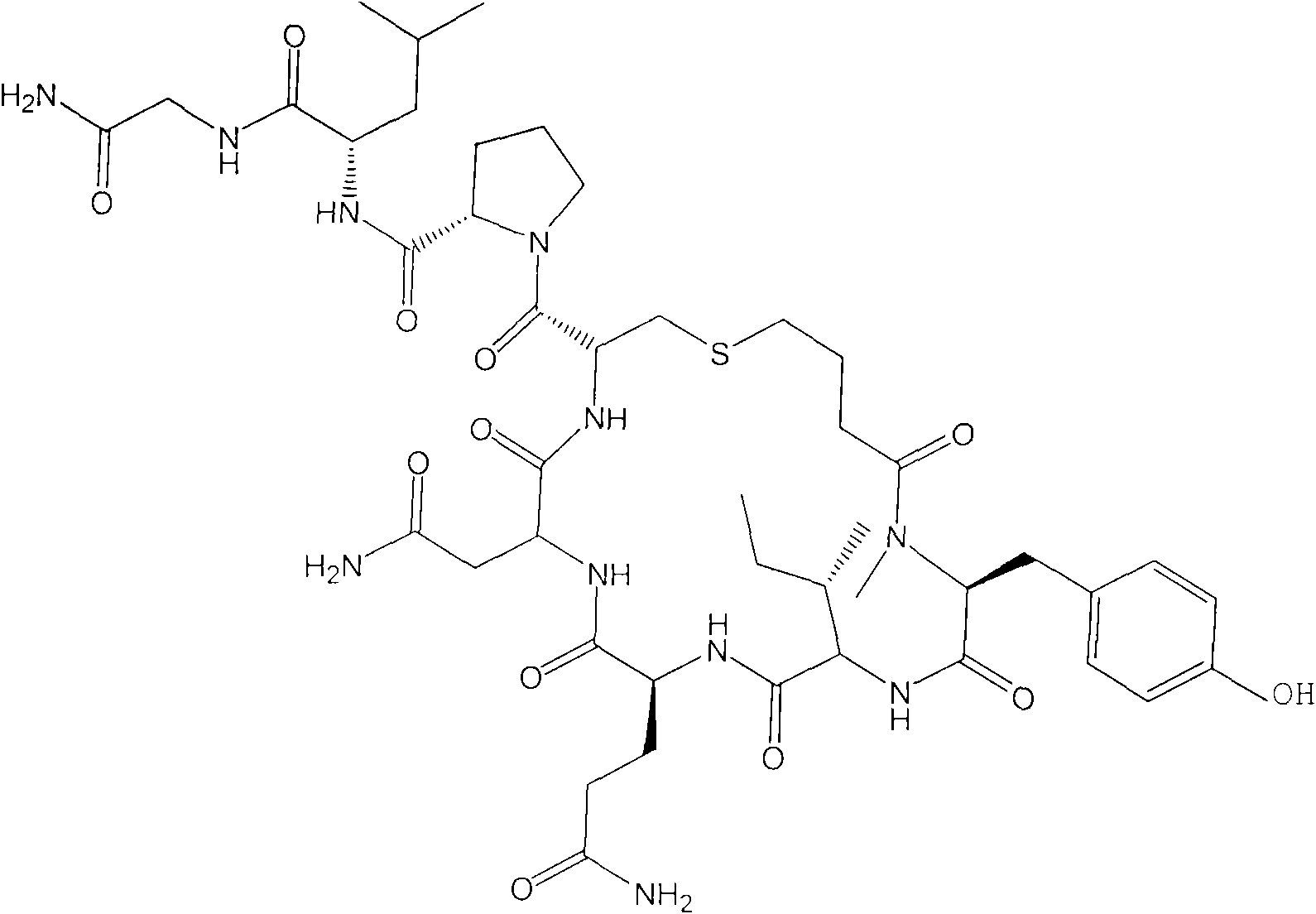
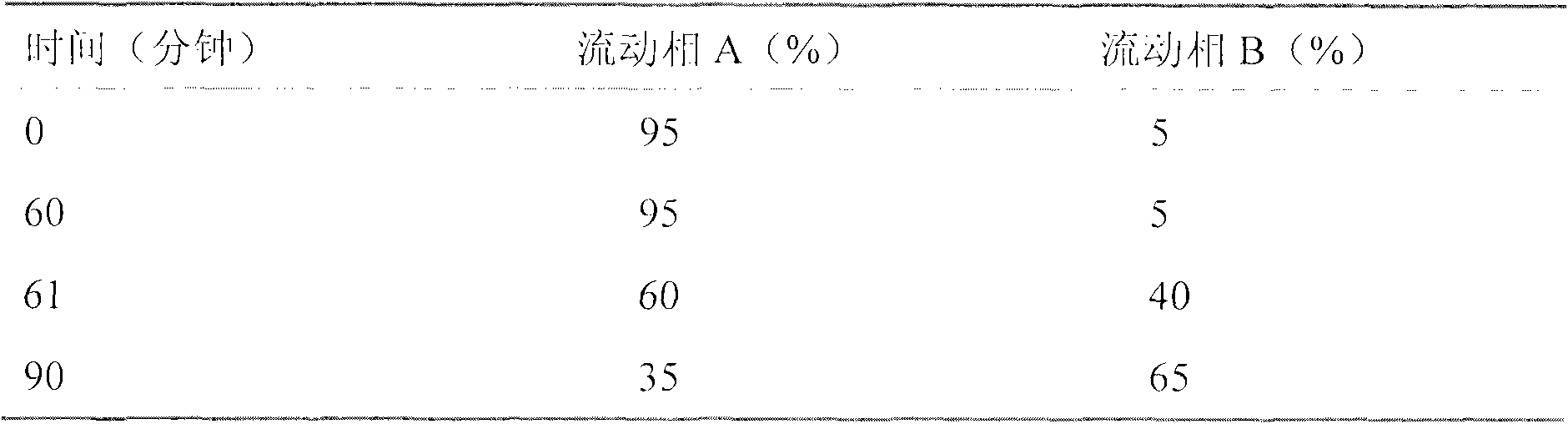
Process for producing medicament with uterine contraction effect
A technology of carbetocin and amino resin, which is applied in the direction of drug combination, bulk chemical production, cyclic peptide composition, etc., and can solve the problems of inability to freeze and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Preparation of Fmoc-Gly-resin
[0062] Add 100.00g of Fmoc-RinkAmide MBHA resin into the reaction column, and the substitution degree of this resin is 1.0mmol / g. Add 500ml DMF for swelling, then add DCM500ml / time for washing 3 times, then add DMF500ml / time for washing 3 times. Add 500ml of the prepared 50% piperidine / DMF solution into the glass reaction column, stir and react for 30min, remove the reaction solution, add DMF500ml / time for washing 6 times. The feeding amount of protected amino acid and condensation reagent is 3 times equivalent. Weigh Fmoc-Gly-OH89.19g and TBTU96.32g, HOBt40.54g, DIEA38.77g, add DMF and stir to dissolve, stir evenly and add to the glass reaction column, The reaction was stirred for 24 hours. The KaiserTest monitors the extent of the reaction until it is complete. After the reaction was completed, the reaction solution was removed, washed 3 times with DMF500ml / time, and then washed 3 times with DCM500ml / time, drained, pour...
Embodiment 2
[0063] Embodiment 2: Preparation of Fmoc-Leu-Gly-resin
