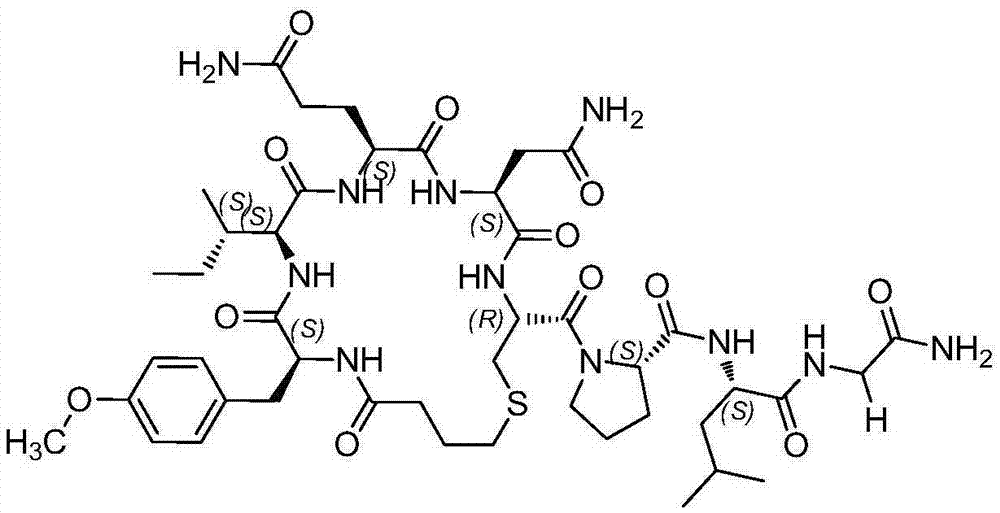
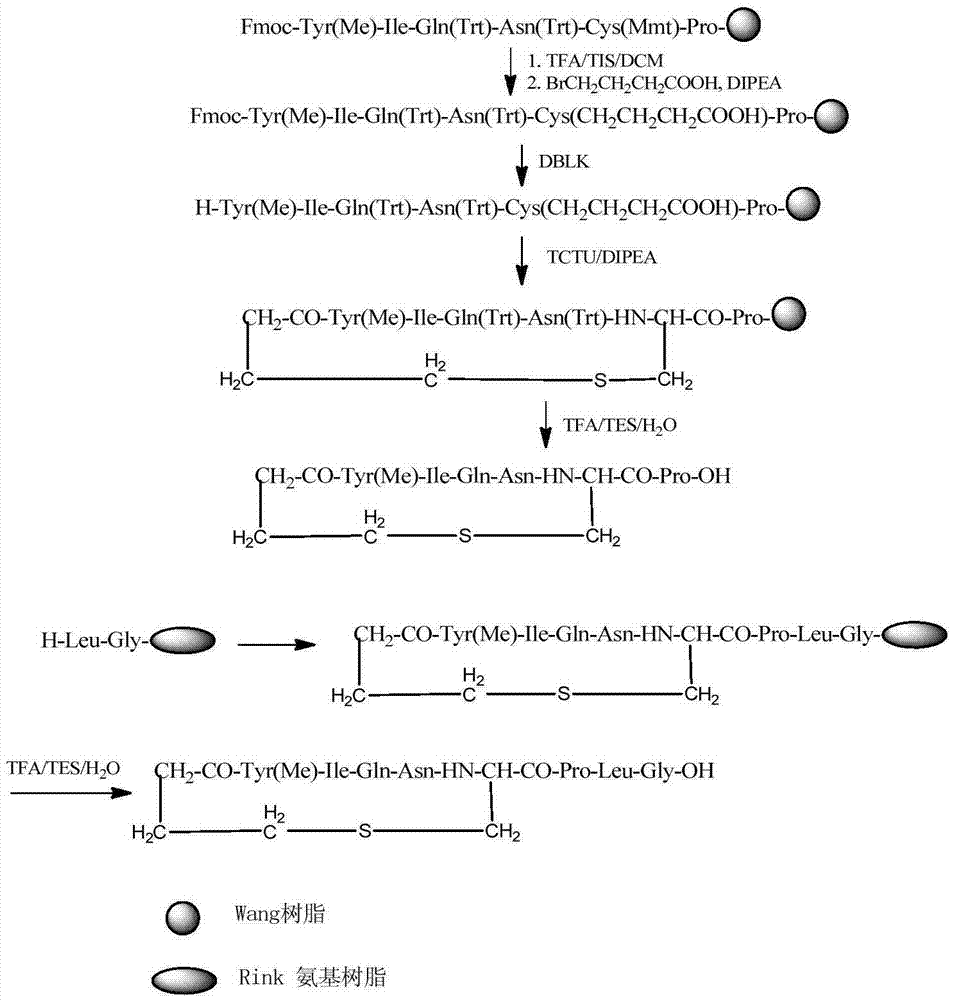
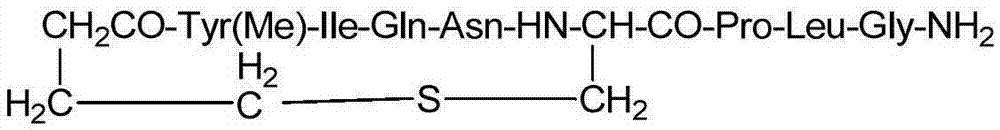Solid-phase fragment synthesis method for carbetocin
A carbetocin and fragment technology, which is applied in the field of solid-phase synthesis of carbetocin, can solve the problems of reducing the yield of refined peptides, increasing glycine-deficient impurities, and large reaction steric hindrance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 prepares Fmoc-Pro-Wang resin
[0035] Weigh 11.949 g (13.9 mmol) of Wang resin with a substitution degree of 1.166 mmol / L, wash with DMF twice, and swell the resin with DMF for 30 minutes. Weigh 0.183g (1.39mmol) of DMAP into the reaction column. Weigh 29.69g (83.4mmol) Fmoc-Pro-OH, add an appropriate amount of DCM to dissolve, add 8.7ml (55.6mmol) DIPCDI under ice-water bath cooling, activate this solution for 3 minutes, add this solution to the reaction column, and react with nitrogen gas bubbling for 3h. The reaction solution was pumped out, washed with DMF three times, and 23ml of pyridine and 28ml of acetic anhydride were added to carry out the blocking reaction overnight. The reaction solution was extracted, DMF was washed 3 times, methanol shrank the resin 3 times, and the resin was drained to obtain 22.51 g of Fmoc-Pro-Wang resin, and the detection substitution degree was 0.627 mmol / g.
Embodiment 2 7
[0036] The preparation of embodiment 2 heptapeptide cyclic peptide
