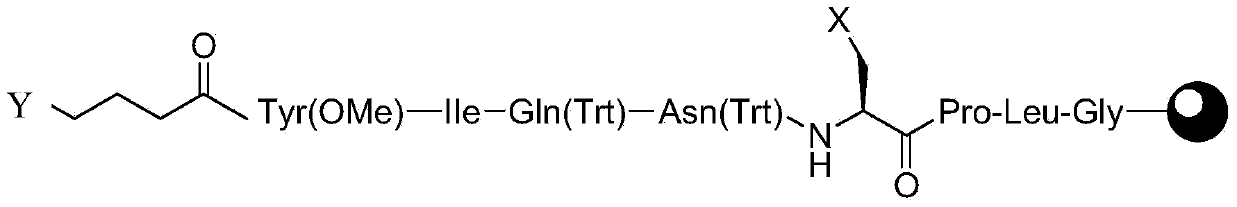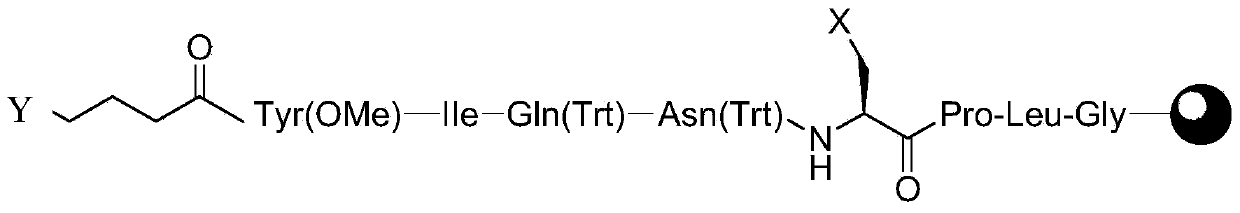Carbetocin preparation method
A technology of carbetocin and solid-phase synthesis, which is applied in the field of preparation of carbetocin, can solve the problems of many protective side reactions, high price, and unfavorable large-scale production, so as to avoid process problems and cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 prepares linear peptide resin A
[0055] Weigh 1 mmol (2 g) of Rinkamide Resin with a substitution degree of 0.50 mmol / g, add it to a solid-phase reaction column, and wash it twice with DMF. After swelling Rinkamide Resin with DMF for 30 minutes, remove the Fmoc protection with DBLK, and then wash it six times with DMF. Dissolve Fmoc-Gly–OH (0.8g, 3mmol), HOBt (0.45g, 3.3mmol) in DMF and add DIC (0.52ml, 3.3mmol) under ice bath conditions, then add the resulting solution to the solid phase reaction column , react at room temperature for 2 hours (the end point of the reaction is determined by the ninhydrin method, if the resin is colorless and transparent, the reaction is complete, and the resin develops color, indicating that the reaction is incomplete, and another 1 hour of coupling reaction is required). Repeat the steps of removing Fmoc protection and adding corresponding amino acids for coupling, and complete Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-A(Cl)-OH, Fmo...
Embodiment 2
[0058] Embodiment 2 prepares peptide resin B
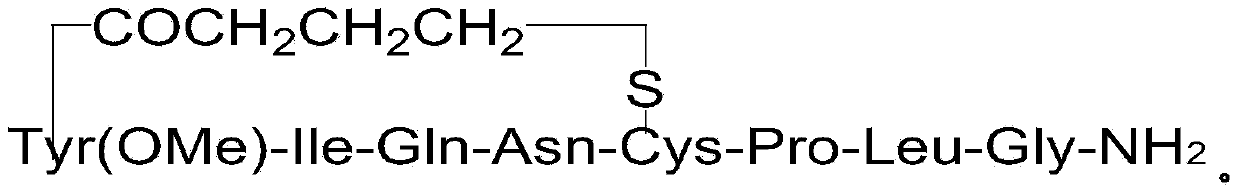
[0059] Weigh Na 2 S (0.32g, 4mmol) was dissolved in 50ml of DMF, and after the dissolution was completed, it was added to the reaction column in Example 1 to start the cyclization reaction. Reacted at room temperature for 24 hours, washed with DMF for 6 times, and finally added methanol for 3 times, and dried to obtain 3.3 g of peptide resin B.
Embodiment 3
[0060] Embodiment 3 prepares crude product carbetocin
[0061] The 3.3g peptide resin B among the embodiment 2 is added in the 50ml flask, configures cracking reagent 35ml (TFA:H 2 O=95:5 (v / v)), pour the lysis reagent into the flask, and react at room temperature for 2 hours. After the reaction, filter the resin and collect the filtrate. The resulting filtrate was added dropwise to 350 ml of ether reagent, centrifuged, washed with anhydrous ether, and dried in vacuo to obtain 0.95 g of crude carbetocin with an HPLC purity of 72.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com