[0064] Add 104.42g of Fmoc-Gly-resin to the reaction column, add DCM500ml / time for washing 3 times, then add DMF500ml / time for washing 3 times. Add 500ml of the prepared 50% piperidine / DMF solution into the glass reaction column, stir for 30min, remove the reaction solution, add DMF500ml / time for washing 6 times. Weigh and collect Fmoc-Leu-OH 94.00g, TBTU85.4lg, HOBt35.94g, DIEA34.38g, add DMF and stir to dissolve. After complete dissolution, the prepared amino acid coupling solution was added to the reaction column, and stirred for 3 hours. Samples were taken and washed 6 times with DMF, and the reaction degree was monitored by Kaiser Test until the reaction was completed. The reaction solution was removed and washed 6 times with DMF500ml / time.
Embodiment 3
[0065] Embodiment 3: Preparation of Fmoc-Pro-Leu-Gly-resin
[0066] Add 500ml of the prepared 50% piperidine / DMF solution into the glass reaction column, stir for 30mm, remove the reaction solution, add DMF500ml / time for washing 6 times. Weigh 89.75g of Fmoc-Pro-OH, 85.41g of TBTU, 35.94g of HOBt, and 34.38g of DIEA, add DMF and stir to dissolve. After complete dissolution, the prepared amino acid coupling solution was added to the reaction column, and stirred for 3 hours. Samples were taken, washed 6 times with DMF, and KaiserTest was used to monitor the degree of reaction until the reaction was completed. The reaction solution was removed and washed 6 times with DMF500ml / time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com