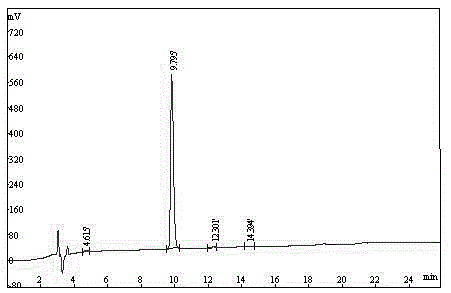
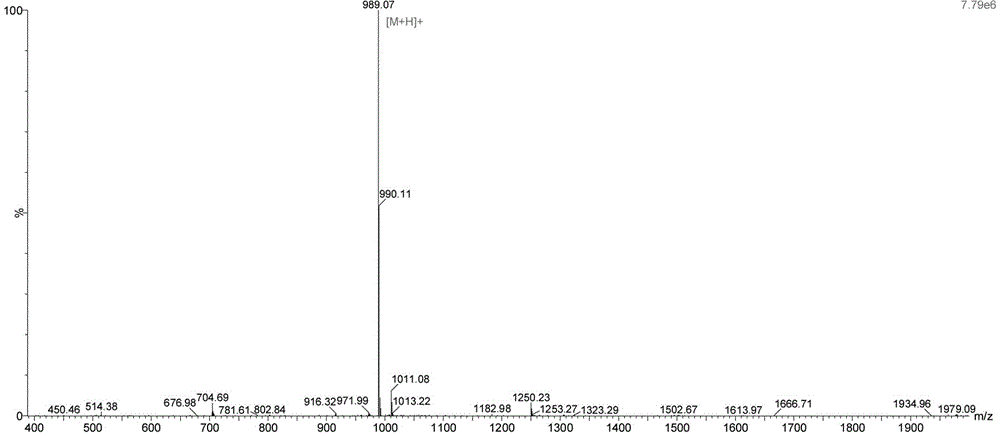
Preparation method of Carbetocin
A carbetocin and solution technology, applied in the field of solid-liquid phase combination synthesis of polypeptides, can solve the problems of high purification cost and low yield, and achieve the effects of reduced purification difficulty, good yield and optimized extraction ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of Fmoc-Gly-amino resin, refer to figure 1 ,
[0029] Add 142 grams of amino resin, with a substitution degree of 0.4 mmol / g, into the solid-phase reaction column, add DCM to swell the resin for 30 minutes, add 34 grams of Fmoc-Gly-OH, 43 grams of HATU,
[0030] 17 g of HOAt was dissolved in DMF and cooled to 5 °C, reacted with nitrogen gas, added 13.7 g of TMP, reacted at room temperature for 50 minutes, washed with DMF for 3 times, and washed with DCM and methanol for 3 times.
[0031] The solid-phase reactor is a sandy funnel, the lower part of which is connected to a tee, one end of the tee is connected to a nitrogen bottle, and the other end of the tee is connected to a suction filter bottle and a vacuum pump, which is convenient for nitrogen and suction filtration.
Embodiment 2
[0032] Embodiment 2: the synthesis of Fmoc-Gly-amino resin, refer to figure 1 ,
[0033]Add 142 grams of amino resin, with a substitution degree of 1.2 mmol / g, into the solid-phase reaction column. After adding DCM to swell the resin for 30 minutes, add 102 grams of Fmoc-Gly-OH, 129 grams of HATU,
[0034] 51 g of HOAt was dissolved in its own DMF and cooled to 5°C, reacted with nitrogen gas, added 41.1 g of TMP, reacted at room temperature for 50 minutes, washed with DMF for 3 times, and washed with DCM and methanol for 3 times alternately.
Embodiment 3
[0035] Embodiment 3, with reference to figure 1 , amino resin substitution degree is 0.8mmol / g, coupling agent DIC / HOBt, all the other are the same as embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com