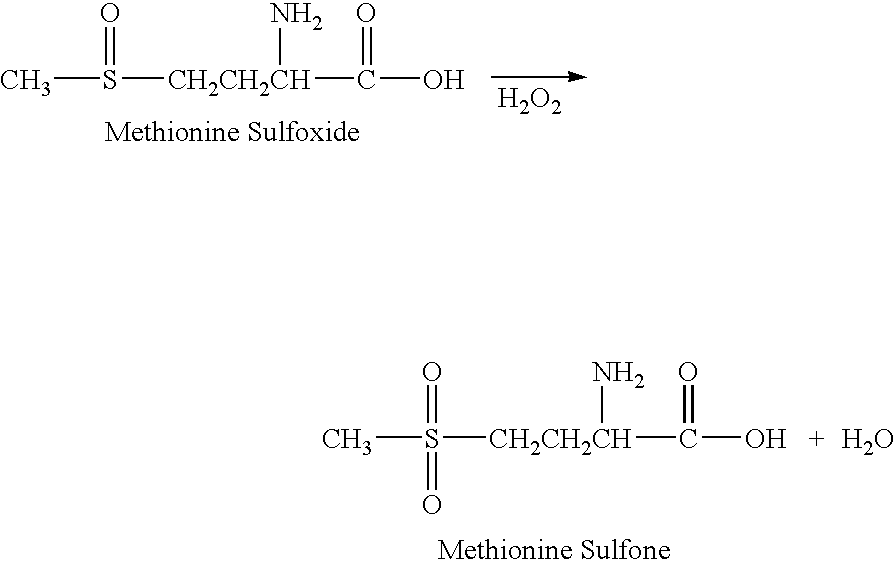
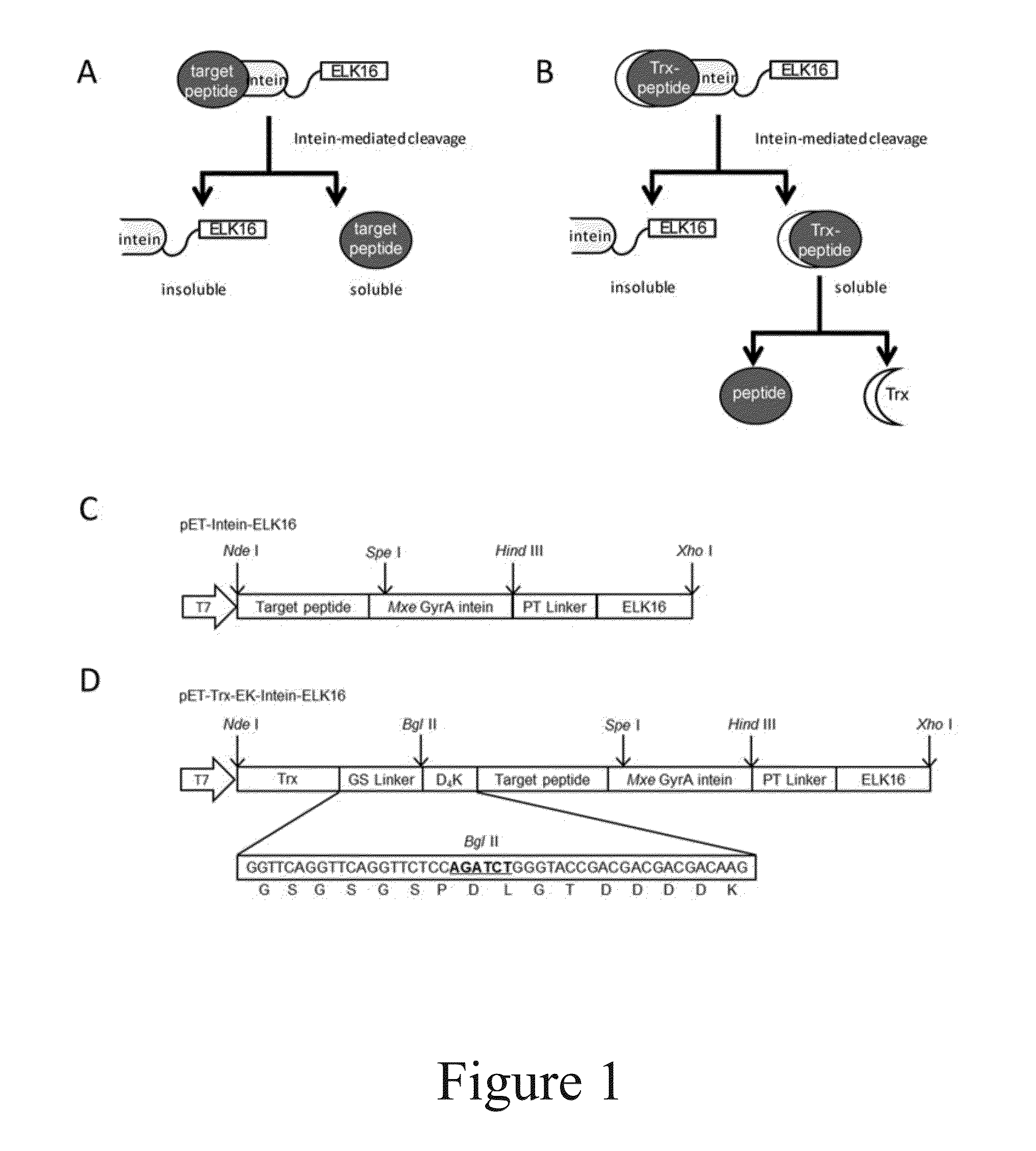
