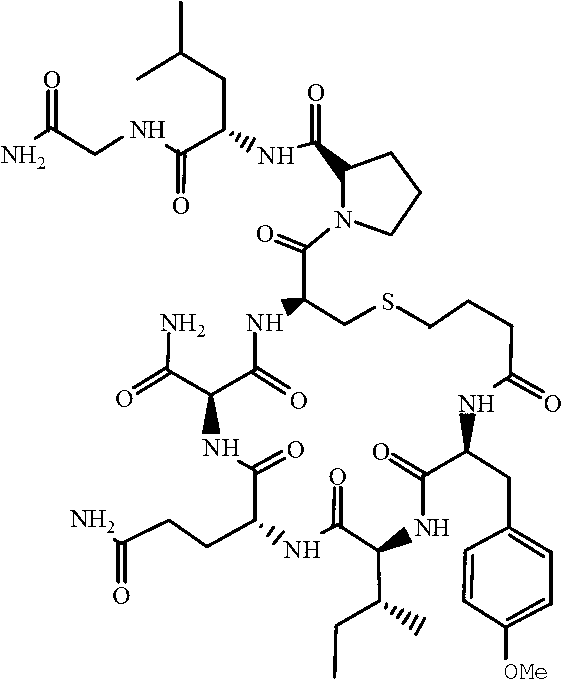
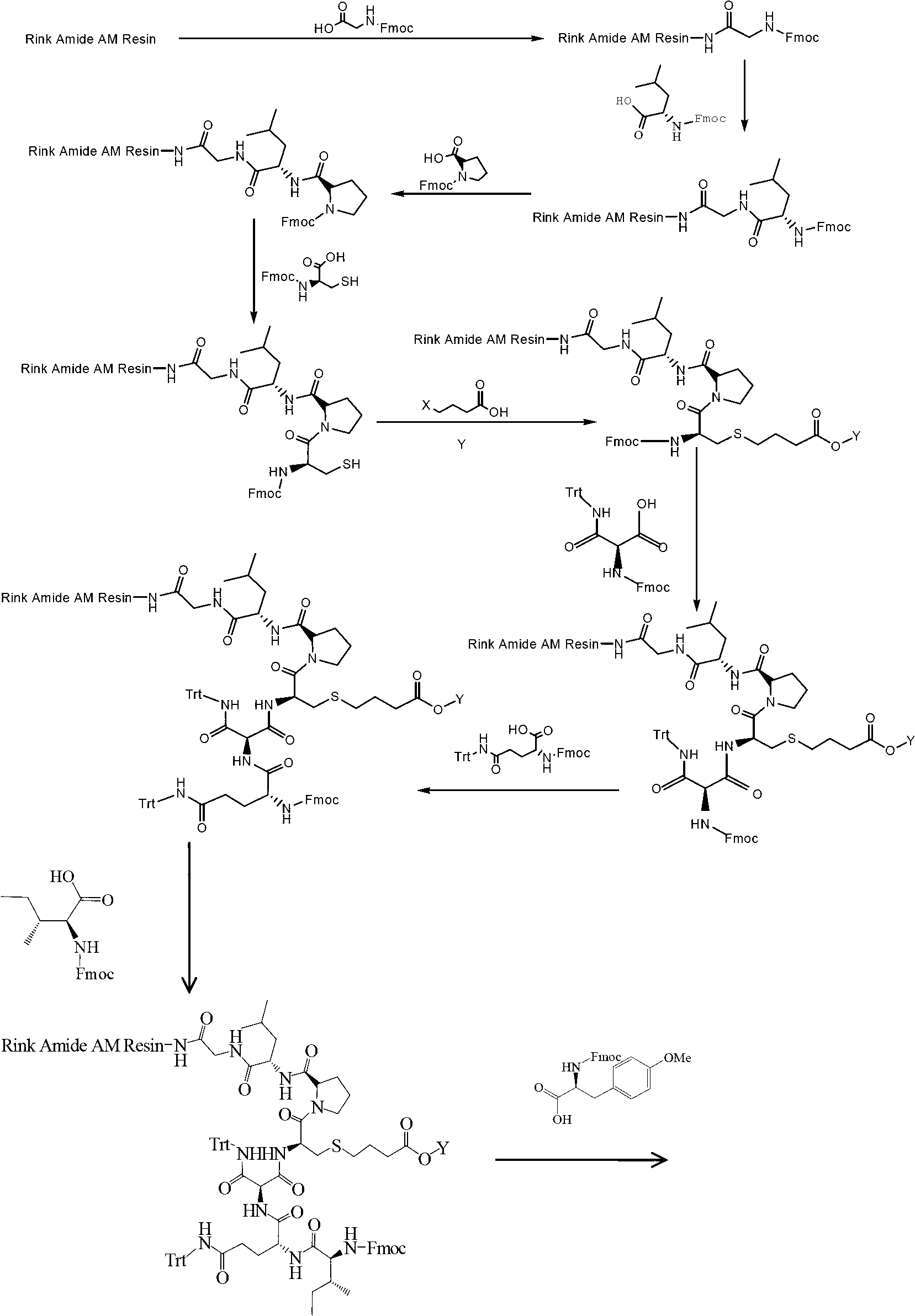
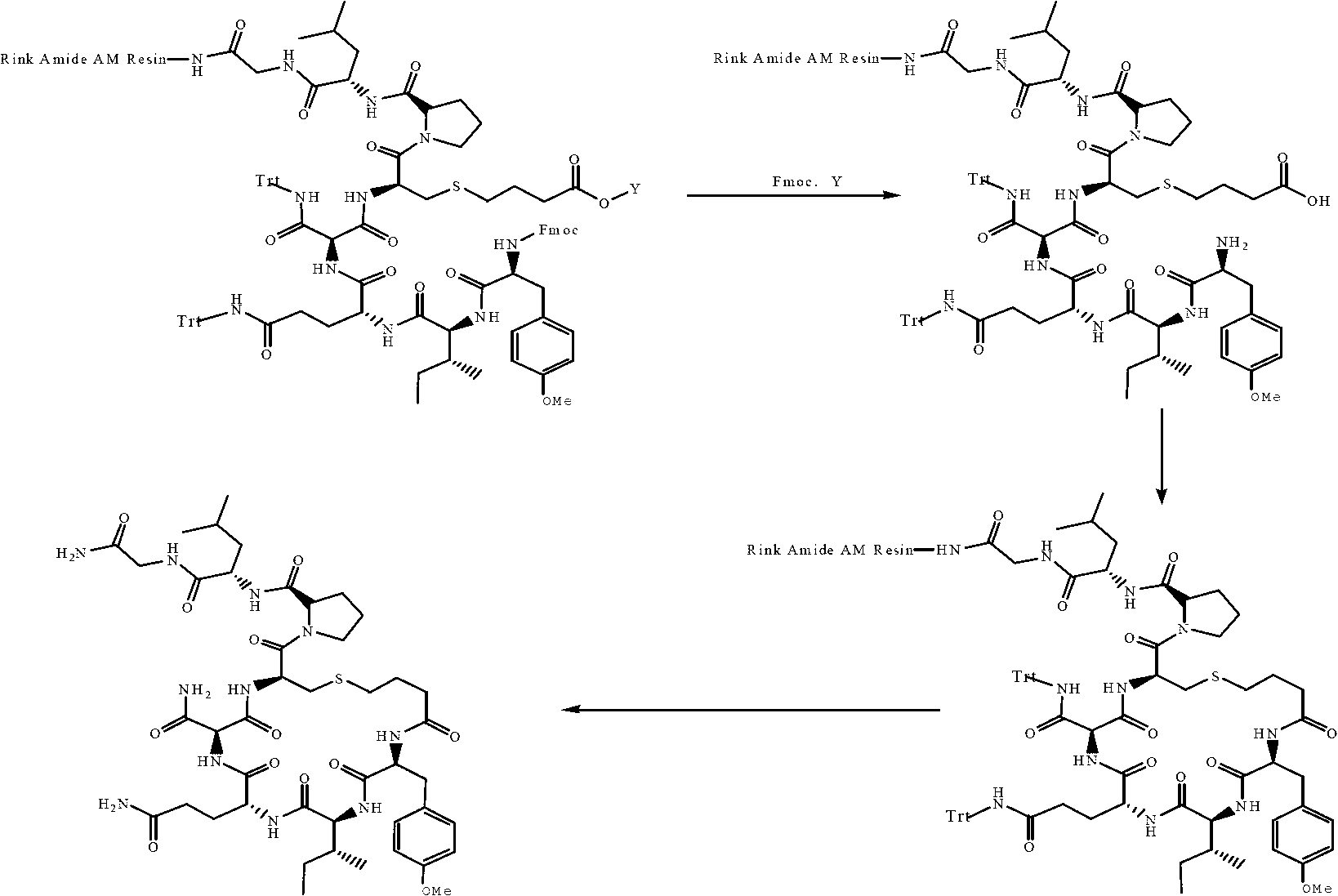
Solid-phase synthesis method of carbetocin
A technology for carbetocin and solid-phase synthesis is applied in the field of solid-phase synthesis of carbetocin, and can solve the problem of being unfavorable for large-scale production, easy to produce polymers, and removing side chain allyl protecting group reagents. Expensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step (1) Preparation of Fmoc-Gly-Rink Amide AM Resin
[0032]Weigh 10g of Rink Amide AM Resin (100 mesh, 0.38mmol / g), soak in 50mL DCM for 30min to fully swell the resin, wash with 70mL DMF twice (not less than 1min each time), and wash with 70mL 25% piperidine / DMF Remove the Fmoc protecting group twice at 30°C, the first time is 5 minutes, the second time is 15 minutes, and the middle is washed once with 70 mL of anhydrous DMF, not less than 1 minute. Wash with 70 mL of anhydrous DMF, MeOH, DCM, and DMF successively 2, 1, 1, and 2 times, each time not less than 1 min.
[0033] Weigh 3.39g of Fmoc-Gly-OH, 1.55g of HOBt, and 1.77mL of DIC into 10mL of anhydrous DMF, and activate at 0~5°C for 5min. Add solid-phase synthesis tube and react at 30°C for 2.0~4.0h. The end point of the reaction is determined by the ninhydrin method. Wash with 70 mL of anhydrous DMF for 2 times, each time not less than 1 min, to obtain Fmoc-Gly-Rink Amide AM Resin.
[0034] Step (2) Tyr(Me)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com