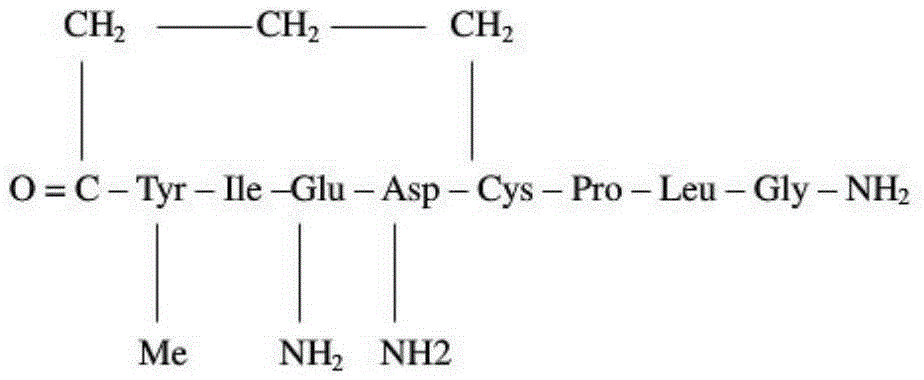
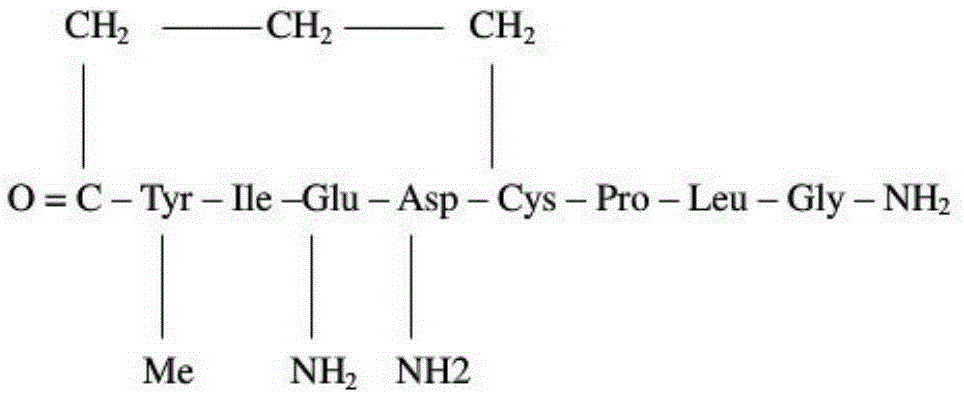
Method for preparing carbetocin through solid phase and liquid combining method
A carbetocin-binding technique, applied in the fields of peptide preparation, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult sample purification, cumbersome steps, inconvenient operation, etc., and avoid glycine-deficient peptide impurities. , The process is easy to operate, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of Fmoc-Cys(Trt)-Wangresin
[0056] The carrier Wanggresin550.0g (sub=1.0mmol / g) was placed in a synthesis column, washed twice with 4LDMF, and swelled with 4LDCM for 30min; after the DCM was filtered off, Fmoc-Cys(Trt)-OH / DIC / HOBt was added Mixed solution [Weigh 644g (1100mmol) Fmoc-Cys(Trt)-OH and 164g (1210mmol) HOBt into a dissolving bottle, add 2L of DMF and DCM mixed solution with a volume ratio of 1:1 and stir to dissolve, add under ice-water bath 190ml (1210mmol) DIC, activated for 5min], add 6.7g (55mmol) DMAP after reacting for 10min; react for 5h, remove the reaction solution, wash 3 times with 4LDMF, add end-capping reagent 4L (1L acetic anhydride and 0.85L pyridine dissolved in 2.15 in DMF) reacted for 1 h, filtered off the reaction solution, washed with DMF, DCM, and methanol three times, and dried in vacuum to obtain 856.0 g of Fmoc-Cys(Trt)-Wangresin; the degree of substitution was determined to be 0.614 mmol / g by sampling. The...
Embodiment 2
[0057] Embodiment 2: Preparation of Fmoc-Cys(Trt)-CTCresin
[0058] Weigh CTCresin63.0g (sub=1.0mmol / g) and place it in a synthesis column, wash it twice with 500mL DMF, add 500mL DCM to swell for 30min; after filtering off the DCM, add 74g (126mmol) of Fmoc-Cys(Trt)- OH DCM / DMF (volume ratio 3:1) solution 300mL, after stirring, add DIEA40ml (250mmol), react for 5h, remove the reaction solution, add DCM / CH 3 400ml of OH / DIEA (volume ratio 17:2:1) mixed solution was capped for 1h; then washed three times with DMF, DCM and methanol, and dried in vacuum to obtain 100.0g of Fmoc-Cys(Trt)-CTCresin. The measured degree of substitution was 0.632mmol / g. The total synthesis scale was calculated to be 61 mmol.
Embodiment 3
[0059] Example 3: Fragment I Peptide Resin:
[0060] Br-(CH 2 ) 3 Preparation of -CO-Tyr(Me)-Ile-Gln(Trt)-Asn(Trt)-Cys(Trt)-Wangresin
[0061] Accurately weigh Fmoc-Cys(Trt)-Wangresin 823g (synthetic scale 500mmol) with a degree of substitution of 0.614mmol / g in Example 1 and place it in a synthesis column, add 4LDCM to swell for 30min; after filtering off the DCM, wash with 800mlDMF twice, Add 4L of 20% piperidine / DMF solution for deprotection twice, react for 10min and 10min respectively; then wash with DMF 6 times, 4L each time; 2.5 LDMF was dissolved, pre-cooled in an ice-water bath for 15-20 minutes, then added DIC170ml (1100mmol) to activate for 5 minutes, added the solution to the resin, and reacted for 2 hours. solution, washed 6 times with DMF, 4L each time; then deprotected. Repeated cyclic operation in this way, the protected amino acids Fmoc-Gln(Trt)-OH, Fmoc-Ile-OH, Fmoc-Tyr(Me)-OH, Br(CH 2 ) 3 -COOH, to obtain the fragment I peptide resin with fully protect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com