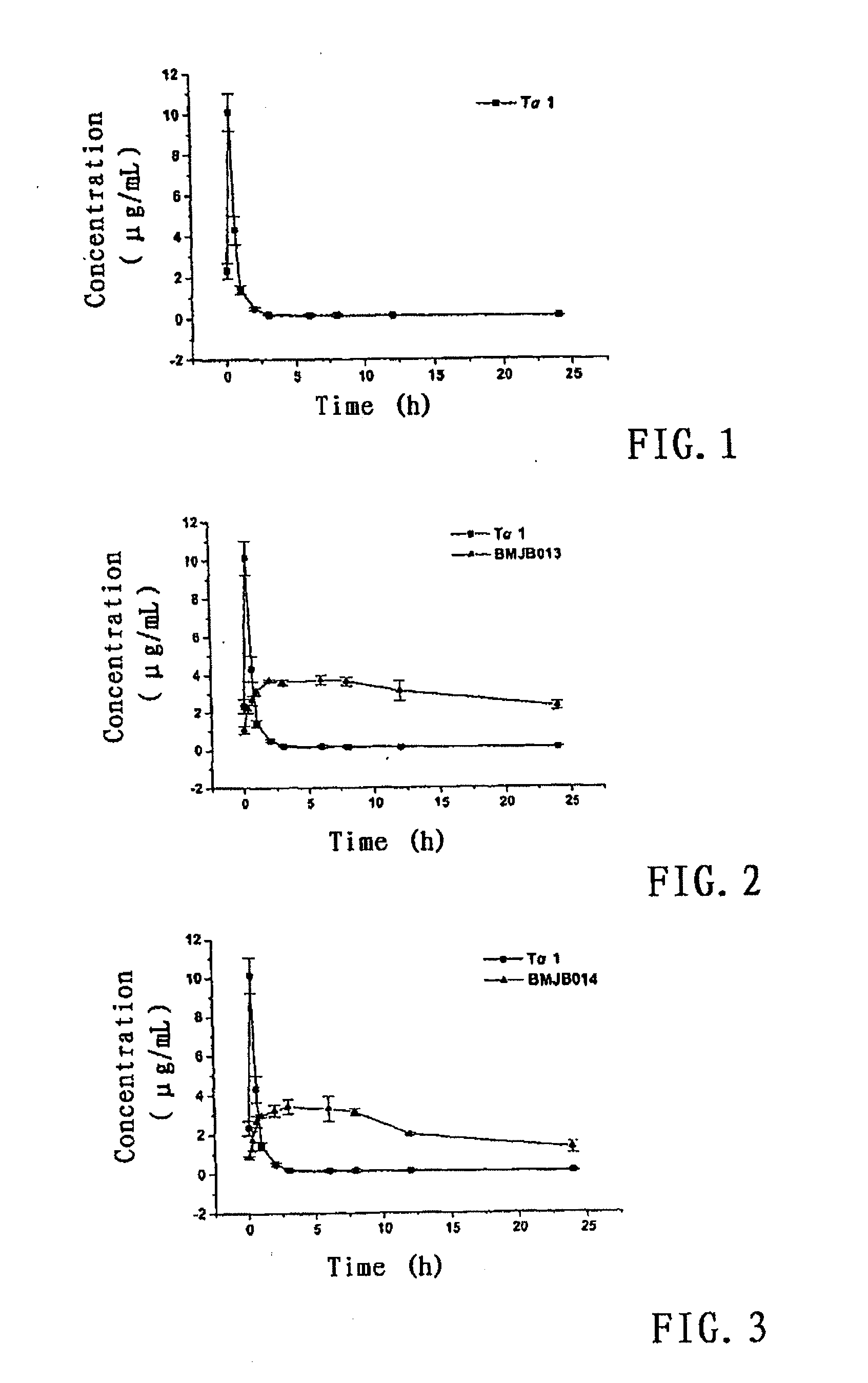
