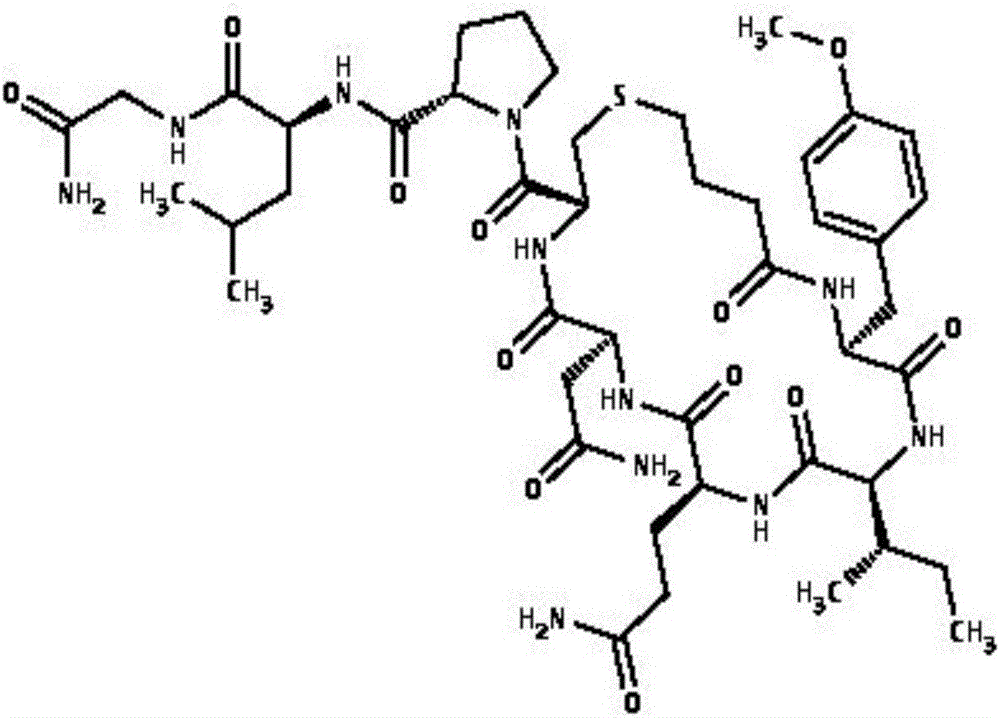
Preparation method for carbetocin
A carbetocin and solution technology, applied in the field of polypeptide drug preparation, can solve problems such as unfavorable large-scale production of carbetocin, poisoning of tetrakistriphenylphosphine palladium catalyst, reduction of product purity and use effect, etc. It is beneficial to industrial production, high product yield and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Swelling of amino resin: Weigh 0.1g of Rink-MBHA-Resin with a substitution degree of 0.38mmol / g, add it to the polypeptide synthesis reactor from the open end, take the DCM reagent and add it to the reactor, so that the resin is completely immersed in the DCM solvent , in full contact with the solvent, swelling for 2h.
[0032] 1. Synthesis of carbetocin precursor peptide Ⅰ-amino resin
[0033] Carbetocin precursor peptide Ⅰ-amino resin is:
[0034] Butyric acid (Cl)-Tyr(Me)-Ile-Gln-Asn-Cys-Pro-Leu-Gly-Rink-AM-Resin
[0035] The protected amino acids used in this example are the protected amino acids corresponding to the 1-8 amino acids from the resin and the 4-chlorobutyric acid at the N-terminal, and the molecular weights are shown in Table 1 below:
[0036] Table 1
[0037]
[0038]
[0039] Some commonly used abbreviations in the present invention have the following meanings:
[0040]
[0041] Activation methods for protected amino acids
[0042] Takin...
Embodiment 2
[0057] Swelling of amino resin: Weigh 2g of Rink-MBHA-Resin with a substitution degree of 0.45mmol / g, add it to the polypeptide synthesis reactor from the open end, take the DCM reagent and add it to the reactor, so that the resin is completely immersed in the DCM solvent , soaked overnight.
[0058] 1. Synthesis of carbetocin precursor peptide Ⅰ-amino resin
[0059] Carbetocin precursor peptide Ⅰ-amino resin is:
[0060] Butyric acid(Cl)-Tyr(Me)-Ile-Gln-Asn-Cys-Pro-Leu-Gly-Rink-AM-Resin
[0061] Activation methods for protected amino acids
[0062] Taking the condensation of Fmoc-Gly-amino resin as an example, weigh 510mg Fmoc-Gly-OH and 255mgHOBt in a centrifuge tube according to the calculated theoretical dosage, add 12ml DMF to dissolve it, and then add 200ul DIC to the solution with a dropper , and mix evenly to obtain an activated protected amino acid solution.
[0063] Resin deprotection and washing method
[0064] After the amino resin solution is drained, add 1 / 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com