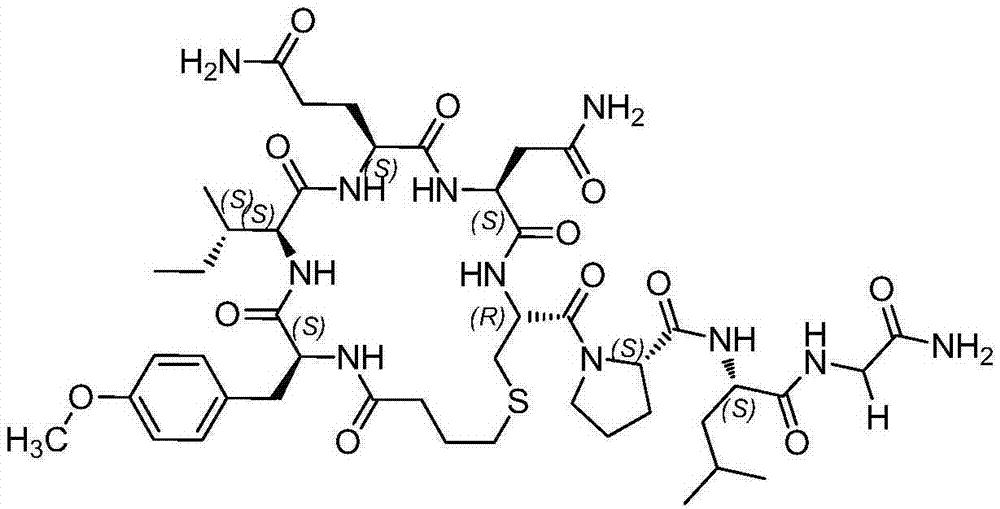
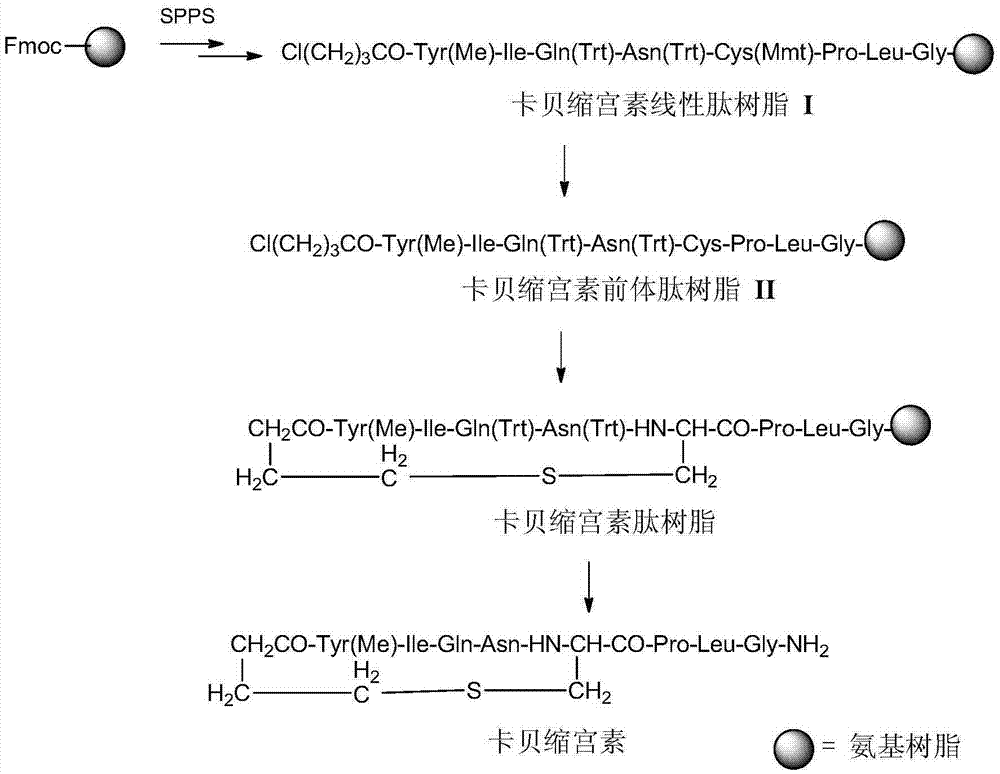
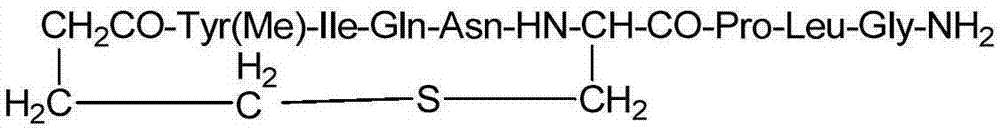All-solid-phase preparation method for carbetocin
A solid-phase preparation technology for carbetocin, applied in the field of polypeptide drug preparation, can solve the problems of many impurities, many side reactions, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of carbetocin peptide resin
[0029] Weigh 52.8g (29.4mmol) of Rink Amide resin with a substitution degree of 0.557mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, deprotect the DBLK for 6min+8min, DMF Wash 6 times. Weigh 26.2g (88.2mmol) Fmoc-Gly-OH and 13.1g (97.0mmol) HOBT dissolved in DMF, add 15.2mL (106mmol) DIPCDI to activate for 3min, then add the mixture into the reaction column, react at room temperature for 2 hours, Use ninhydrin to detect the end of the reaction (if the resin is colorless and transparent, terminate the reaction; if the resin develops color, prolong the reaction for 1 hour).
[0030] After the reaction is over, wash the resin with DMF for 3 times, add DBLK for deprotection for 5min + 7min, wash the resin with DMF for 6 times, and detect the color of the resin with ninhydrin. Weigh 31.2g (88.2mmol) Fmoc-Leu-OH and 13.1g (97.0mmol) HOBT dissolved in DMF...
Embodiment 2
[0036] Embodiment 2: the preparation of carbetocin essence peptide
[0037] Add 78.5 grams of the carbetocin peptide resin obtained in Example 1 into a 2000ml three-necked bottle, and protect it with nitrogen. Add pre-made TFA:TIS:H 2 O=90:5:5 (V:V) 785ml, react at room temperature for 2 hours, filter the resin, and