Method for preparing carbetocin
A carbetocin and resin technology, which is applied in the field of solid-phase synthesis of peptides, can solve problems such as unfavorable large-scale production, high equipment requirements, high price, etc., and achieves the advantages of being beneficial to industrialized large-scale production, ensuring production safety, and responding mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
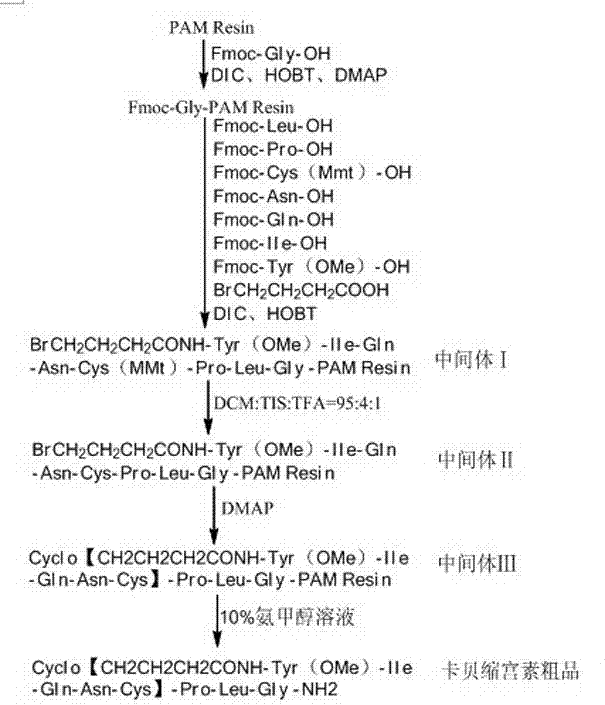
[0039] Embodiment 1: Preparation of Fmoc-Gly-PAM resin
[0040] Add 22.2g of PAM resin (degree of substitution: 0.9mmol / g) into the solid-phase reaction column, swell with DMF for 30 minutes, put 11.88g of Fmoc-Gly-OH, 5.4g of HOBT, 0.122g of DMAP into the container, and keep the mixture at room temperature Shake for 2.5-3 hours, filter the reaction solution, wash 3 times with 200ml of DMF, then wash with 200ml of pyridine / Ac 2 O / DMF= 6:5:50 (v / v / v) solution was reacted for 2 hours, after the reaction was completed, the DMF was filtered off, washed 3 times with DMF (250 ml), washed 3 times with DCM (250 ml), washed with Methanol (250 ml) was washed 3 times and filtered to obtain 27.81 g of Fmoc-Gly-PAM resin, the measured substitution degree was 0.69 mmol / g, and the yield was 95.94%.
[0041]
Embodiment 2
[0042] Example 2: BrCH 2 CH 2 CH 2 Preparation of CONH-Tyr(OMe)-Ile-Gln-Asn-Cys(MMt)-Pro-Leu-Gly-PAM Resin (Intermediate Ⅰ)
[0043] Weigh 1.45 g of the prepared Fmoc-Gly-PAM resin and add it to the reactor, swell it with DMF for 30 minutes, remove the Fmoc protection with 20% DBLK for 30 minutes, and connect Fmoc-Leu-OH after washing 5 times with DMF (0.71g Fmoc -Leu-OH, 0.27g HOBT was put into the container in an ice-water bath and dissolved with DMF, added 0.32ml DIC to activate for 10 minutes and then added to the reactor), reacted for about 1h. The end point of the reaction was determined by the ninhydrin method. Repeat the above steps, and use the amino acids of the Fmoc protecting group to complete the remaining connections in turn to obtain BrCH 2 CH 2 CH 2 CONH-Tyr(OMe)-Ile-Gln-Asn-Cys(MMt)-Pro-Leu-Gly-PAM Resin (Intermediate I).
[0044]
Embodiment 3
[0045] Example 3: Preparation of BrCH2CH2CH2CONH-Tyr(OMe)-Ile-Gln-Asn-Cys-Pro-Leu-Gly-PAM Resin (Intermediate II)
[0046] Use 50 ml of DCM:TIS:TFA=95:4:1 (v / v / v) solution to remove the MMt protecting group on Cys from the intermediate I prepared in Example 2 for 10 minutes, and repeat 3 times to obtain BrCH 2 CH 2 CH 2 CONH-Tyr(OMe)-Ile-Gln-Asn-Cys-Pro-Leu-Gly-PAM Resin (Intermediate II).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com