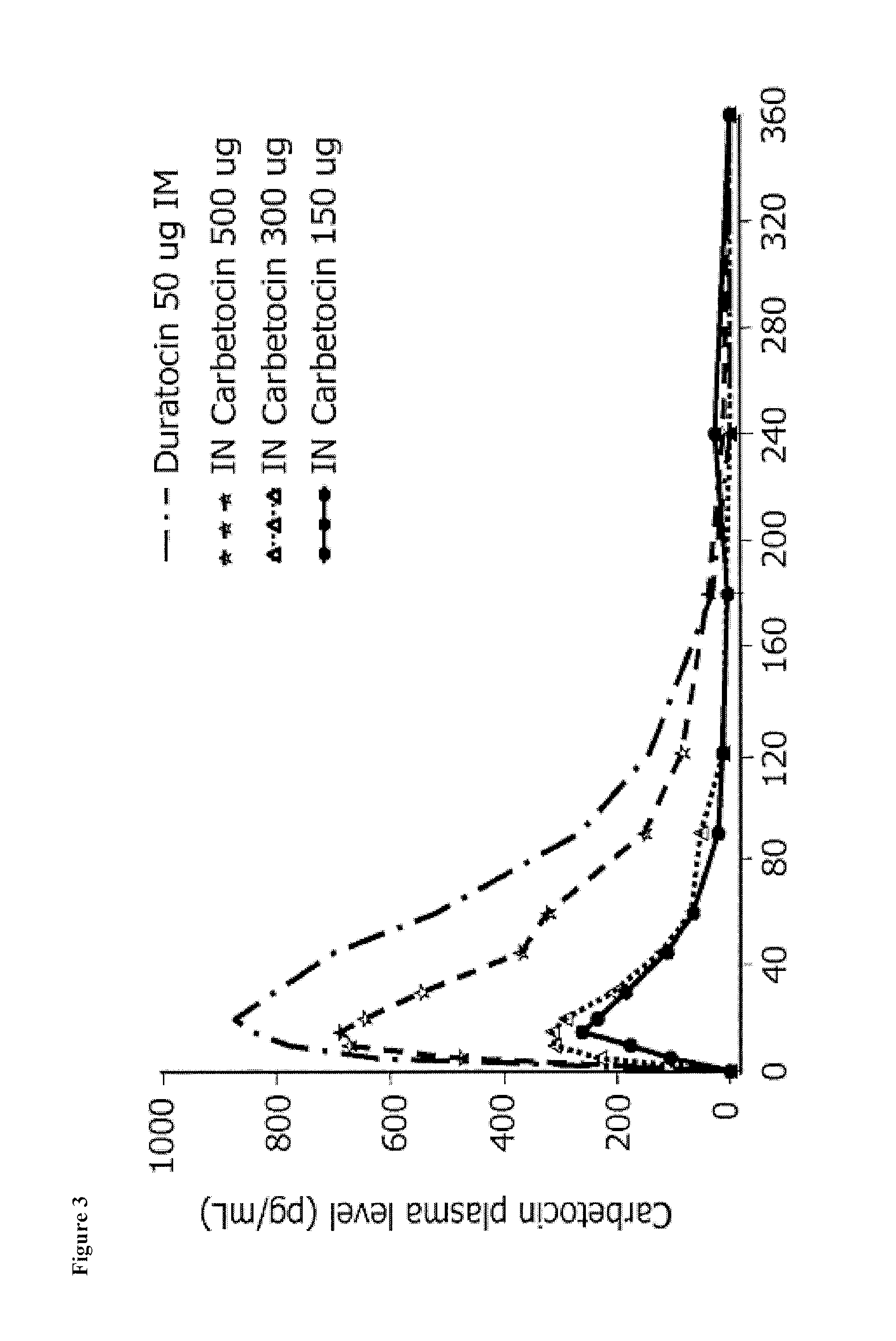
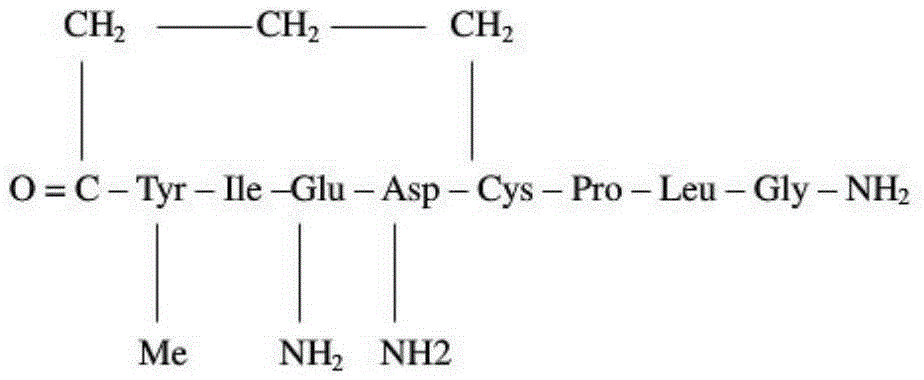
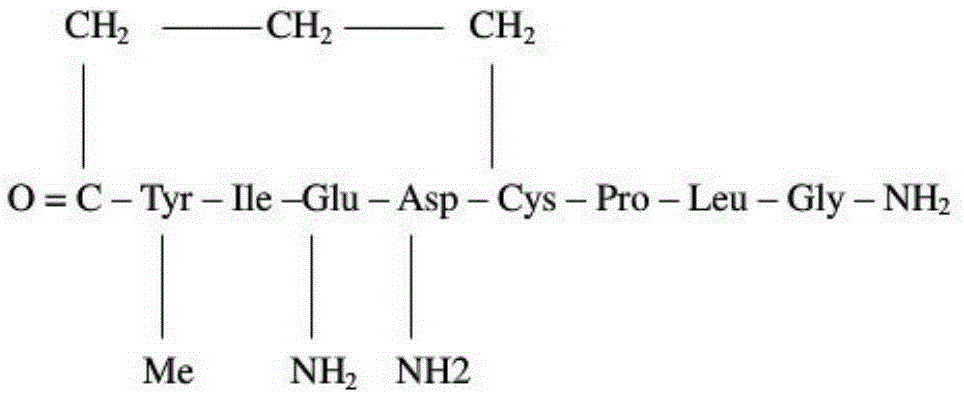
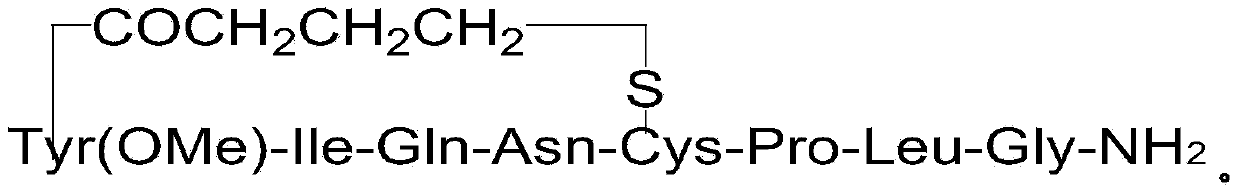
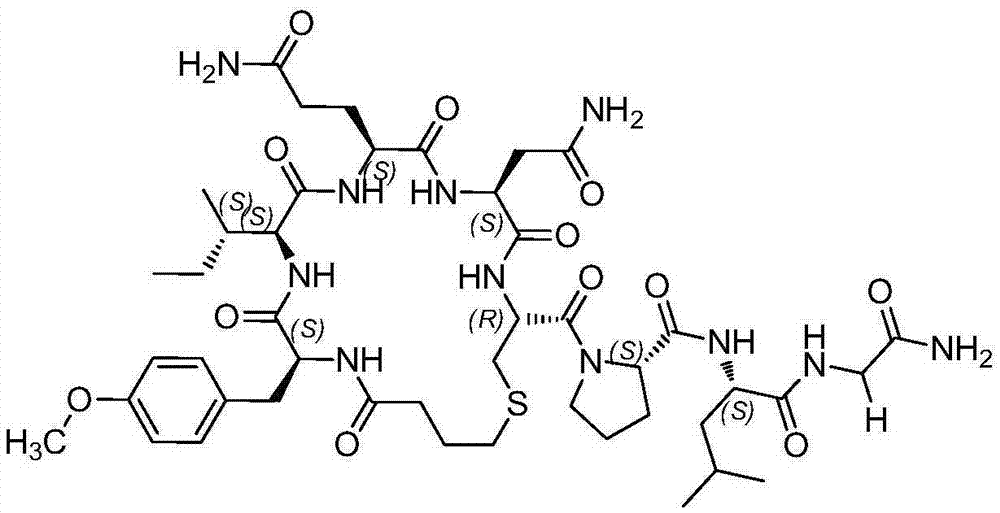
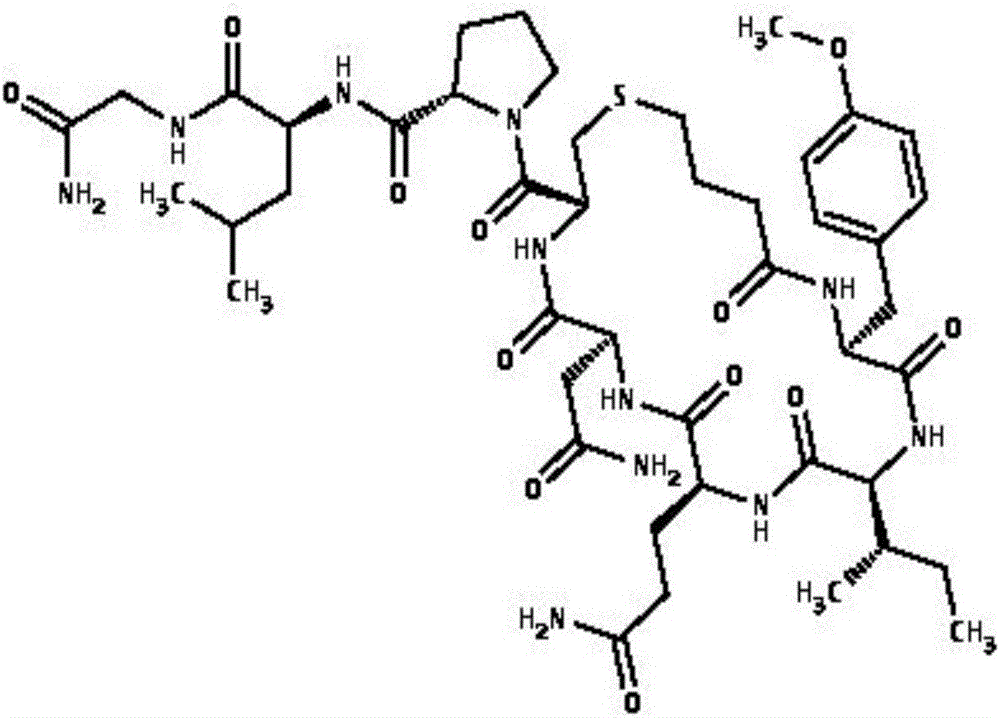
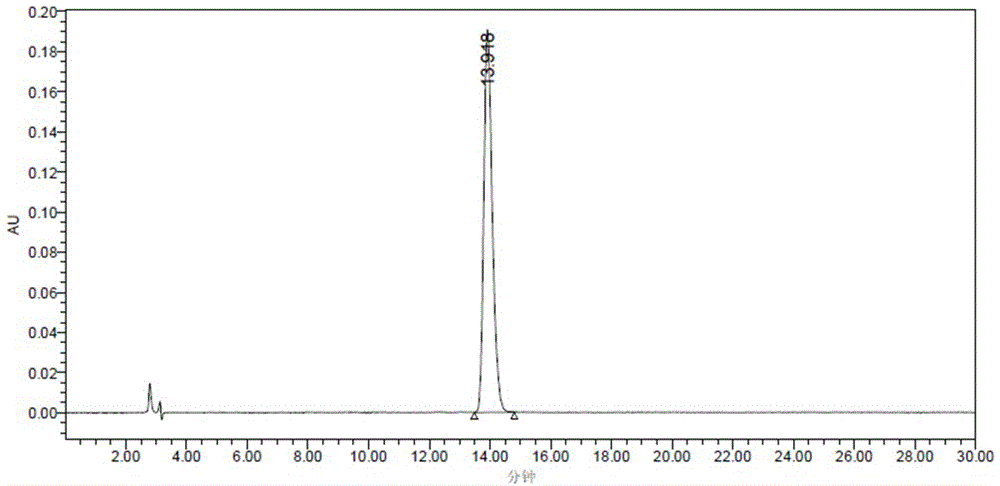
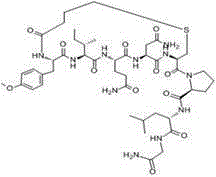
Carbetocin (INN, BAN) (brand names Duratocin, Pabal, Lonactene, Depotocin, Comoton, Decomoton), or 1-butanoic acid-2-(O-methy-L-tyrosine)-1-carbaoxytocin, is an oxytocic used in obstetrics to control postpartum hemorrhage and bleeding after giving birth, particularly following Cesarean section. It is an eight amino acid long analogue of oxytocin (a nonapeptide) and has a similar mechanism of action. Carbetocin is an agonist at peripherally expressed oxytocin receptors. It is manufactured by Ferring Pharmaceuticals and is available in Canada and the United Kingdom and many other countries throughout the world, but not in the United States.
Compositions and methods for the treatment of psychiatric disorders
InactiveUS20070032410A1Prevent and reduce occurrencePrevent and reduce and symptomBiocideNervous disorderDiseaseSocial withdrawal