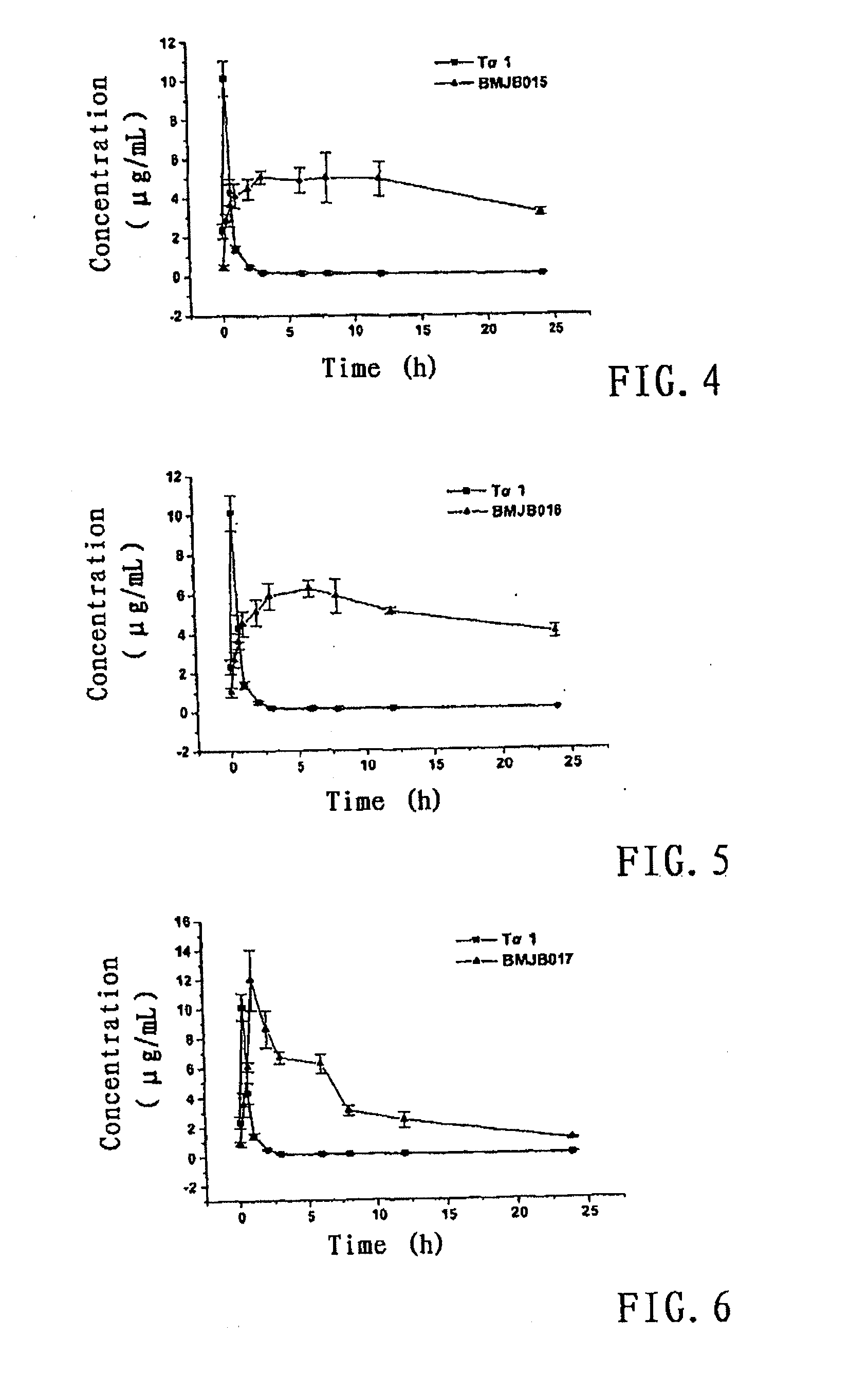
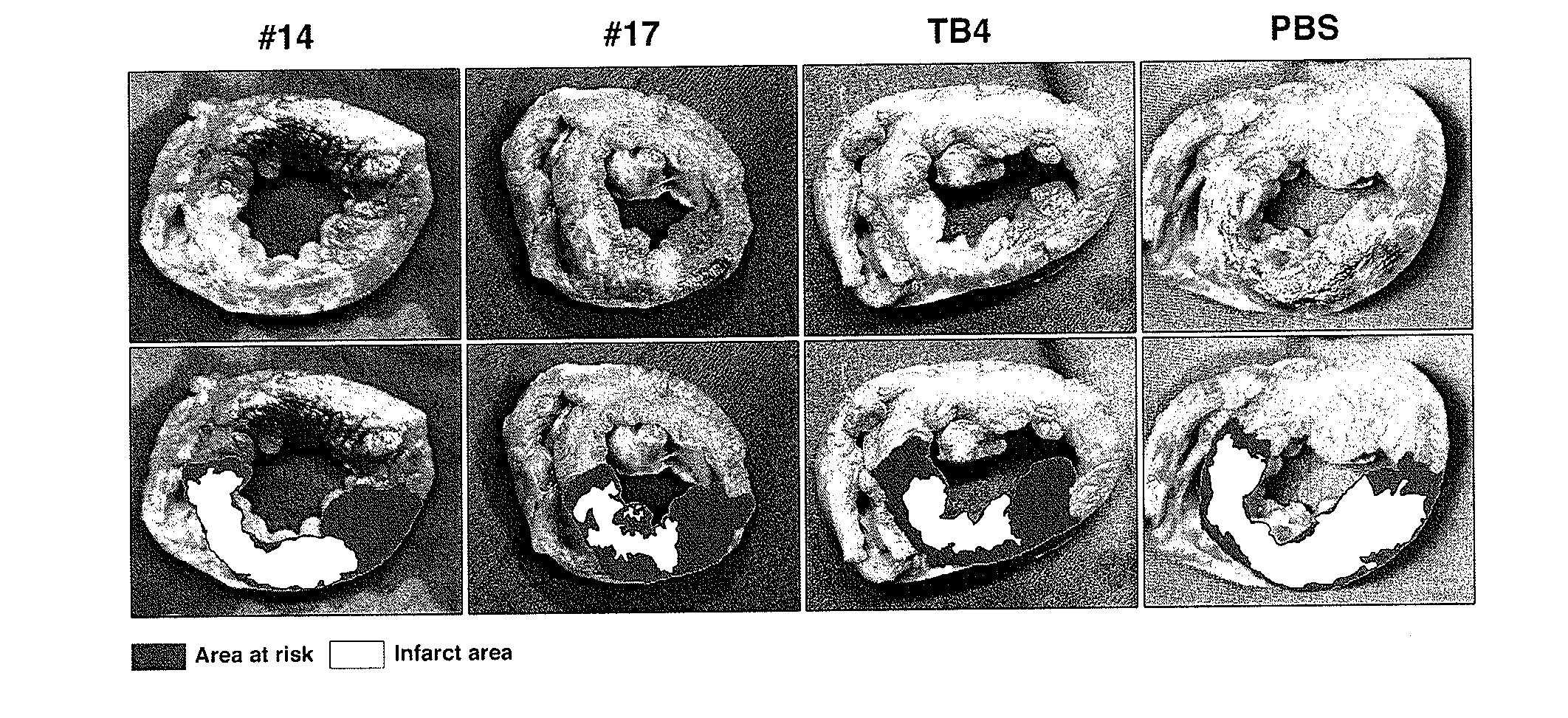
