Method for purifying carbetocin
A technology of carbetocin and pure product, which is applied to the preparation method of peptide, chemical instrument and method, organic chemistry and other directions, can solve the problem that the yield of carbetocin product is not high and carbetocin cannot be effective. Separation, low purity of carbetocin finished products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
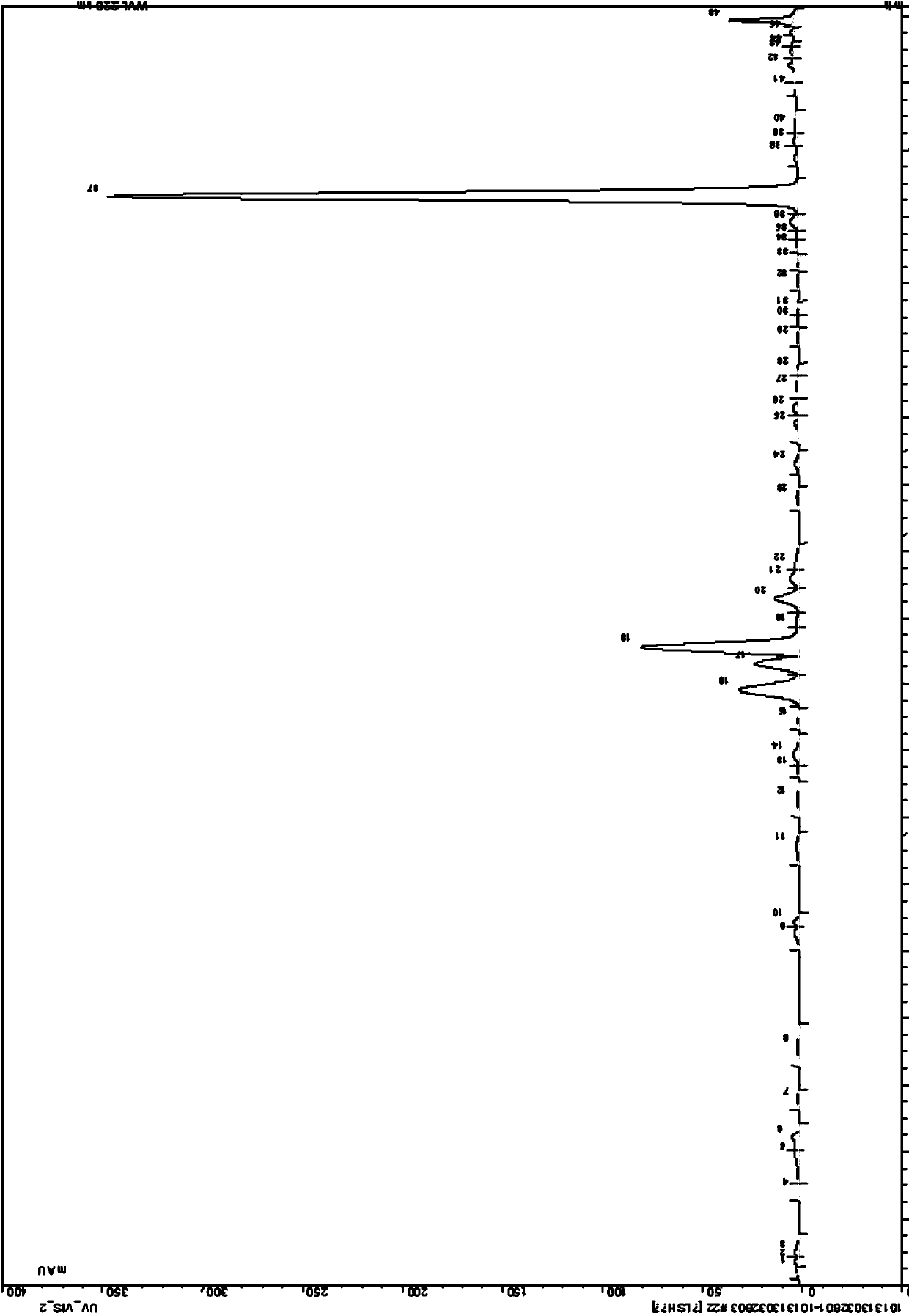
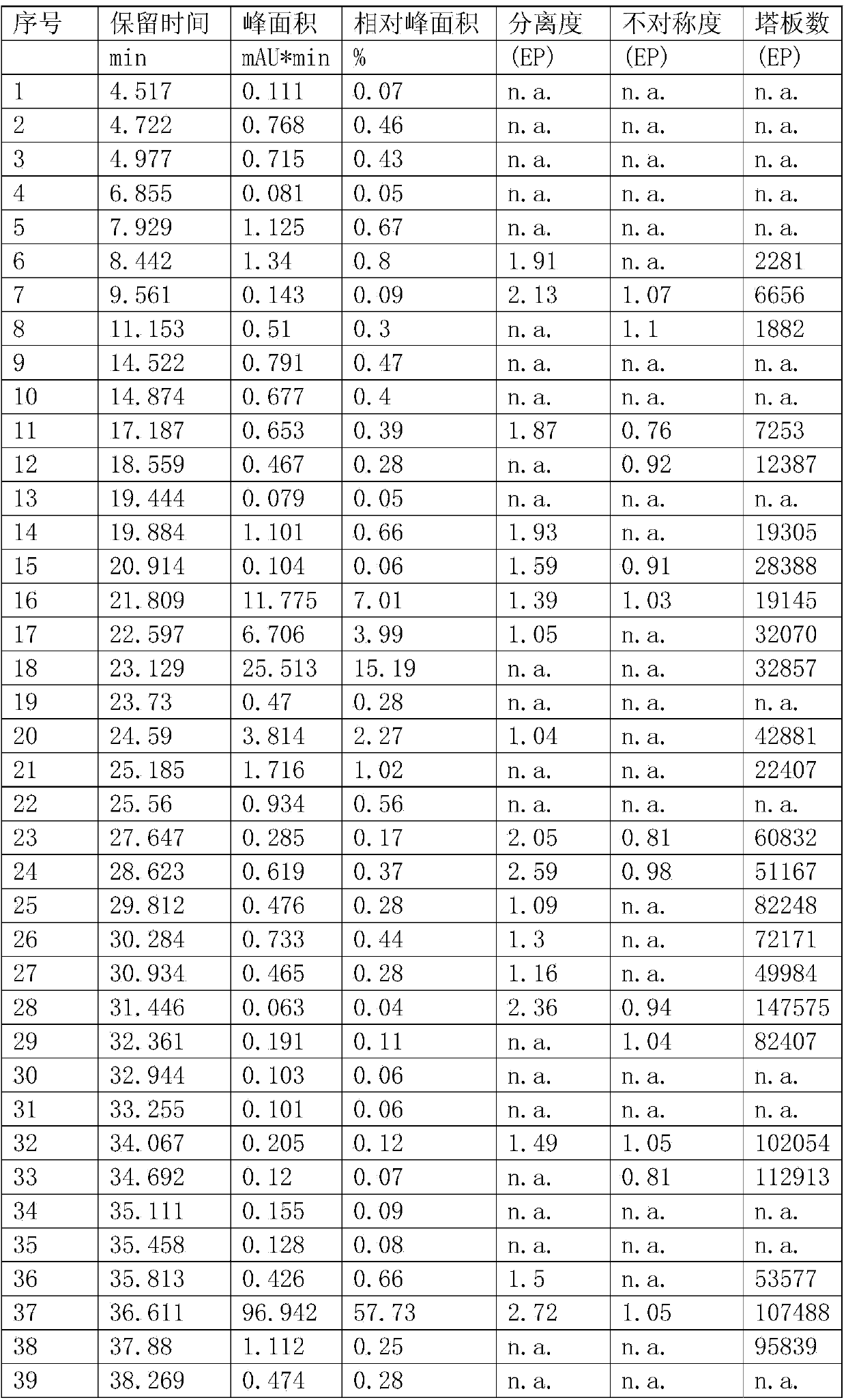
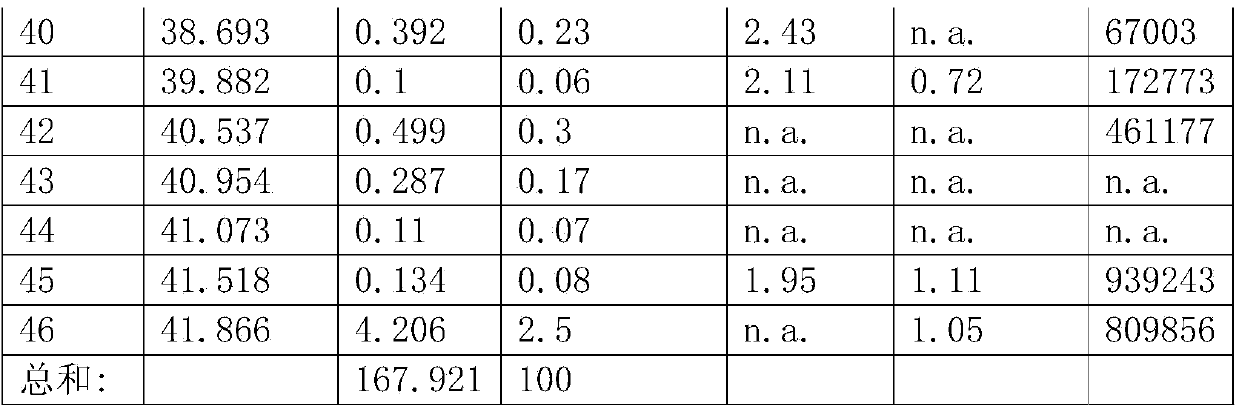
Image
Examples
Embodiment 1
[0095] Solid phase synthesis of carbetocin:
[0096] Scale: 1.0 mmol carbetocin Synthesis:
[0097] Step (1) Peptide Resin Reaction: Weigh 1.38g (1.1mmol) Wang Resin (the degree of substitution is 0.80mmol / g) into the reaction kettle, add 15ml DCM, stir and swell at 15-35°C for 30min, and remove by suction filtration liquid. Weigh 1.17g (3.3mmol) Fmoc-Leu-OH and 0.45g (3.3mmol) HOBt into a dissolution tank. Measure 6ml of DMF solvent into the tank, stir to dissolve, cool down to about 0-15°C in an ice bath after complete dissolution, add 510μl (3.3mmol) of DIC, stir well, control the temperature at 10-25°C for 5min, add 0.04g (0.3mmol) DMAP, then added to the reaction kettle, reacted at 25-35°C for 5h, took a sample to detect that the degree of substitution was 0.73mmol / g, and pumped out the reaction solution. Measure 10ml of DMF into the reaction kettle, stir and wash for 3 minutes, and repeat the washing 3 times. Measure 525 μl (5.5 mmol) of acetic anhydride, 445 μl (5.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com