Methods and compositions containing oxytocin or an oxytocin analog, specifically carbetocin, are provided for the prevention and treatment of autism spectrum disorders, related disorders and symptoms of such disorders. The methods and compositions of the invention are effective in the treatment of social withdrawal, eye contact avoidance, repetitive behaviors, anxiety, attention deficit, hyperactivity, depression, loss of speech, verbal communication difficulties, aversion to touch, visual difficulties, comprehension difficulties, and sound and light sensitivity. Additional compositions and methods are provided which employ oxytocin or an oxytocin analog in combination with a secondary or adjunctive therapeutic agent to yield more effective treatment tools against autism spectrum disorders and related disorders.
Owner:KYALIN BIOSCI
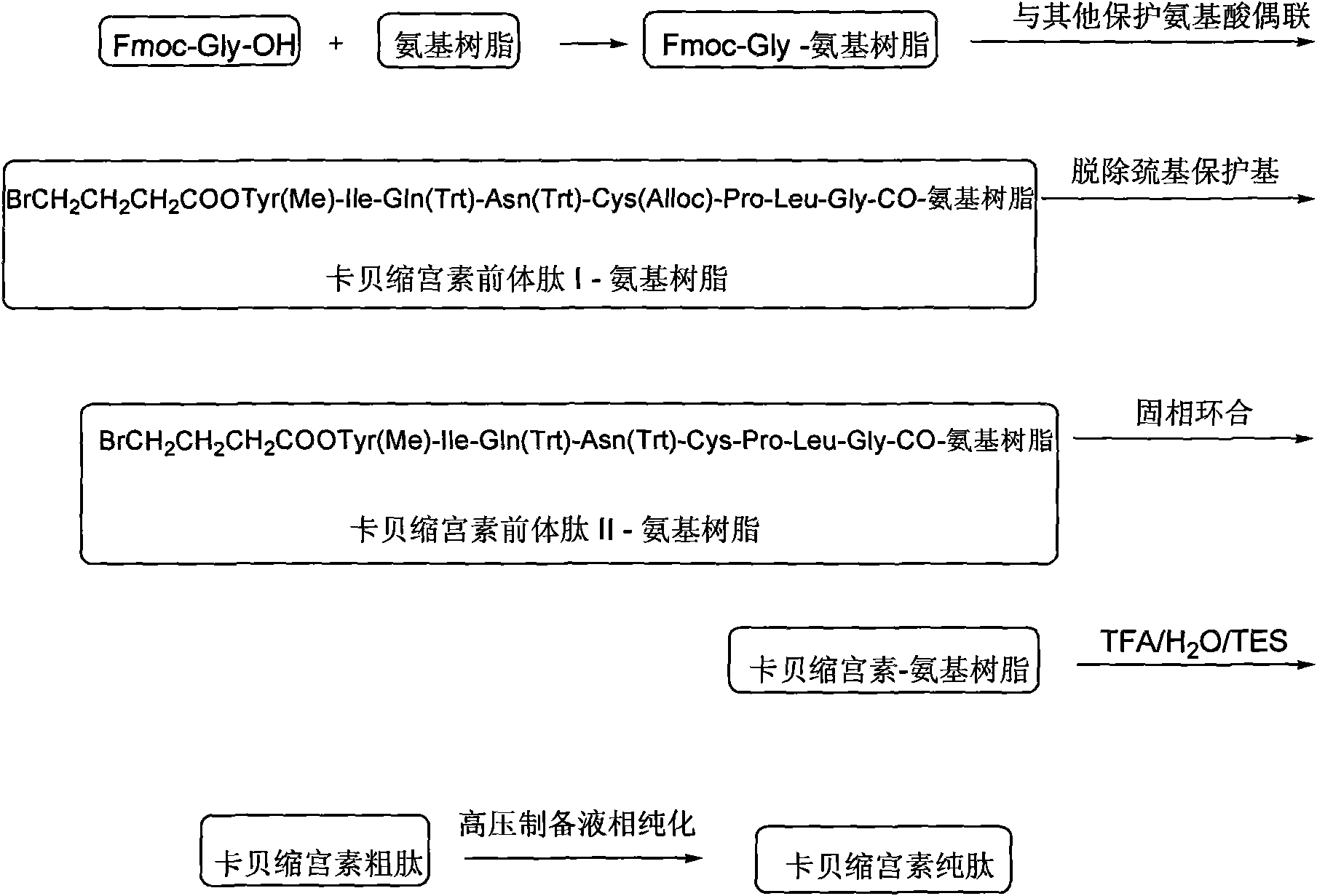
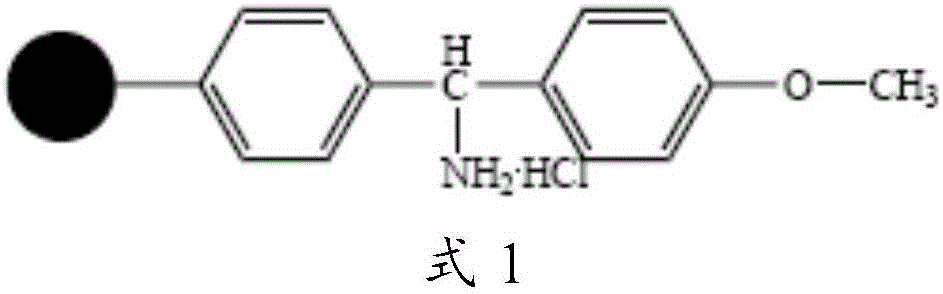
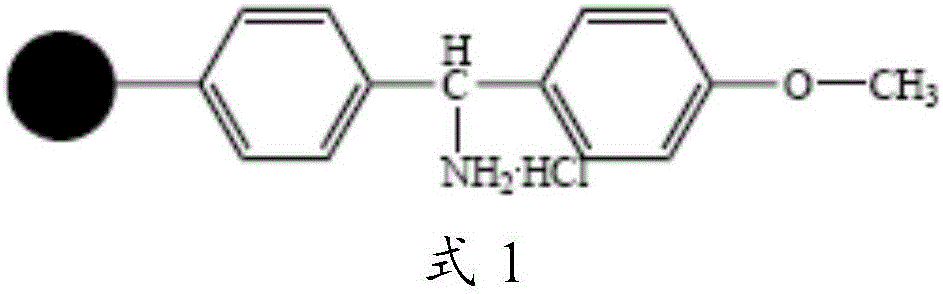
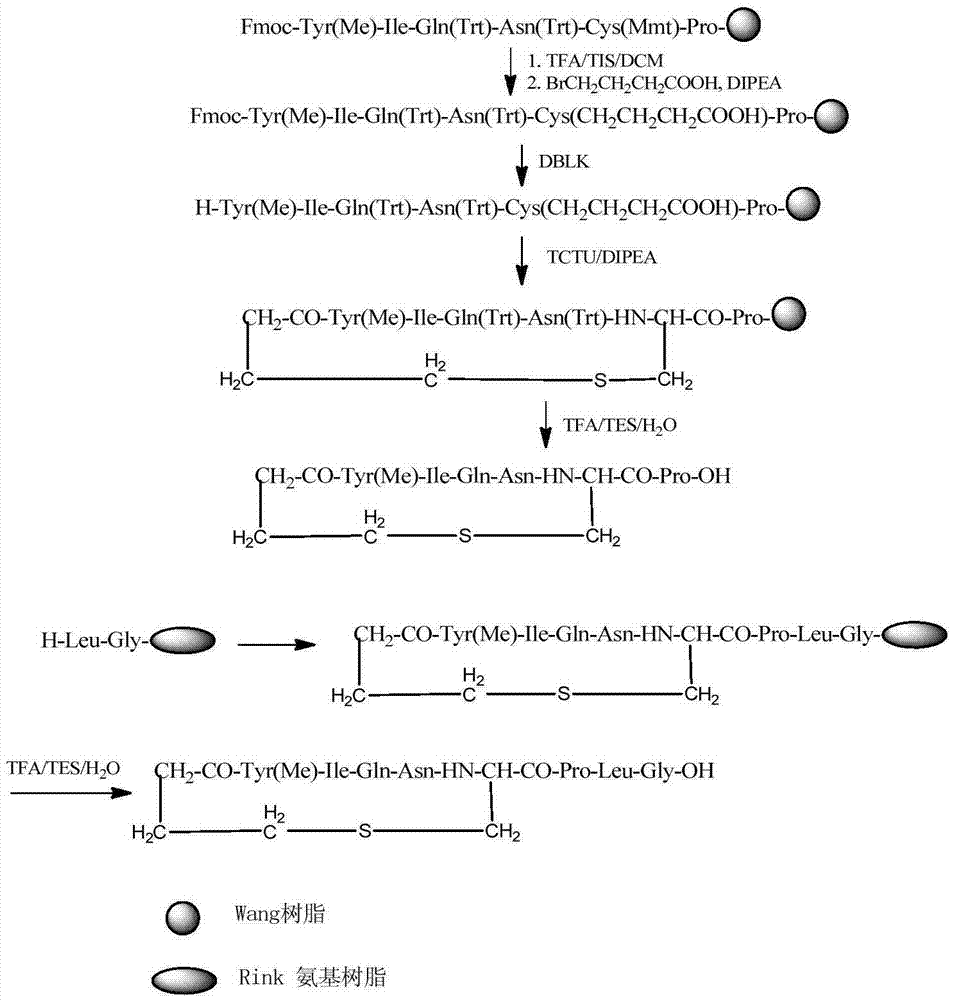
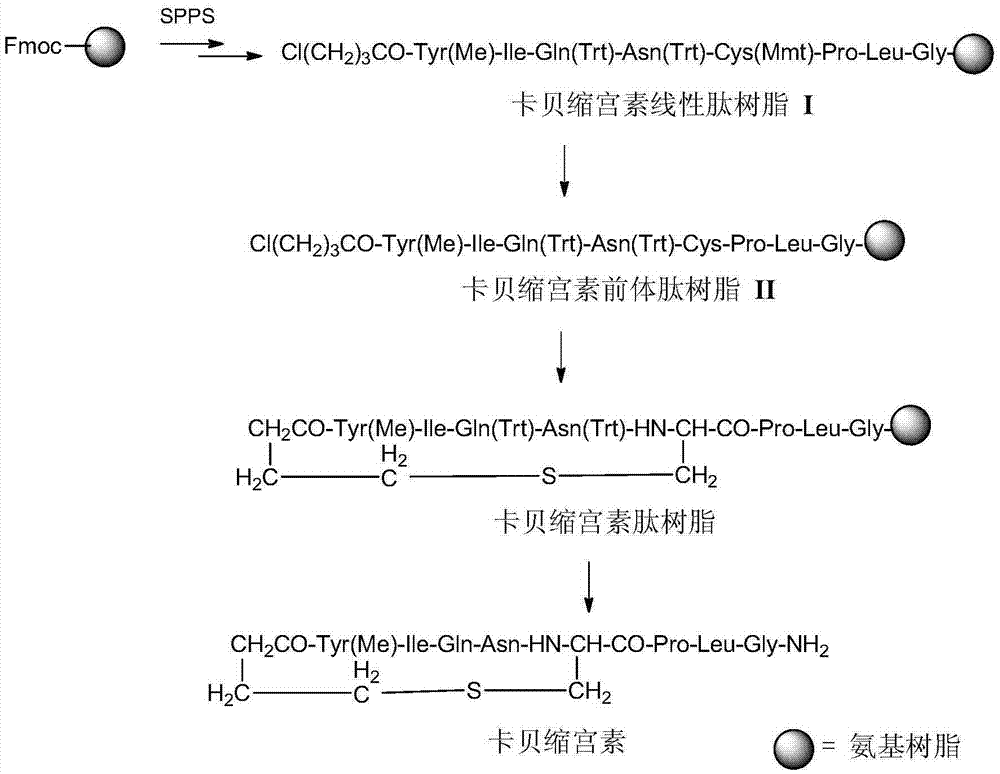
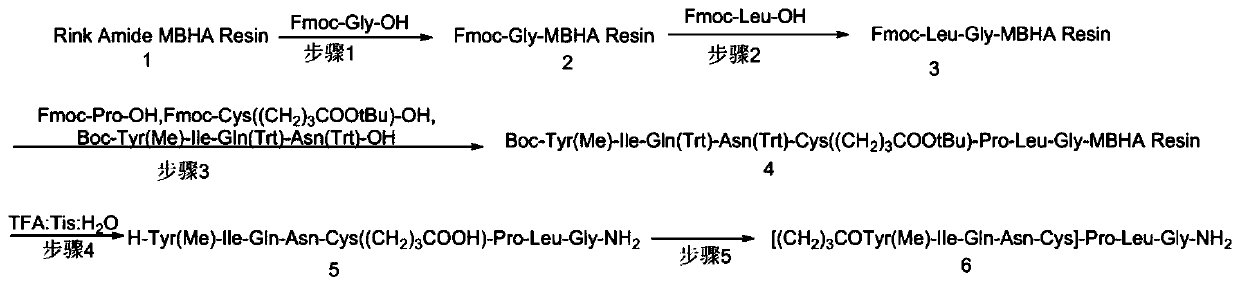
Solid phase preparation method of carbetocin
ActiveCN101555272AReduce investmentEasy to operatePeptide preparation methodsSexual disorderLithium chlorideFreeze-drying
The invention discloses a solid phase synthesis method of carbetocin. The technical proposal comprises the following steps of: obtaining Fmoc-Gly-amino resin by reaction of Fmoc-Gly-OH and amino resin with the substitutability being 0.2 mmol / g-0.9 mmol / g; sequentially connecting amino acids with Fmoc protecting groups by the solid phase synthesis method to obtain carbetocin precursor peptide I-amino resin; stripping off cysteine side chain protecting groups to obtain carbetocin precursor peptide II-amino resin; adding organic alkali and lithium chloride in solvent for cyclization to obtain carbetocin-amino resin; cracking to obtain carbetocin crude peptide; and purifying and freeze-drying to obtain the carbetocin. The method adopts the amino resin to synthesize carbetocin by the solid phase cyclization technology. The process is characterized by simple operation, easy post-treatment, high yield, low cost, and the like, and has considerable economical and practical value and broad application prospect.