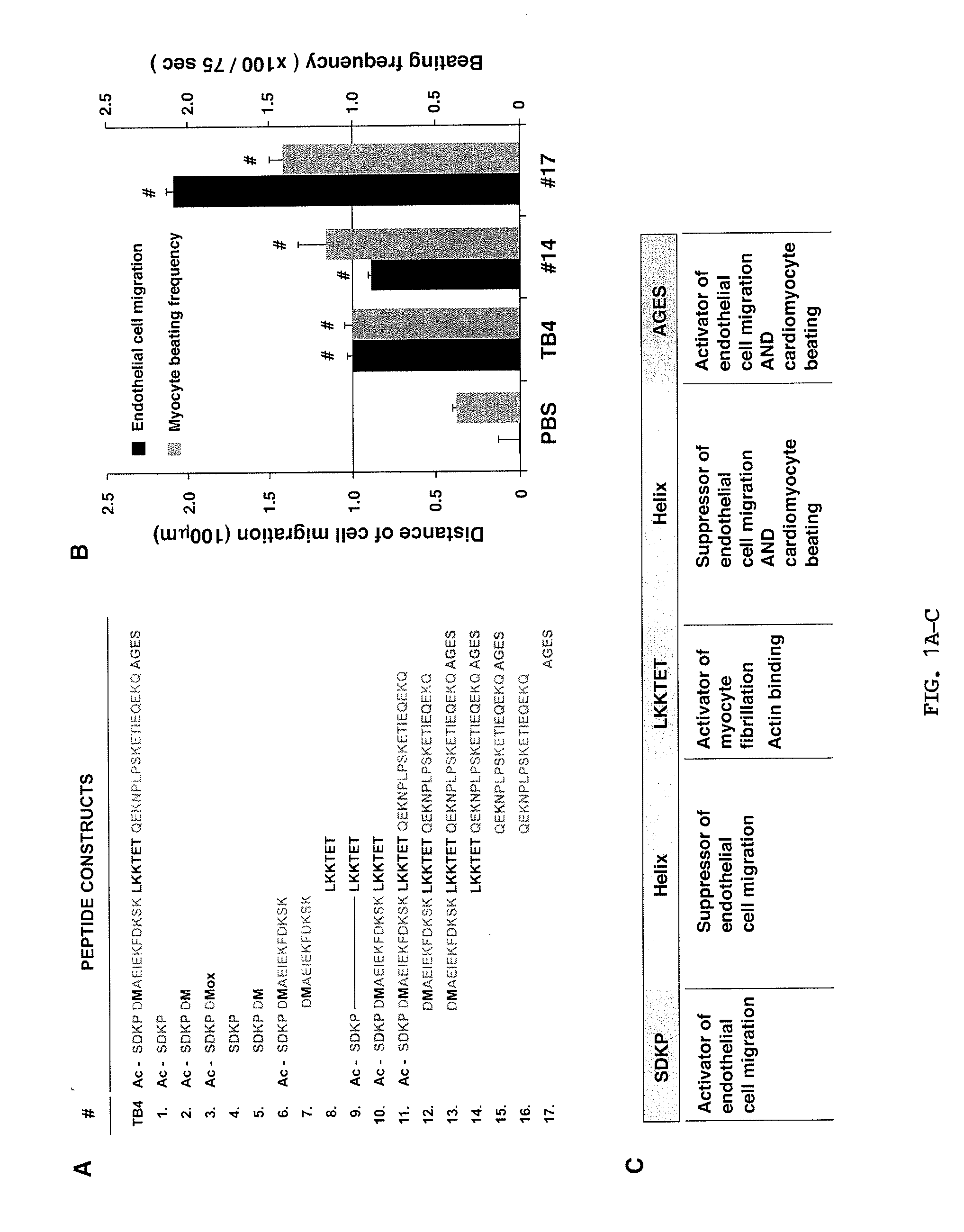
