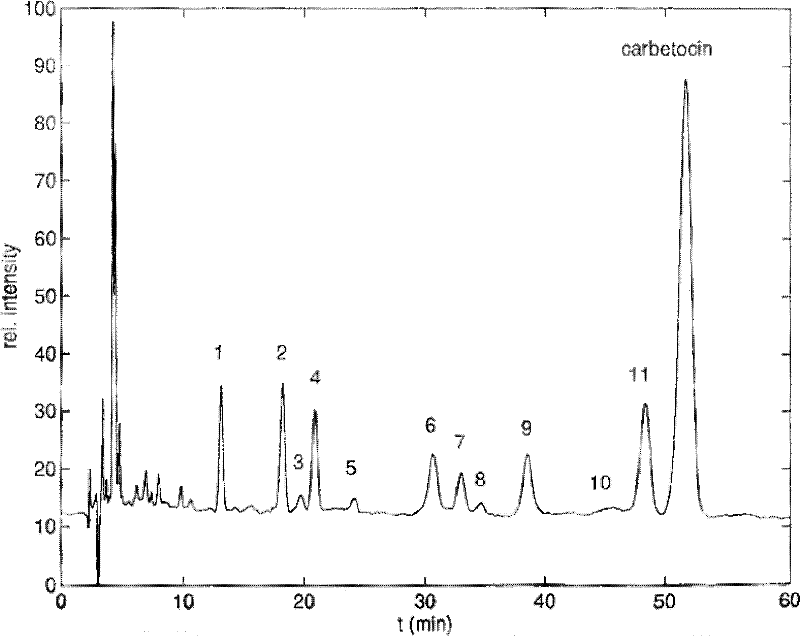
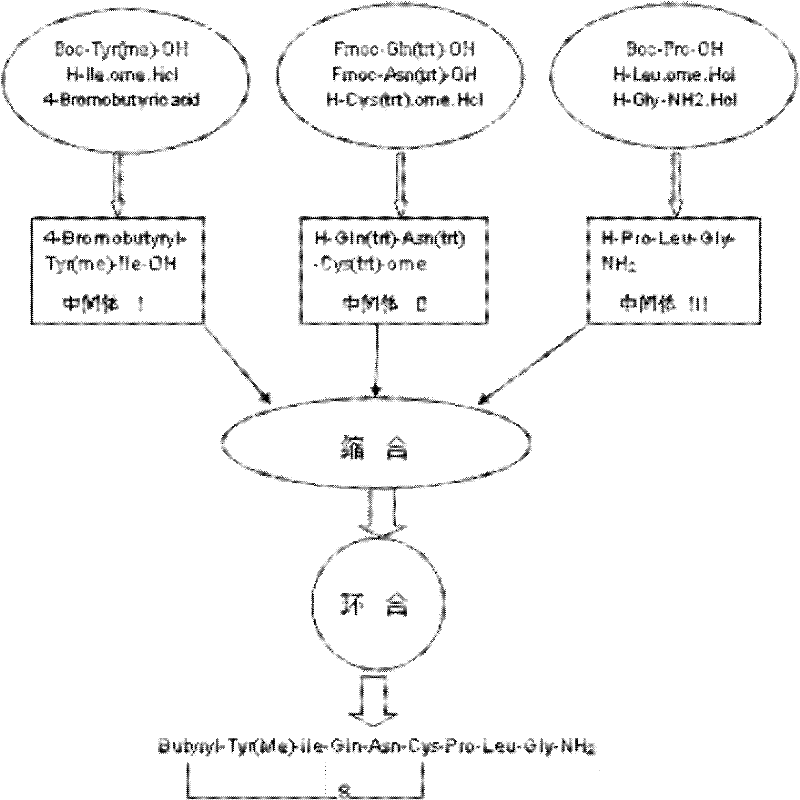
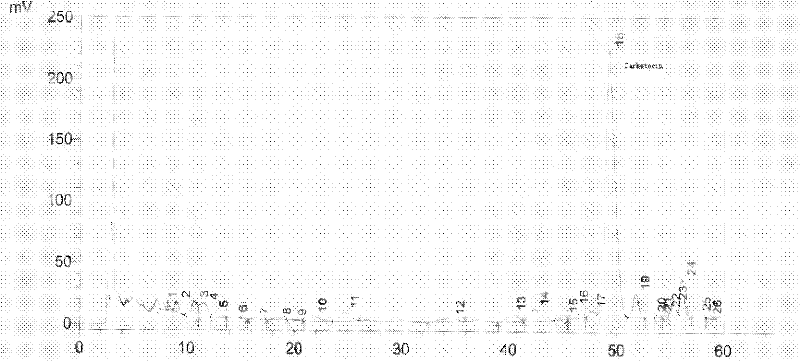
Method for synthesizing carbetocin through liquid-phase synthetic method
A technology of carbetocin and a synthesis method, which is applied to the synthesis field of polypeptide drugs, can solve the problems of high toxicity, difficult purification, low efficiency and purity, etc., and achieves the effects of large differences in amino acids, easy separation and purification, and high product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Synthesis of embodiment one 4-Bromobutyryl-Tyr(me)-Ile-OH
[0084] Add 1.48g Boc-Tyr(me)-OH (5mmol) and an appropriate amount of DMF that can basically dissolve under magnetic stirring in turn into a 250ml round bottom flask, and take another 0.91g H-Ile.ome.Hcl (5mmol) and 0.68 Add gHOBT (5mmol) to a small amount of DMF, then add 2 times the number of moles of NMM and add it to the flask together, continue to adjust the pH to 7 to 8, add 1.03g DCC (5mmol) to stir the reaction, and track and monitor the completion of the reaction by TLC. After 12 hours, the stirring was stopped, and TLC detected that the reaction was incomplete, adding BOP to continue the reaction for 2 hours, and standing at room temperature. Suction filtration (filter insoluble matter DCU), the filtrate is extracted with ethyl acetate and water with a separatory funnel, the upper layer is the product, and the lower layer is extracted twice with ethyl acetate, the ethyl acetate layers are combined, was...
Embodiment 2
[0085] Example 2 Synthesis of H-Gln(trt)-Asn(trt)-Cys(trt)-ome