Owner:HYBIO PHARMA
Solid-phase synthesis method of carbetocin
InactiveCN102796178ACheap and easy to getMild conditionsOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingCarbetocin
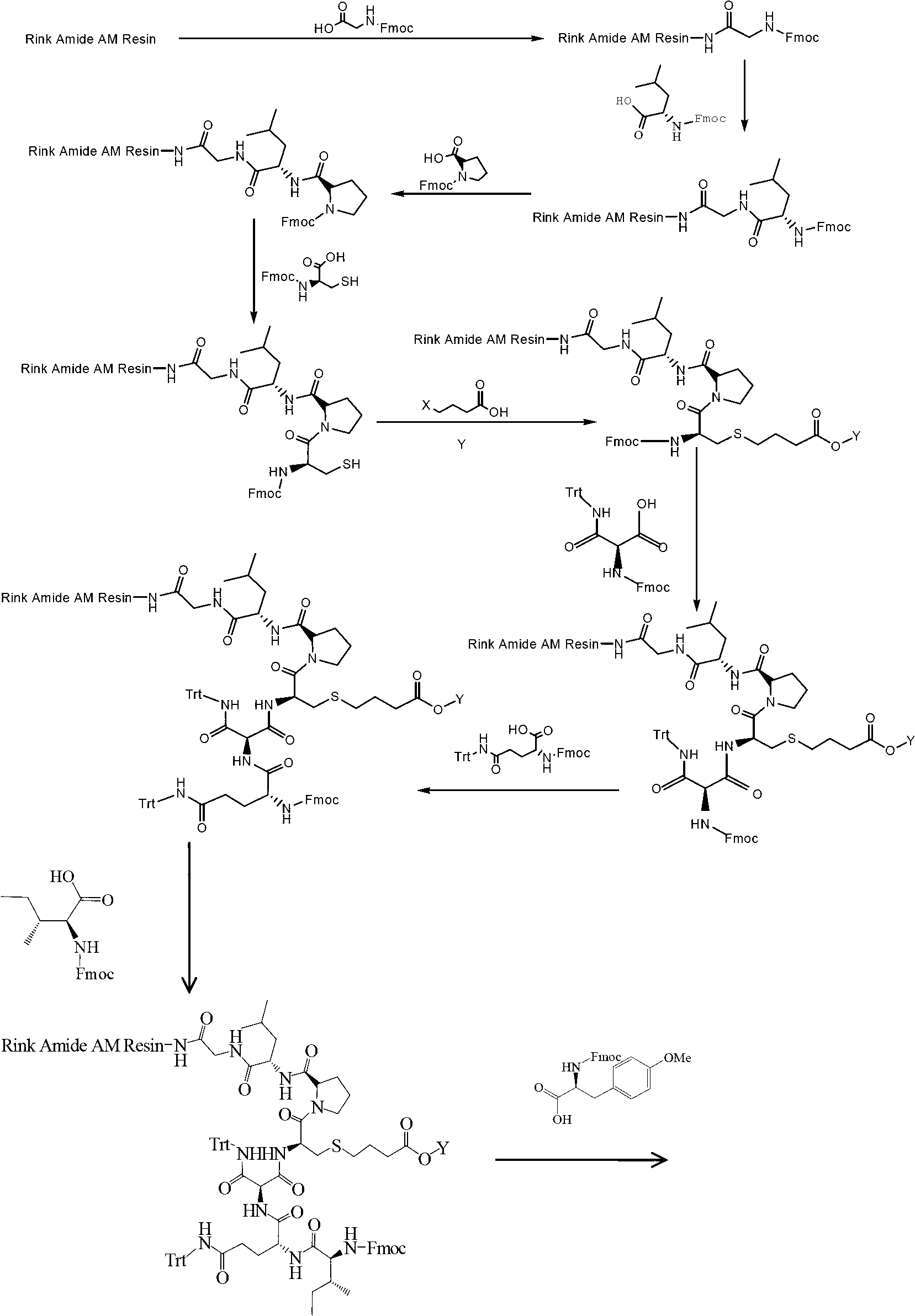
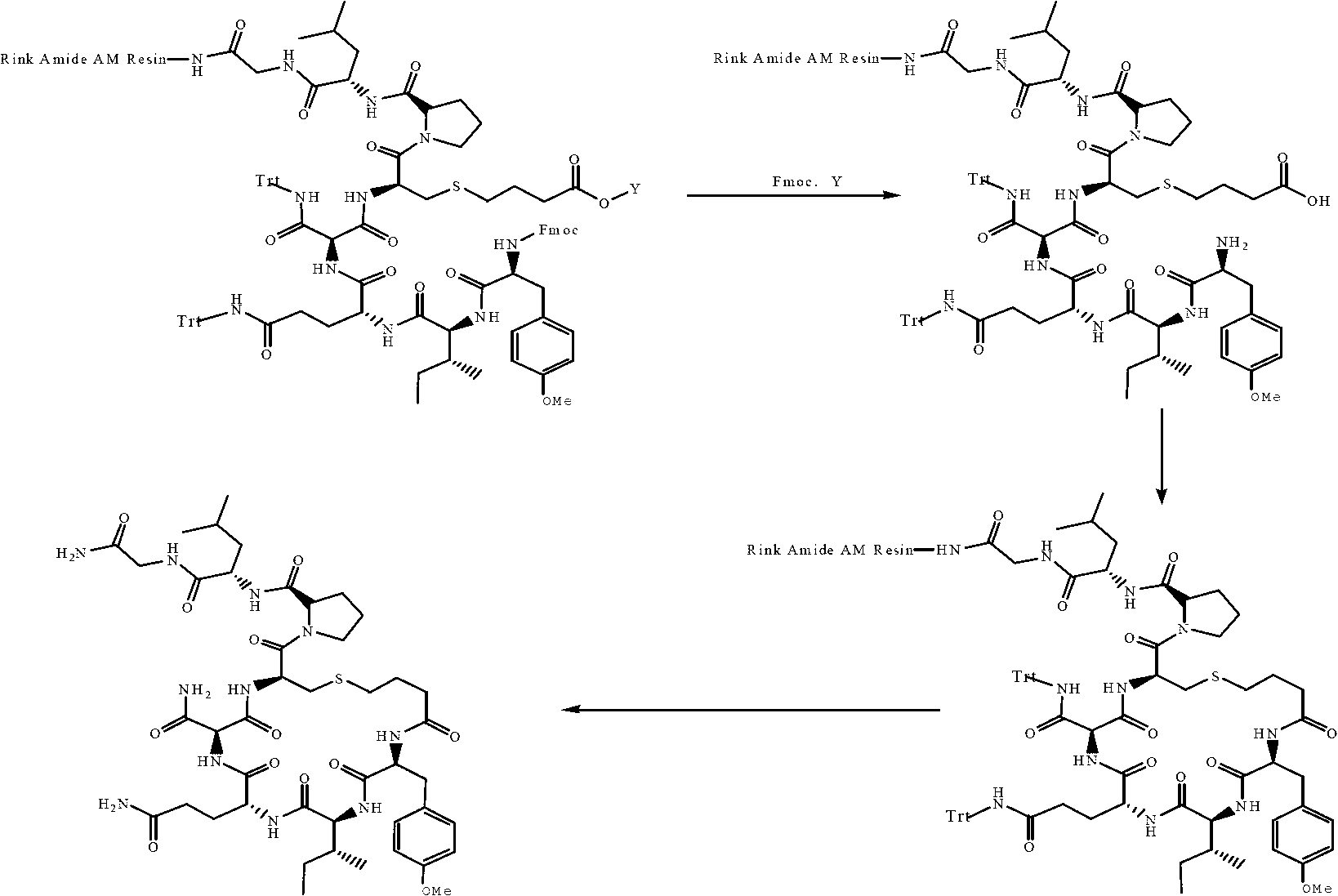
The invention provides a solid-phase synthesis method of carbetocin, and the method comprises the following steps of: sequentially coupling amino acid containing Fmoc (fluorenylmethoxycarbonyl) protecting group by Rink Amide AM Resin to obtain Fmoc-Cys(CH2CH2CH2COOCH2CH2CN)-Pro-Leu-Gly-Rink Amide AM Resin; then sequentially coupling the remaining Fmoc-amino acid, removing the protecting group, and cyclizing to obtain carbetocin-Amide AM Resin; and cracking, purifying and freeze-drying to obtain the carbetocin. The carbetocin is subjected to solid-phase cyclization synthesis by using a novel mercapto protecting group and a false solid-phase dilution principle. The solid-phase synthesis method is simple to operate, low in cost and environmentally-friendly, and meanwhile can be used for greatly improving the purity and the yield as a solid-phase cyclization technology is used at the same time.
Owner:WUXI KAILI PHARMA
Long-acting oxytocin analogues for the treatment and prevention of breast cancer and psychiatric disorders
InactiveUS6894026B1Prevent and alleviate symptomInhibit initiation and growthOrganic active ingredientsNervous disorderDiseaseVein
Methods and compositions are provided for prophylaxis and treatment of breast cancer involving administration of a therapeutically effective amount of carbetocin and / or other long-acting oxytocin analogues. 1-Butanoic acid-2-(O-methyl-L-tyrosine)-1-carbaoxytocin (carbetocin) and / or other long-acting oxytocin analogues are formulated with a pharmaceutically acceptable carrier and administered in an amount sufficient to inhibit initiation or growth of breast cancer in the patient. The carbetocin and / or other long-acting oxytocin analogues may also be formulated with a pharmaceutically acceptable carrier and administered in an amount sufficient to treat, prevent or alleviate the symptoms of a psychiatric disorder in the patient. Carbetocin may be administered prophylactically or to treat existing conditions in patients by a variety of administration modes, including intramuscular, intravenous, intranasal, intrapulmonary, subcutaneous, parenteral, oral, or transdermal delivery methods and formulations. Preferably, the carbetocin is administered to a mucosal surface of the patient via intranasal delivery. For this purpose, pharmaceutical compositions are provided for intranasal delivery that incorporate carbetocin in a powder or aqueous formulation for intranasal delivery.
Owner:KYALIN BIOSCI
Preparation method of polypeptide synthesis carbetocin
ActiveCN102167723AConvenient sourceHigh peptide yieldPeptide preparation methodsCarbetocinDiethyl ether
The invention discloses a preparation method of polypeptide synthesis carbetocin. The technical scheme of the method comprises the steps of taking Rink Amide MBHA (methylbenzene hydrogen amine) resin as an initial raw material; taking Fmoc-protected amino acid as a monomer; sequentially connecting amino acid, wherein Cys adopts Fmoc-Cys (CH2CH2CH2COOR)-OH, and the last amino acid uses Fmoc-Tyr (Me)-OH, and obtaining protected nonapeptide resin; removing the Fmoc azyl protected group of Tyr; cutting straight chain peptide from the resin by peptide cutting reagents such as FTA (fault tree analysis) and the like; precipitating a straight chain peptide rough product by adding diethyl ether; performing ring formation reaction by adding BOP (butyl octyl phthalate) / HOBt (oxhydryl benzotriazole) / DMF (dimethyl formamide); and purifying the preparation type HPLC (high performance liquid chromatography) in a separating way to obtain a target product. After the method is used, the each-step peptide connecting yield is more than 99%, the peptide cutting yield is 96.2%, and the gross yield is as high as more than 35%. The preparation method is convenient to industrial implementation, and has a higher industrial prospect.