collect the filtrate. The resin was washed with a small amount of TFA, and the filtrates were combined. The filtrate was slowly added to 7850 ml of glacial ether for precipitation, centrifuged, washed twice with ether, and dried under reduced pressure to obtain 28.7 g of crude peptide with an HPLC purity of 87.23%. After preparation and purification by high-pressure liquid phase, 19.7 g of carbetocin refined peptide was obtained by lyophilization, with a purity of 99.42% and a maximum of 0.15%. The theoretical yield is 29.04g, and the total yield is 67.8%.
Embodiment 3
[0038] Embodiment 3: adopt bromobutyric acid as the comparative example of raw material
[0039] According to the method of Example 1, after changing 4-chlorobutyric acid to 4-bromobutyric acid for solid-phase coupling, BrCH 2 CH 2 CH 2 CO-Tyr(Me)-Ile-Gln(Trt)-Asn(Trt)-Cys(Mmt)-Pro-Leu-Gly-RinkAmide resin, followed by removal of the Mmt protecting group. In the solid-phase cyclization step, DIPEA was used for cyclization at room temperature for 3 hours, and the end of the reaction was detected by DTNB (if the resin is colorless and transparent, stop the reaction; if the resin develops color, prolong the reaction for 0.5 hours until the resin is colorless). After the reaction was completed, the reaction solution was drained, the resin was washed with DMF for 3 times, and the liquid was drained. Methanol shrinks the resin 3 times, and the peptide resin is vacuum-dried. The crude peptide was cleaved by the method of Example 3, and the purity of the crude peptide was 60.4%, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com