[0037] Weigh 15.959 g (10 mmol) of the Fmoc-Pro-Wang resin obtained in Example 1, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, deprotect DBLK for 6 min+8 min, and wash with DMF 6 times. Weigh 18.47g (30mmol) Fmoc-Cys(Mmt)-OH and 4.46g (33mmol) HOBT and dissolve them in DMF / DCM (V:V=1:1), add 5.2mL (36mmol) DIPCDI under ice-water bath to activate for 3min , the mixed solution was added to the reaction column, and reacted at room temperature for 2 hours, and the end point of the reaction was detected by ninhydrin.
[0038] After the reaction is over, wash the resin with DMF for 3 times, add DBLK for deprotection for 5min + 7min, wash the resin with DMF for 6 times, and detect the color of the resin with ninhydrin. Weigh 17.90g (30mmol) Fmoc-Asn(Trt)-OH and 4.46g (33mmol) HOBT and dissolve them in DMF / DCM (V:V=1:1), add 5.2mL (36mmol) DIPCDI unde...
Embodiment 3
[0046] Embodiment 3: Preparation of carbetocin peptide resin
[0047] Weigh 5.40g (3.0mmol) of Rink Amide resin with a substitution degree of 0.557mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, deprotect the DBLK for 6min+8min, DMF Wash 6 times. Weigh 2.68g (9mmol) Fmoc-Gly-OH and 1.34g (9.9mmol) HOBT to dissolve in DMF, add 1.55mL (10.8mmol) DIPCDI to activate for 3min, then add the mixture to the reaction column, react at room temperature for 2 hours, The end point of the reaction was detected with ninhydrin.
[0048] After the reaction is over, wash the resin with DMF for 3 times, add DBLK for deprotection for 5min + 7min, wash the resin with DMF for 6 times, and detect the color of the resin with ninhydrin. Weigh 3.18g (9mmol) Fmoc-Leu-OH and 1.34g (9.9mmol) HOBT and dissolve them in DMF / DCM (V:V=1:1), add 1.55mL (10.8mmol) DIPCDI to activate for 3min under ice-water bath, The mixed solution was added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com