Owner:SHANGHAI SOHO YIMING PHARMA
Synthesis process of carbetocin
InactiveCN104592362AHigh purityReduced operating requirementsOxytocins/vasopressinsPeptide preparation methodsSide chainCarbetocin
The invention provides a synthesis process of carbetocin. The synthesis process comprises the following steps: performing a coupling reaction on Fmoc-Gly-OH with Rink Amide-AM Resin obtained in the first step to obtain Fmoc-Gly-Rink Amide-AM Resin; performing deprotection (20% piperidine) with DBLK to obtain H-Gly-Rink Amide-AM RFesin, and orderly completing the coupling of the H-Gly-Rink Amide-AM Resin with Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ile-OH, Fmoc-Tyr(Me)-OH and tetrachlorobutyric acid until carbetocin linear peptide resin is synthesized; mixing a cracking agent with the carbetocin linear peptide resin obtained in the fourth step to have a cracking reaction, thereby removing the Rink Amide-AM Resin and side chain protecting groups; cyclizing the carbetocin linear crude peptide into a carbetocin crude product, and separating and purifying the carbetocin crude product to obtain the carbetocin. The synthesis process has the advantages that the polymerization side reaction is prevented, the process route is greatly simplified, the production cost is reduced and the synthesis efficiency is improved; in addition, the purity of the finished product is high; in short, the synthesis process is convenient for large-scale production, and meanwhile, advantageous for environmental protection, and has remarkable economic and social benefits.
Owner:苏州天马医药集团天吉生物制药有限公司
Process for producing medicament with uterine contraction effect
The invention relates to a process for producing a uterine contraction medicament which uses carbetocin as a main drug. The process comprises a synthesis process and a preparation process. The technical scheme of solid-phase synthesis comprises the following steps of: 1, carrying out deprotection by using amino resin as a vector; 2, connecting carboxyl of Fmoc-Gly-OH with amino of the resin to obtain Fmoc-Gly-amino resin; 3, carrying out solid-phase synthesis sequentially to form sequence residual protection amino acid; 4, removing side-chain protecting groups of cysteine; 5, connecting brombutyl acid onto sulfydryl of cysteine; 6, removing terminal amino Fmoc protection; 7, carrying out solid-phase cyclization; 8, cutting to obtain carbetocin crude peptides; and 9, refining to obtain a carbetocin pure product. The process has the characteristics of simple reaction operation, easy post treatment, high yield, low cost and the like. The invention also discloses a process for preparing a carbetocin freeze-dried powder injection which mainly comprises the following material compositions: carbetocin serving as the main drug, an excipient, a pH (potential of hydrogen) value regulator and water for injection. The powder injection has the advantages of high dispersion degree, high stability and the like.
Owner:SHENZHEN JYMED TECH
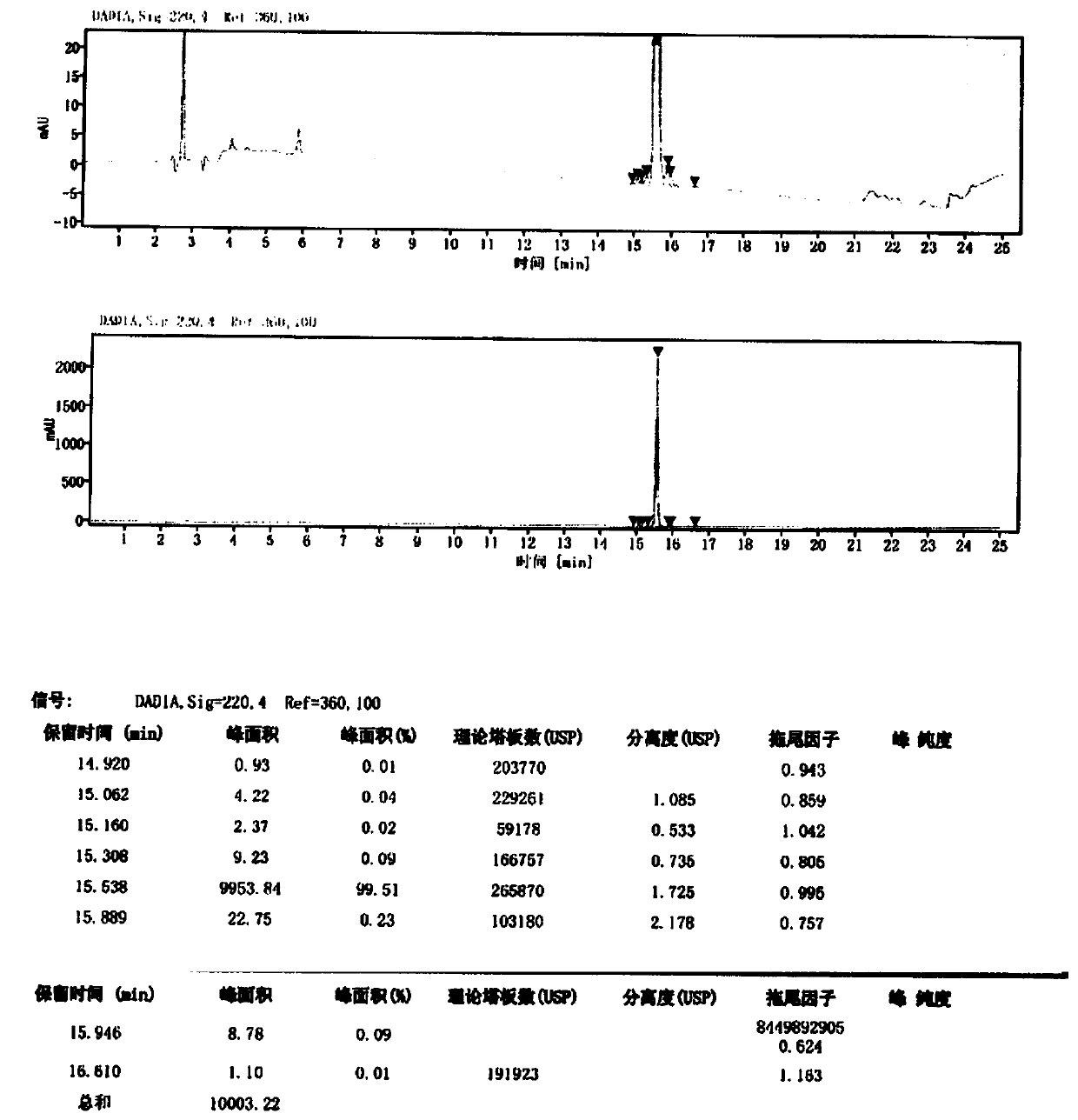
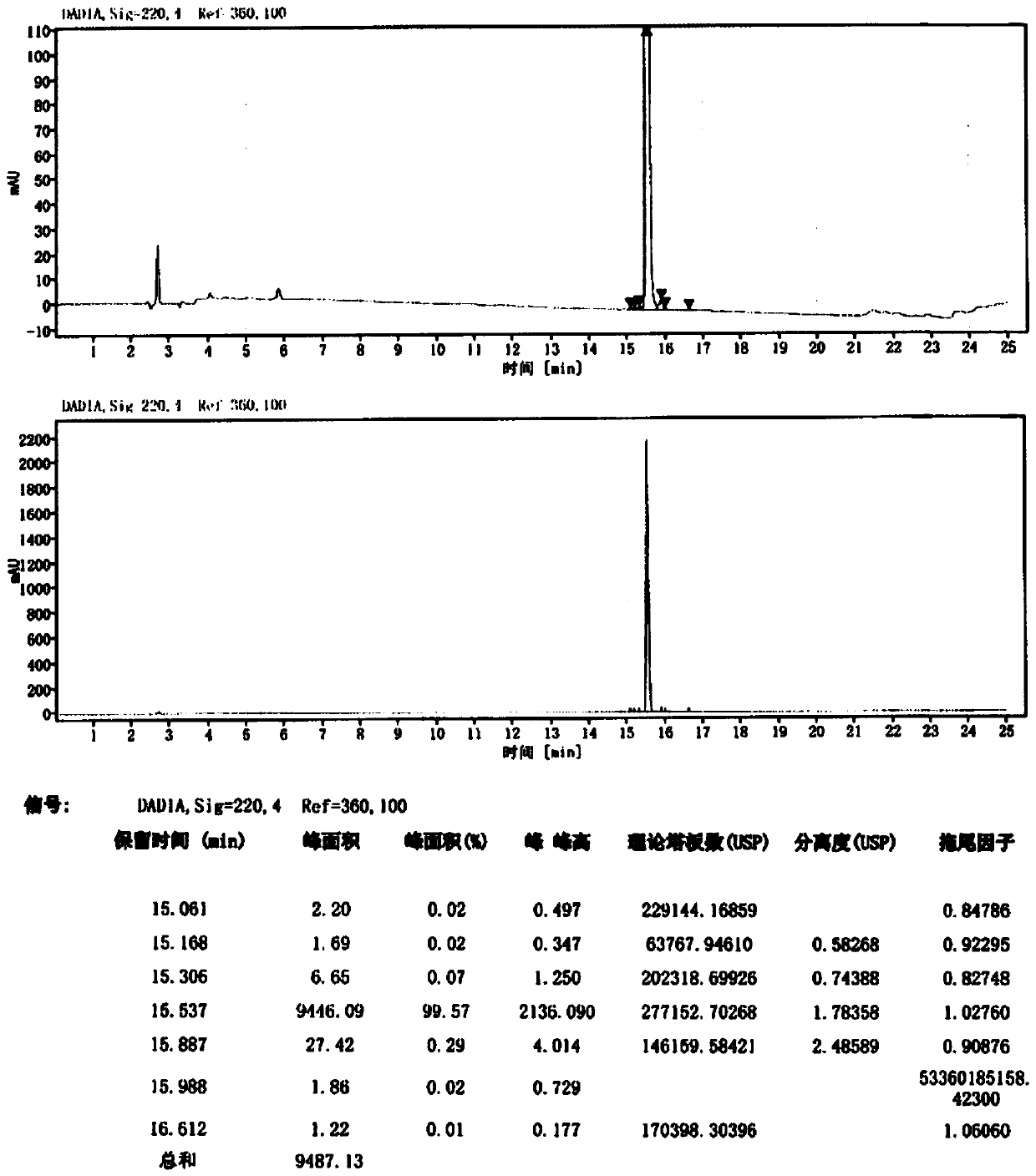
Method for purifying Carbetocin
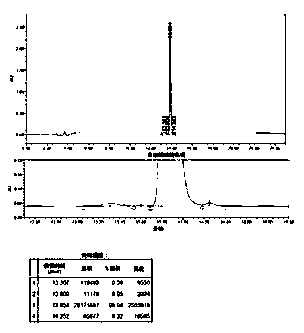
The invention provides a technological method suitable for the industrialized purification of Carbetocin, a reversed-phase efficient liquid chromatography is used to purify the Carbetocin to result in high purity and good yield in order to meet industrialized demands. A crude peptide solution of the Carbetocin is filled with materials by a reversed-phase chromatographic column to be a stationary phase, a phosphate buffer solution is regarded as phase A and acetonitrile is regarded as phase B to implement gradient elution purification, wherein, the gradient: B%:20-40%, the pH of the phase A is 2.5-3.5; an anion exchange method is employed to convert the phosphate and trifluoroacetate to acetate. The invention employs one-step reversed-phase efficient liquid chromatography to purify followed by the acetate conversion by an anion exchange column in one step, thus obtaining high-purity acetate Carbetocin at high yield and offering an efficient purification technology for the massive purification and preparation of the peptide raw drugs.