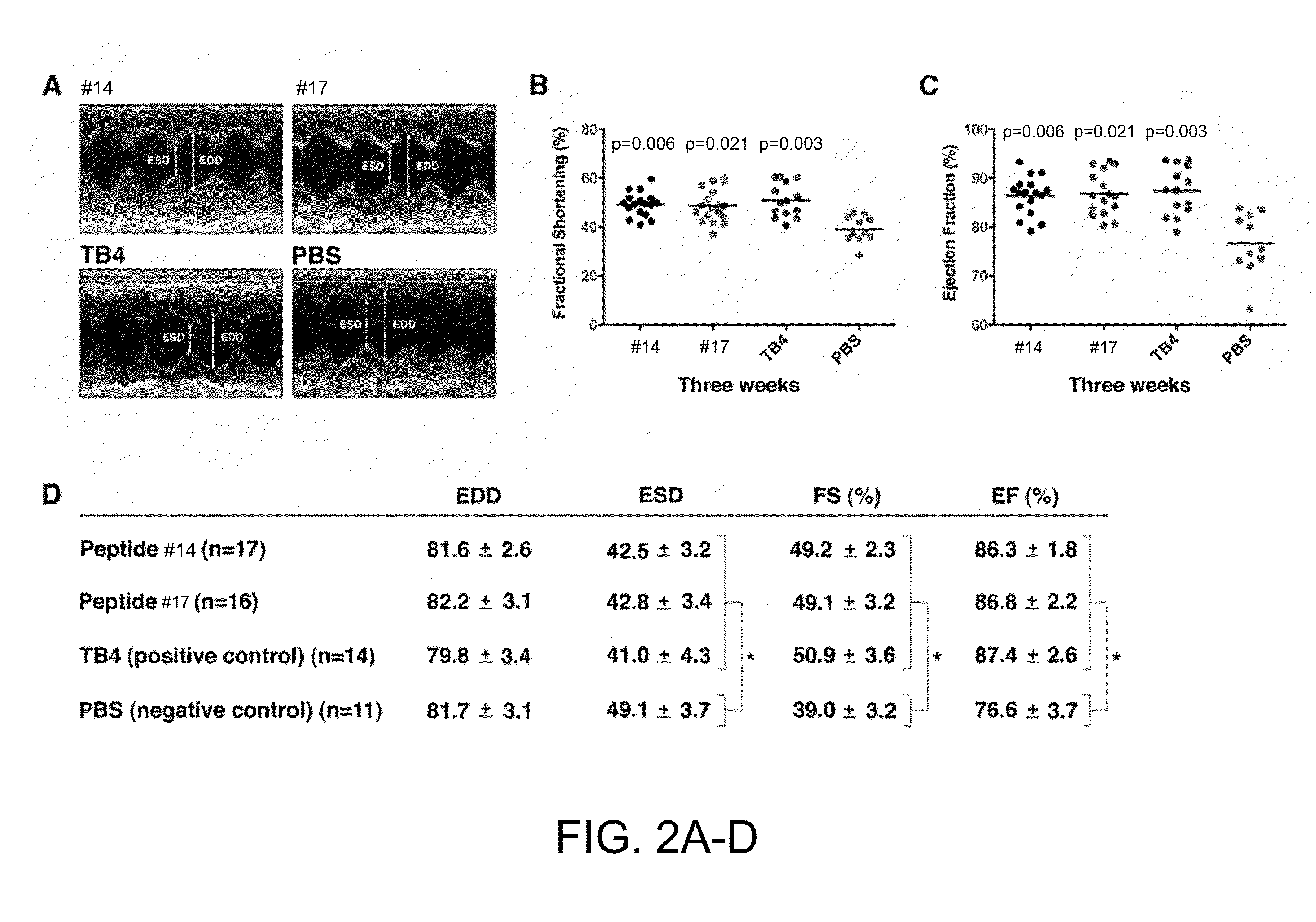
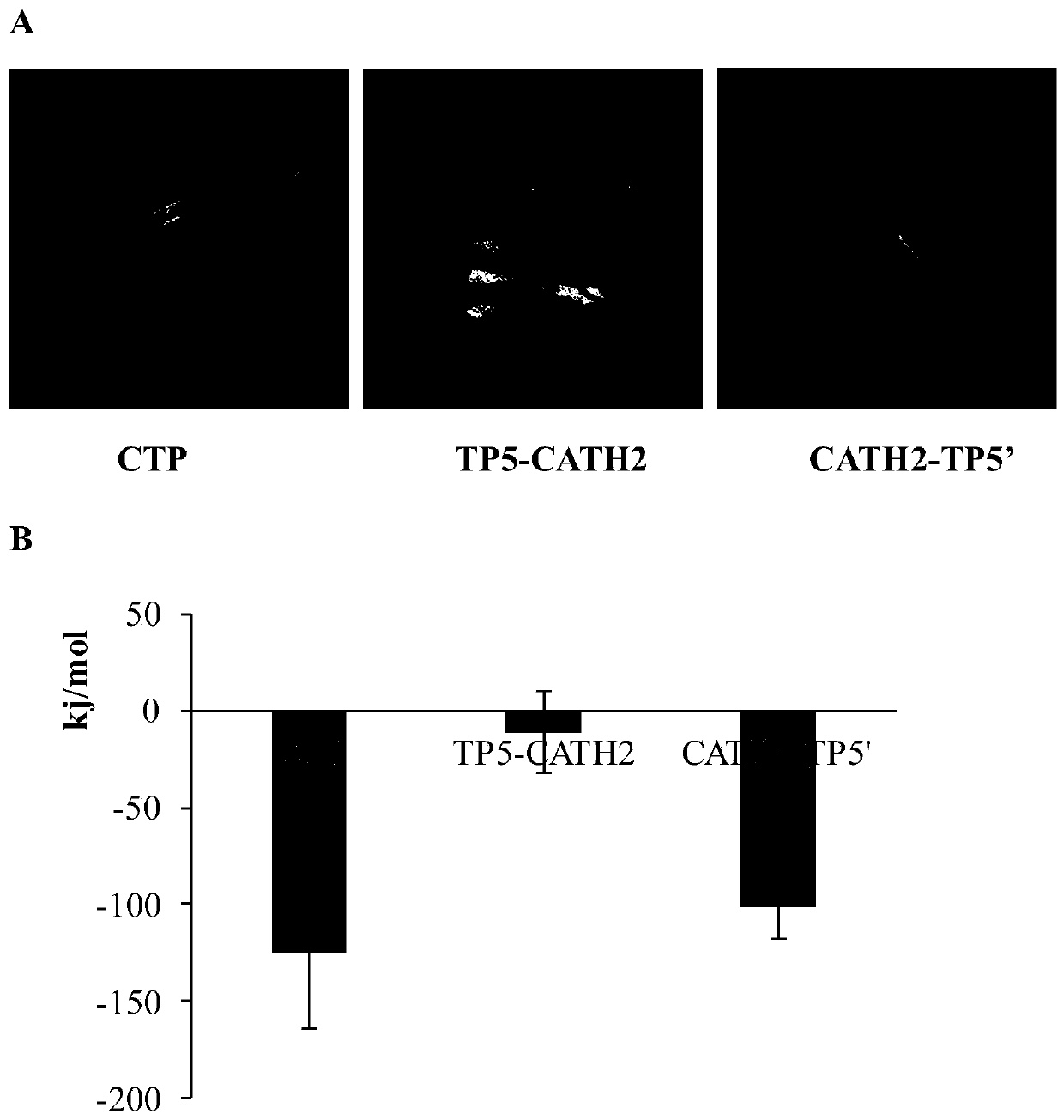