[0086] Dissolve 3g of Fmoc-Asn(trt)-OH (5mmol) in 10ml of dichloromethane, add 7ml of diethylamine to react for 2 hours, spot the plate after 2 hours, and directly concentrate to obtain 1.8g of solid after the reaction is complete, with a yield of 98%. An appropriate amount of DMF that can be basically dissolved under magnetic stirring, another 3g Fmoc-Gln(trt)-OH (5mmol) and 0.68g HOBT (5mmol) were added to a small amount of DMF, and then N-methylmorpholine (NMM) was added to adjust the pH From 7 to 8, 1.03 g of DCC (5 mmol) was added to stir the reaction, and TLC was followed to monitor the completion of the reaction. After 12 hours, the stirring was stopped, and TLC detected that the reaction was incomplete, adding BOP to continue the reaction for 2 hours, and standing at room temperature. Suction filtration (filter insoluble matter DCU), the filtrate is extracted with ethyl acetate and water with a separator...
Embodiment 3
[0087] Example 3 Synthesis of H-Pro-Leu-Gly-NH2
[0088] Add 1.1g Boc-Pro-OH (5mmol) and an appropriate amount of DMF that can basically dissolve under magnetic stirring in turn in a 250ml round bottom flask, and take another equimolar 0.91g H-Leu.ome.Hcl (5mmol) and 0.68g Add HOBT (5mmol) to a small amount of DMF, then add 2 times the number of moles of N-methylmorpholine (NMM) and add it to the flask together, continue to adjust the pH to 7 to 8, add 1.03gDCC (5mmol) to stir the reaction, TLC tracking monitoring The completion status of the reaction. After 12 hours, the stirring was stopped, and TLC detected that the reaction was incomplete, adding BOP to continue the reaction for 2 hours, and standing at room temperature. Suction filtration (filter insoluble matter DCU), the filtrate is extracted with ethyl acetate and water with a separatory funnel, the upper layer is the product, and the lower layer is extracted twice with ethyl acetate, the ethyl acetate layers are comb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com