Owner:HYBIO PHARMA
Carbetocin injection and preparation method thereof
ActiveCN104055732APeptide/protein ingredientsPharmaceutical delivery mechanismUterus atonyGynecology
The invention belongs to the technical field of medicines, relates to a carbetocin analogue, particularly relates to carbetocin, and more particularly relates to a carbetocin injection with excellent pharmaceutical performance. The carbetocin can be used for preventing uterine atony and postpartum hemorrhage after selective epidural or lumbar anesthesia cesarean section. The invention also relates to a preparation method of the carbetocin injection, further relates to medical application of the carbetocin. Particularly relates to the carbetocin injection disclosed by the invention contains carbetocin, saccharides and injection water.
Owner:CHENGDU TIANTAISHAN PHARMA
Intranasal carbetocin formulations and methods for the treatment of autism
InactiveUS20120172304A1Prevent and reduce occurrencePrevent and reduce and symptomOrganic active ingredientsNervous disorderSocial withdrawalAttention deficits
Methods and compositions containing oxytocin or an oxytocin analog, such as carbetocin, are provided for the prevention and treatment of autism spectrum disorders, related disorders and symptoms of such disorders. The methods and compositions of this disclosure are effective in the treatment of social withdrawal, eye contact avoidance, repetitive behaviors, anxiety, attention deficit, hyperactivity, depression, loss of speech, verbal communication difficulties, aversion to touch, visual difficulties, comprehension difficulties, and sound and light sensitivity. Additional compositions and methods are provided which employ oxytocin or an oxytocin analog in combination with a secondary or adjunctive therapeutic agent to yield more effective treatment tools against autism spectrum disorders and related disorders.
Owner:RETROPHIN
Carbetocin pharmaceutical composition and preparation method thereof
The invention relates to a stable pharmaceutical composition containing carbetocin, and a preparation method thereof. The stable pharmaceutical composition containing carbetocin contains carbetocin, a pH regulator, an isoosmotic regulator and pharmaceutically acceptable auxiliary materials. The composition is used for preventing uterine atony and postpartum hemorrhage after selective epidural or spinal anesthesia cesarean section. The pharmaceutical composition mainly exists in a liquid form and is used through parenteral administration forms, mainly injection and nasal spray.
Owner:HYBIO PHARMA
Intranasal carbetocin formulations and methods for the treatment of autism
InactiveUS20100311655A1Prevent and reduce occurrencePrevent and reduce and symptomNervous disorderPeptide/protein ingredientsSocial withdrawalAttention deficits
Methods and compositions containing oxytocin or an oxytocin analog, such as carbetocin, are provided for the prevention and treatment of autism spectrum disorders, related disorders and symptoms of such disorders. The methods and compositions of this disclosure are effective in the treatment of social withdrawal, eye contact avoidance, repetitive behaviors, anxiety, attention deficit, hyperactivity, depression, loss of speech, verbal communication difficulties, aversion to touch, visual difficulties, comprehension difficulties, and sound and light sensitivity. Additional compositions and methods are provided which employ oxytocin or an oxytocin analog in combination with a secondary or adjunctive therapeutic agent to yield more effective treatment tools against autism spectrum disorders and related disorders.
Owner:KYALIN BIOSCI
Purification method of carbetocin
InactiveCN102977192AEasy to operateObvious cost advantageOxytocins/vasopressinsPeptide preparation methodsIon exchangeCombinatorial chemistry
The invention discloses a purification method of carbetocin. The method comprises the following steps in sequence: purifying and synthesizing rough titanium through reversed-phase ion-pair chromatography, and removing an ion-pair agent through ion exchange resin, so as to obtain fine carbetocin peptide with purity of 99.5%. According to the purification method of carbetocin, the reversed-phase ion-pair chromatography is adopted, so that the purification yield and the purity of the carbetocin can be greatly improved.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Method for synthesizing carbetocin through liquid-phase synthetic method
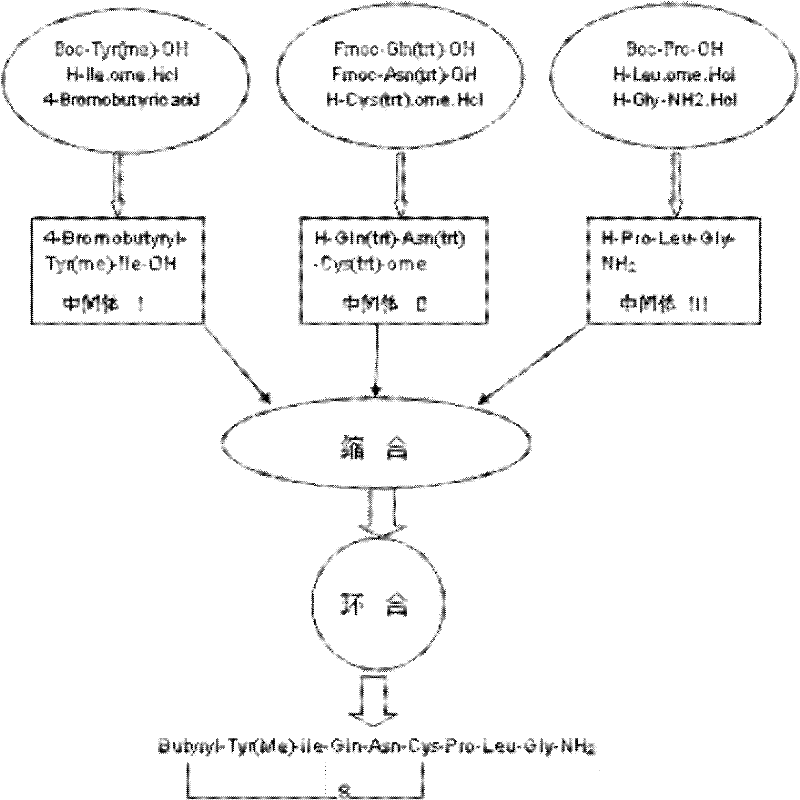
The invention discloses a method for synthesizingcarbetocin, comprising the following steps: first, respectively synthesizing an intermediate I: 4-Bromobutyryl-Tyr(me)-Ilw-OH, an intermediate II: H-Gln(trt)-Asn(trt)-Cys(trt)-ome, and an intermediate III: H-Pro-Leu-Gly-NH2, putting the intermediate I, II and III in a solvent, condensing a noncyclized product IV in the presence of condensing agent, finally adding a catalyst to cyclize in alkaline condition to obtain carbetocin. According to the invention, the yield of carbetocin is more than 85 %, the product purity is more than 80 %, the impurities are easy to remove, and the method has remarkable advantages.
