Method of synthesizing carbetocin
A technology of carbetocin and condensation reagents, which is applied in the field of pharmaceutical synthesis, can solve the problems of high production cost, complicated preparation process, and high single impurity, and achieve the effects of reduced content, high total yield, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Synthesis of Peptide Resin 1
[0054] Take 0.15mol Fmoc-Gly and 0.15mol HOBt, and dissolve them with an appropriate amount of DMF; take another 0.15mol DIC, slowly add them to the protected amino acid DMF solution under stirring, and stir and react at room temperature for 30 minutes to obtain the activated protected amino acid solution ,spare.
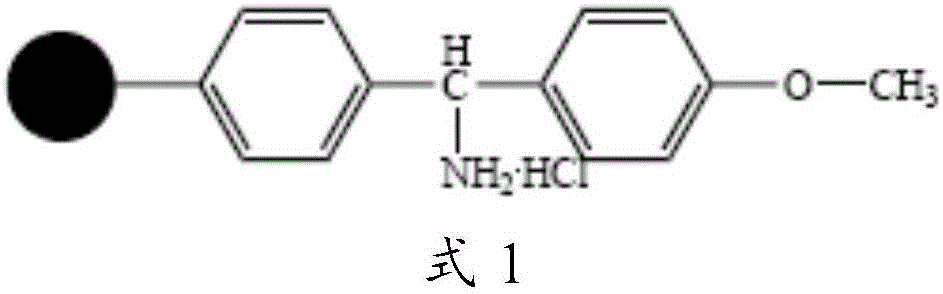
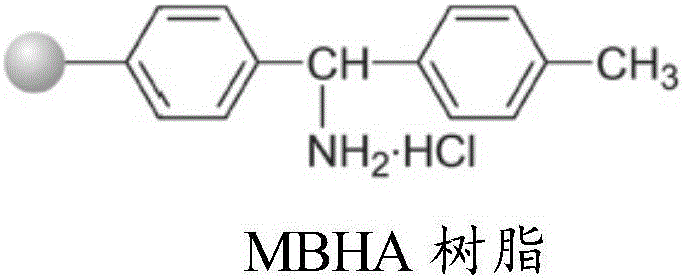
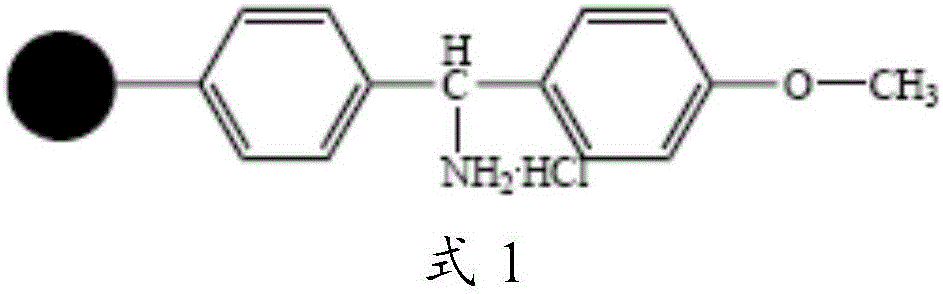
[0055] Take 0.05mol of MOBHA resin (substitution value about 0.6mmol / g), swell with DMF for 25 minutes, wash and filter, add activated Fmoc-Gly solution, stir and react at room temperature for 3 hours, remove the reaction solution, wash with DMF for 3 times, DCM was washed 3 times, and the washing time was 3 minutes each time to obtain Fmoc-Gly-MBHA resin, that is, peptide resin 1. Before the next coupling reaction, use 20% PIP / DMF solution to remove Fmoc protection for 25 minutes, wash and filter to obtain Gly - MOBHA resin.
Embodiment 2
[0056] Example 2: Synthesis of Peptide Resin 1
[0057] Take 0.15mol Boc-Gly and 0.15mol HOBt, and dissolve them with an appropriate amount of DMF; take another 0.15mol DIC, slowly add it to the protected amino acid DMF solution under stirring, and stir and react at room temperature for 30 minutes to obtain the activated protected amino acid solution ,spare.
[0058] Take 0.05mol of MOBHA resin (substitution value about 0.6mmol / g), swell with DMF for 25 minutes, wash and filter, add activated Fmoc-Gly solution, stir and react at room temperature for 3 hours, remove the reaction solution, wash with DMF for 3 times, DCM was washed 3 times, and the washing time was 3 min each time to obtain Boc-Gly-MOBHA resin, that is, peptide resin 1. Before the next coupling reaction, 30% TFA / DCM solution was used to remove Boc protection for 30 minutes, and DIEA / DCM The solution was neutralized, washed and filtered with DMF and DCM to obtain Gly-MOBHA resin.
Embodiment 3
[0059] Example 3: Synthesis of Peptide Resin 2
[0060] Take 0.15mol Fmoc-Leu and 0.15mol HOBt, and dissolve them with an appropriate amount of DMF; take another 0.15mol DIC, slowly add them to the protected amino acid DMF solution under stirring, and stir and react at room temperature for 30 minutes to obtain the activated protected amino acid solution .
[0061]Add the above-mentioned activated protected amino acid solution to the Gly-MOBHA resin prepared in Example 1, stir and react at room temperature for 3 hours, remove the reaction solution, wash 3 times with DMF, and wash 3 times with DCM, each washing time is 3min, Then use 20% PIP / DMF solution to remove Fmoc protection for 25 minutes, wash and filter to complete the access of Leu.
[0062] Access Fmoc-Pro in the same way, Fmoc-Cys ((CH 2 ) 3 COOtBu), Fmoc-Asn (Trt), Fmoc-Gln (Trt), Fmoc-Ile and Fmoc-Tyr (OMe), Fmoc was removed using 20% PIP / DMF solution, washed and filtered to obtain peptide resin 2, Tyr (OMe )-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com