Carbetocin pharmaceutical composition and preparation method thereof
A carbetocin and composition technology are applied in the field of pharmaceutical preparation compositions and preparations containing carbetocin, and can solve the problems of large increase in impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
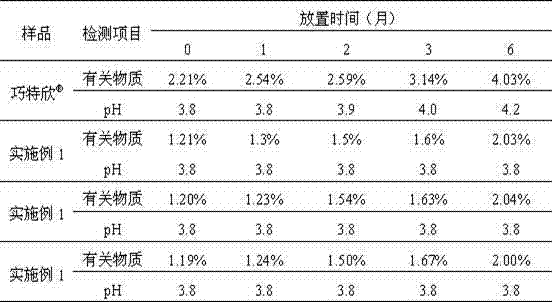
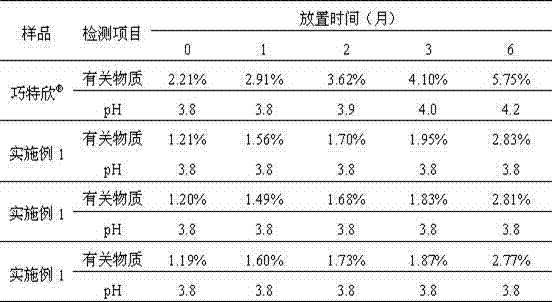
Embodiment 1
[0047] Carbetocin 0.1g Sodium chloride 9g glacial acetic acid 0.41g Sodium acetate 0.1g Add water for injection to 1000ml
[0048] Preparation Process:
[0049] Weigh glacial acetic acid and sodium acetate according to the prescription amount, prepare a pH buffer solution, and set aside. The buffer solution is added with the prescribed amount of sodium chloride, dissolved, and mixed evenly, so as to prepare the auxiliary material liquid. The excipient liquid is ultrafiltered with an ultrafilter to remove heat source. Carbetocin was dissolved and added to the ultrafiltrate of the excipient that had been ultrafiltered and made to volume. Sterile filtration, sub-packaging, and filled with inert gas protection.
Embodiment 2
[0051] Carbetocin 0.1g Sodium chloride 9g tartaric acid Adjust pH4.0 Add water for injection to 1000ml
[0052] Preparation Process:
[0053] Weigh the raw and auxiliary materials according to the prescription quantity, and set aside. Dissolve the sodium chloride of prescription quantity, carry out ultrafiltration with ultrafilter instrument and remove heat source. Dissolve carbetocin and add it to the ultrafiltrate of the excipient that has been ultrafiltered, add water for injection close to the total volume, adjust the pH to 4.0 with tartaric acid, and finally add water for injection to constant volume. Sterile filtration, sub-packaging, and filled with inert gas protection.
Embodiment 3
[0055] Carbetocin 0.1g Mannitol 45.5g glacial acetic acid 0.41g Sodium acetate 0.1g Methionine 0.05g Add water for injection to 1000ml
[0056] Preparation Process:
[0057] Weigh glacial acetic acid and sodium acetate according to the prescription amount, prepare a pH buffer solution, and set aside. The buffer solution is added with prescribed amount of mannitol, dissolved, mixed evenly, and prepared as auxiliary material liquid. The excipient liquid is ultrafiltered with an ultrafilter to remove heat source. Dissolve methionine and carbetocin and add them to the ultrafiltered excipient solution and make to volume. Sterile filtration, sub-packaging, and filled with inert gas protection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com