Owner:HANGZHOU HEJIN TECH
Method for preparing carbetocin
ActiveCN104262464AReduce usageLow costOxytocins/vasopressinsPeptide preparation methodsHigh concentrationFreeze-drying
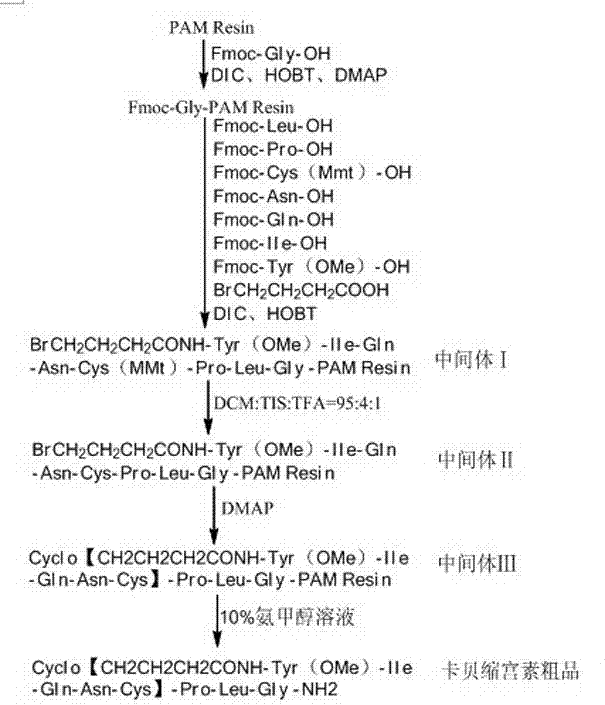
The invention relates to a solid-phase synthesis method of carbetocin, which comprises the following steps: reacting a PAM (polyacrylamide) resin and Fmoc-Gly-OH to obtain an Fmoc-Gly-PAM resin; sequentially connecting amino acid with Fmoc protective group by solid-phase synthesis to obtain a BrCH2CH2CH2CONH-Tyr(OMe)-Ile-Gln-Asn-Cys(Mmt)-Pro-Leu-Gly-PAM resin; removing the Mmt protective group in the Cys; carrying out cyclization reaction by using DMAP (dimethylaminopyridine) as a cyclization reagent to obtain Cyclo[CH2CH2CH2CO-Tyr(OMe)-Ile-Gln-Asn-Cys]-Pro-Leu-Gly-PAM; carrying out ammonolysis on the cyclization product with an ammonia methanol solution to obtain a carbetocin crude product; and purifying and freeze-drying to obtain the carbetocin. The total yield is up to higher than 45%. Compared with the conventional preparation method, the method provided by the invention avoids using abundant high-concentration strongly-corrosive acid and flammable and explosive aether, has the advantages of high yield, low cost, mild reaction conditions and small environmental pollution, and is beneficial to industrialized production.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
Intranasal carbetocin formulations and methods for the treatment of autism
Methods and compositions containing oxytocin or an oxytocin analog, such as carbetocin, are provided for the prevention and treatment of autism spectrum disorders, related disorders and symptoms of such disorders. The methods and compositions of this disclosure are effective in the treatment of social withdrawal, eye contact avoidance, repetitive behaviors, anxiety, attention deficit, hyperactivity, depression, loss of speech, verbal communication difficulties, aversion to touch, visual difficulties, comprehension difficulties, and sound and light sensitivity. Additional compositions and methods are provided which employ oxytocin or an oxytocin analog in combination with a secondary or adjunctive therapeutic agent to yield more effective treatment tools against autism spectrum disorders and related disorders.
Owner:NASTECH PHARMA
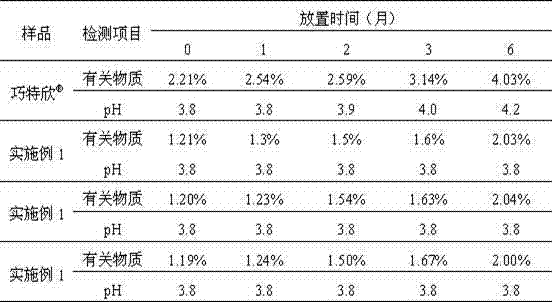
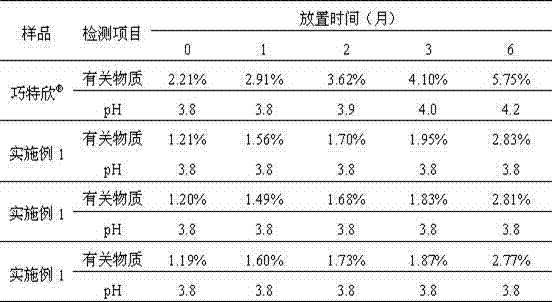
More-stable carbetocin acetate injection
InactiveCN102144965AQuality improvementDefinite curative effectPeptide/protein ingredientsPharmaceutical delivery mechanismActivated carbonAntioxidant
The invention relates to a more-stable carbetocin acetate injection. The preparation consists of carbetocin acetate, an isoosmotic adjusting agent, a pH value regulating agent, an antioxidant, a local analgetic and a solvent, wherein the addition of the antioxidant can improve the stability of the preparation, and the addition of the local analgetic can relieve pains of a patient but does not affect the basic remedy. The process for preparing the preparation mainly comprises the following steps: weighing, dissolving, adsorbing a heat source by activated carbon, filtering, filtering by a terminal, subpackaging and sealing. The preparation is used for preventing and treating insufficient uterus tension and postpartum hemorrhage. The preparation has stable quality and an exact curative effect, and can be accepted by patients.
Owner:SHENZHEN JYMED TECH
Method for purifying carbetocin
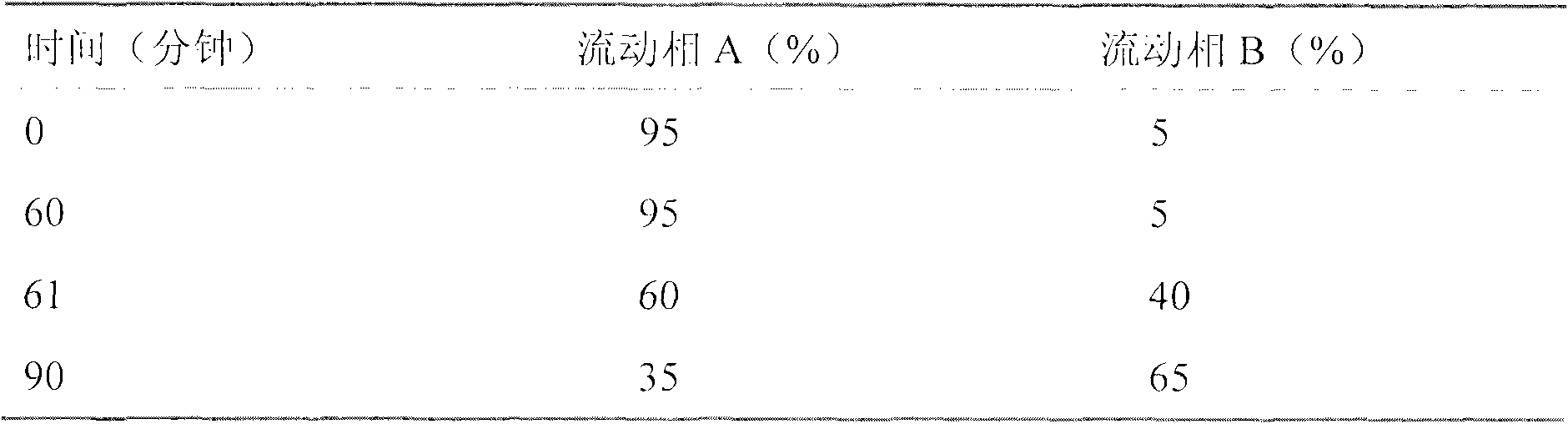
The invention relates to a method for purifying carbetocin. The method is characterized by comprising the following steps of: 1, dissolving a carbetocin crude product by using water, and regulating pH to be acidic; 2, arranging gradients according to volume fraction, and flushing a polymer reverse-phase filled column by using a mobile phase A; 3, loading a solution obtained from the step 1 into a polymer reverse-phase filler; 4, arranging the gradients according to the volume fraction, wherein a mobile phase B is 5% in the initial state of the eluted gradients and is kept for 2 minutes, then the proportion of the mobile phase B is increased to 25% within 5 minutes, and then the proportion of the mobile phase B is increased to 40% within 30 minutes; collecting all eluted distillates; 5, drying the eluted distillates to obtain the pure product carbetocin.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Method for preparing carbetocin
ActiveCN103833831AMeet the requirementsEasy to operateOxytocins/vasopressinsPeptide preparation methodsImidePolymer science
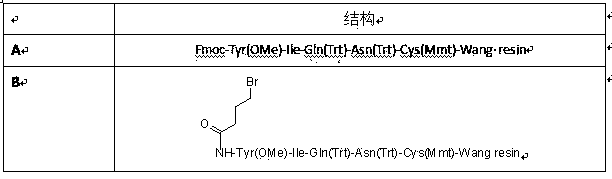
The invention discloses a method for preparing carbetocin. The method comprises the following steps: coupling Fmoc-Cys(Mmt)-OH with hydroxyl function group resin to obtain Fmoc-Cys(Mmt)-resin; removing Fmoc from the Fmoc-Cys(Mmt)-resin to obtain H-Cys(Mmt)-resin; coupling the Fmoc-Asn(Trt)-OH with the H-Cys(Mmt)-resin to obtain Fmoc-Asn(Trt)-Cys(Mmt)-resin; repeating the steps of removing Fmoc and condensation reaction according to a solid-phase synthesis method, so as to obtain peptide resin with a structure shown in A; removing the Fmoc from the A, and then reacting with bromine succinimide butyrate to obtain B; obtaining C from the B in a manner of weak acid cracking; obtaining a segment as shown in a formula D in manners of cyclizing and cracking, and obtaining E from the D by cracking in a strong acid system; mixing the Fmoc-Gly-OH with amino resin and then removing the Fmoc to obtain H-Gly-resin by coupling reaction; repeating the steps according to the solid-phase synthesis method, so as to obtain F; coupling the segment E with the F, so as to obtain the peptide resin in the formula G; and obtaining the carbetocin H after cracking the G. The method disclosed by the invention is high in total yield, less in impurity content, and high in product purity, and has a great industrial prospect.
Owner:HAINAN SHUANGCHENG PHARMA
Method for preparing carbetocin through solid phase and liquid combining method
The invention belongs to the field of polypeptide synthesis, and particularly relates to a method for preparing carbetocin through a solid phase and liquid combining method. A solid-phase synthesis method is firstly adopted for preparing an X-(CH2)3-CO-Tyr(Me)-Ile-Gln-Asn-Cys-OH fragment which is subjected to a coupled reaction with an H-Pro-Leu-Gly-NH2 fragment prepared through a liquid-phase method, carbetocin linear peptide is obtained, and then a cyclization reaction of linear peptide is carried out in the liquid phase to prepare carbetocin. According to the method, the operation process is easy and convenient, production cost is low, the situation that glycine is in lack of peptide impurities can be avoided, the subsequent purifying process is facilitated, and the method is suitable for large-scale production.
