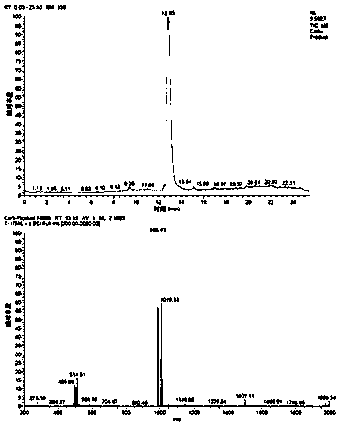
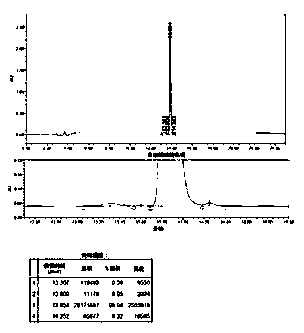
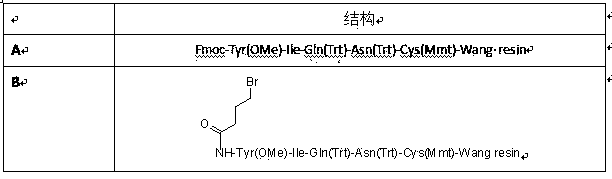
Method for preparing carbetocin
A carbetocin and resin technology, which is applied in the field of polypeptide drug preparation and achieves the effects of low price, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Preparation of Fmoc-Cys(Mmt)-resin
[0043] Weigh 30g Wang resin (0.50 mmol / g) and place it in a solid-phase reactor, add 300 ml of DMF to swell for about 10 minutes, and drain it; add 18.5 g (30 mmol) of Fmoc-Cys(Mmt)-OH, 4.05 g (30 mmol) of HOBt, 7.0 mL of DIC (45.0 mmol), 183.2 mg of DMAP (1.5 mmol), the mixture was shaken at room temperature for 2.5-3 hours, the reaction solution was drained, washed 3 times with 200 ml of DMF, and then washed with 200 mL pyridine / Ac 2 O / DMF=6:5:50 (v / v / v) solution was reacted for 2 hours. After the reaction was completed, the DMF was filtered off with suction, washed with DMF (250 ml) 3 times, and sucked dry.
[0044] (2) Preparation of Fmoc-Asn(Trt)-Cys(Mmt)-resin
[0045] Add about 200 mL of 20% PIP / DMF solution, shake at room temperature for about 5 minutes; then add about 200 mL of 20% PIP / DMF solution, shake for about 5 minutes at room temperature; drain, wash with DMF 3 times, and drain , the Kaiser test (Kaiser test) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com