Owner:JINAN KANGHE MEDICAL TECH
Method of synthesizing carbetocin
ActiveCN106084015AHigh purityReduce contentOxytocins/vasopressinsPeptide preparation methodsCarbetocinMedicinal chemistry
The present invention relates the field of synthesis of medicine and discloses a method of synthesizing carbetocin. According to the method, group tBu is selected to be protected to replace Alloc and HOCH2CH2CN used in the prior art so as to protect (CH2)3COOH, and new amino acid resin, deprotection agent, acid-splitting agent and the like are utilized to complete the whole synthetic process, and at the premise of ensuring total yield, the purity of carbetocin is improved and the content of single impurites is significantly reduced.
Owner:CHENGDU SHENGNUO BIOPHARM
Carbetocin preparation method
ActiveCN103467573ASolve process problemsAvoid expensiveOxytocins/vasopressinsPeptide preparation methodsBiochemical engineeringCombinatorial chemistry
The invention belongs to the technical field of medicinal chemistry and particularly relates to a carbetocin preparation method. The carbetocin preparation method comprises the following steps of: (1) sequentially coupling to an amino resin by adopting an Fmoc sold-phase synthesis process so as to obtain a linear peptide resin A the structural formula of which is shown in the specification, wherein X is C1, Br or I, and Y is C1, Br or I; (2) adding a Na2S solution to the linear peptide resin and carrying out cyclization reaction so as to obtain peptide resin B; and (3) splitting the peptide resin B so as to obtain a carbetocin crude product. The carbetocin preparation method has the advantages that the process problems of expensive raw materials, need of heavy metal reagents and the like caused by using sulfydryl protected amino acids are avoided, the cost is low, no lots of waste liquid is generated, reaction conditions are mild, and the large-scale production is facilitated.
Owner:HYBIO PHARMA
Solid-phase fragment synthesis method for carbetocin
The invention relates to a solid-phase fragment synthesis method for carbetocin. The method comprises the following steps: 1) with solid-phase synthetic resin as a carrier, successively connecting the solid-phase synthetic resin with proline, cysteine, asparagine (Asn), glutamine, isoleucine and tyrosine all having protected amino groups and side chains; 2) removing the mercapto protection group of cysteine; 3) carrying out coupling with bromo-butyric acid under an alkaline condition; 4) after removal of a terminal amino protection group, coupling a terminal amino group with a carboxyl group at the side chain of cysteine so as to form a ring; 5) subjecting cyclic seven-peptide fragment resin to cracking; 6) with the solid-phase synthetic resin as a carrier, successively connecting the solid-phase synthetic resin to glycine and leucine with protected amino groups; 7) connecting a cyclic seven-peptide fragment obtained in the step 5) to dipeptide resin; and 8) carrying out cracking so as to obtain carbetocin. According to the invention, sites located between Pro to Leu are selected; and carbetocin prepared by using the preparation method has high purity.
Owner:HYBIO PHARMA
Purification method of carbetocin
InactiveCN104744567ALow toxicityLow costOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingIon exchange
The invention discloses a method for purifying carbetocin by virtue of a reversed-phase high-performance liquid chromatography. According to the method, the technical problems that separation methods are single, the product purity is low or the product yield is low by virtue of multiple measures in the prior art are solved. The method comprises the following steps: (1) filtering a carbetocin crude product solution by virtue of a 0.45-micron filter membrane for later use, carrying out gradient elution purification by virtue of a reverse phase silica gel column, and collecting a peptide solution with a target peak value, wherein a stationary phase of the reverse phase silica gel column is stearyl bonded silica gel, a mobile phase of the reverse phase silica gel column comprises a phase A and a phase B, the phase A is 0.1% sodium perchlorate, and the phase B is chromatographic grade acetonitrile; (2) converting the peptide solution into acetate by virtue of an ion exchange method; and (3) carrying out reduced pressure rotary evaporation concentration and freeze drying on the final high-purity peptide solution, so as to obtain powdery finished peptide. The method is applied to the industrial production of carbetocin.
Owner:上海吉尔多肽有限公司 +1
Preparation method of Carbetocin
ActiveCN105622725AOptimizing Extraction SolventsOptimizing the Extraction RatioOxytocins/vasopressinsPeptide preparation methodsCarbetocinButyric acid
The invention relates to a method for synthesizing polypeptide by combining solid and liquid phases, in particular to a preparation method of Carbetocin, and mainly solves the technical problems that the existing preparation method is low in yield and high in purifying cost. The invention adopts a technical scheme as follows: the preparation method of the Carbetocin comprises the following steps: 1) sequentially coupling amino resin with Fmoc-Gly-OH, Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Cys(Alloc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH and Fmoc-Ile-OH by using a coupling agent to obtain resin polypeptide; 2) performing de-cysteine protection; 3) carrying out reaction with 4-halogeno butyric acid under an alkaline condition, and coupling with H-Tyr(OMe)-OtBu; 4) cutting polypeptide, and performing ring closure on a liquid phase; 5) performing efficient countercurrent chromatographic purification on crude peptide, and lyophilizing to obtain the Carbetocin. The preparation method is mainly used for preparing the Carbetocin.
Owner:GL BIOCHEM SHANGHAI
Preparation method for carbetocin
ActiveCN106478779AHigh yieldEasy to sampleOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingCarbetocin
The invention discloses a preparation method for carbetocin. The preparation method comprises the following steps: subjecting swelled, deprotected and washed amino resin to reacting with an activated protected amino acid solution in a constant-temperature vibrator, carrying out successive introduction into protected amino acids corresponding to first-to-eighth amino acids accounting from the resin and into butyric acid at the tail end of N; subjecting prepared carbetocin precursor peptide 1-amino resin to deprotection, carrying out washing, then carrying out drying until the dried carbetocin precursor peptide 1-amino resin is of a granular shape, adding a cutting reagent, and carrying out a cutting reaction and settling; dissolving a carbetocin precursor peptide II in a solvent, removing impurities by adopting a reversed phase highly-efficient liquid chromatography method, and carrying out liquid-phase cyclizing so as to obtain a crude carbetocin peptide III; and subjecting the crude carbetocin peptide III to purifying and freeze-drying so as to obtain a pure carbetocin polypeptide. The preparation method provided by the invention utilizes an Fmoc solid-phase synthetic principle to develop a solid-phase synthetic technology; and through process optimization, the yield of crude carbetocin is up to 90% or above, so the yield of carbetocin is greatly improved.
Owner:合肥国肽生物科技有限公司
Purifying method for carbetocin
InactiveCN105399799AReduce the chance of high temperature degradationEasy to operateOxytocins/vasopressinsPeptide preparation methodsBiochemical engineeringMembrane technology
The invention discloses a purifying method for carbetocin. The method employs a purifying scheme of a combination of semi-permeable membrane dialysis purification and reversed phase chromatographic purification to eventually obtain carbetocin with a purity of greater than 99.5%. Semi-permeable membrane dialysis purification is simple to operate and does not need special equipment, and a semi-permeable membrane is repeatedly usable, so cost is reduced. Meanwhile, through preliminary purification by using semi-permeable membrane technology, chromatographic purification can be carried out without concentration, so the step of high-temperature concentration is omitted, the possibility of high-temperature degradation of polypeptide is reduced, and yield and purity are ensured.
Owner:郑州大明药物科技有限公司
Intranasal carbetocin formulations and methods for the treatment of autism
Methods and compositions containing oxytocin or an oxytocin analog, such as carbetocin, are provided for the prevention and treatment of autism spectrum disorders, related disorders and symptoms of such disorders. The methods and compositions of this disclosure are effective in the treatment of social withdrawal, eye contact avoidance, repetitive behaviors, anxiety, attention deficit, hyperactivity, depression, loss of speech, verbal communication difficulties, aversion to touch, visual difficulties, comprehension difficulties, and sound and light sensitivity. Additional compositions and methods are provided which employ oxytocin or an oxytocin analog in combination with a secondary or adjunctive therapeutic agent to yield more effective treatment tools against autism spectrum disorders and related disorders.
Owner:RETROPHIN
All-solid-phase preparation method for carbetocin
The invention relates to an all-solid-phase preparation method for carbetocin. The method comprises the following steps: subjecting chlorobutyric acid to amide coupling with the amino group of tyrosine of a peptide chain; and then with DBU as alkali, carrying out cyclization under the condition of a solid phase so as to obtain crude peptide of carbetocin.
Owner:HYBIO PHARMA
Method for synthesizing cyclopeptide drug carbetocin
InactiveCN110343149AHigh yieldHigh product purityOxytocins/vasopressinsPeptide preparation methodsSynthesis methodsCombinatorial chemistry
The invention discloses a method for synthesizing a cyclopeptide drug carbetocin. The method comprises the following steps that (1) carbetocin main chain resin is prepared with Y-Tyr(Me)-Ile-Gln(X)-Asn(X)-OH, Fmoc-Cys((CH2)3COOtBu)-OH, Fmoc-Pro-OH, Fmoc-Leu-OH and Fmoc-Gly-OH as starting raw materials by adopting a polypeptide solid-phase synthesis method; (2) the main chain resin is subjected topyrolysis to obtain a crude product of a carbetocin main chain, and a crude product of the carbetocin is obtained through one-step liquid-phase condensation cyclization. The method is a process combining solid-phase synthesis and liquid-phase synthesis, the operation is convenient, and the synthesis steps of the process are greatly shortened; through a fragment synthesis method, the total yield ofthe carbetocin is greatly increased, the product purity of the carbetocin is greatly improved, the occurrence of side reactions and the types of by-products are reduced, and the industrial scale-up synthesis is facilitated.
Owner:CHENGDU SINTANOVO BIOTECHNOLOGV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com