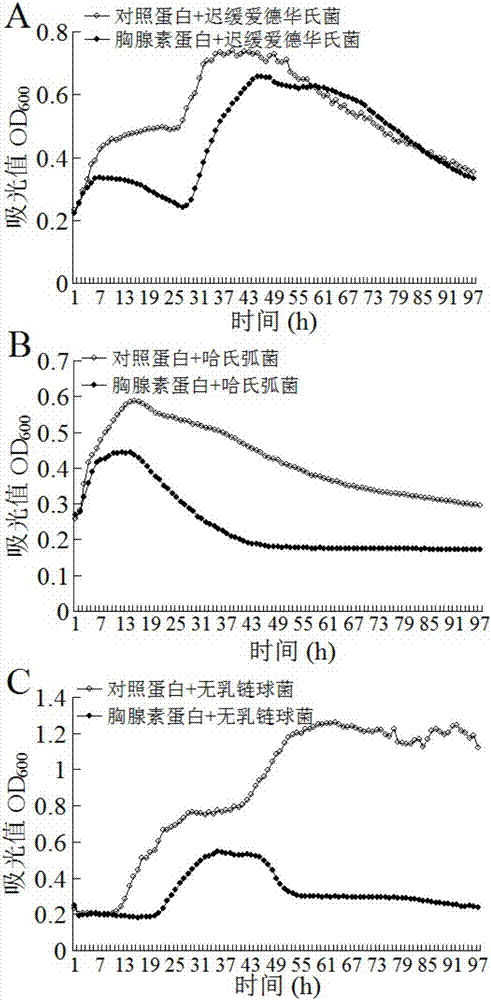
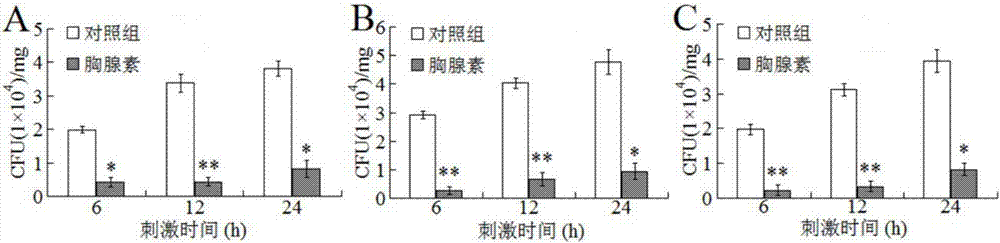
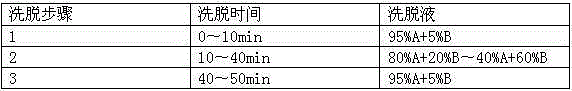
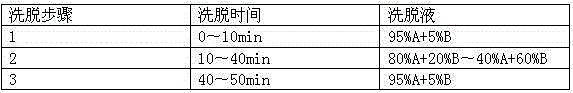
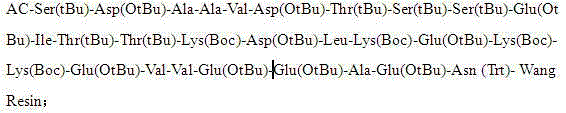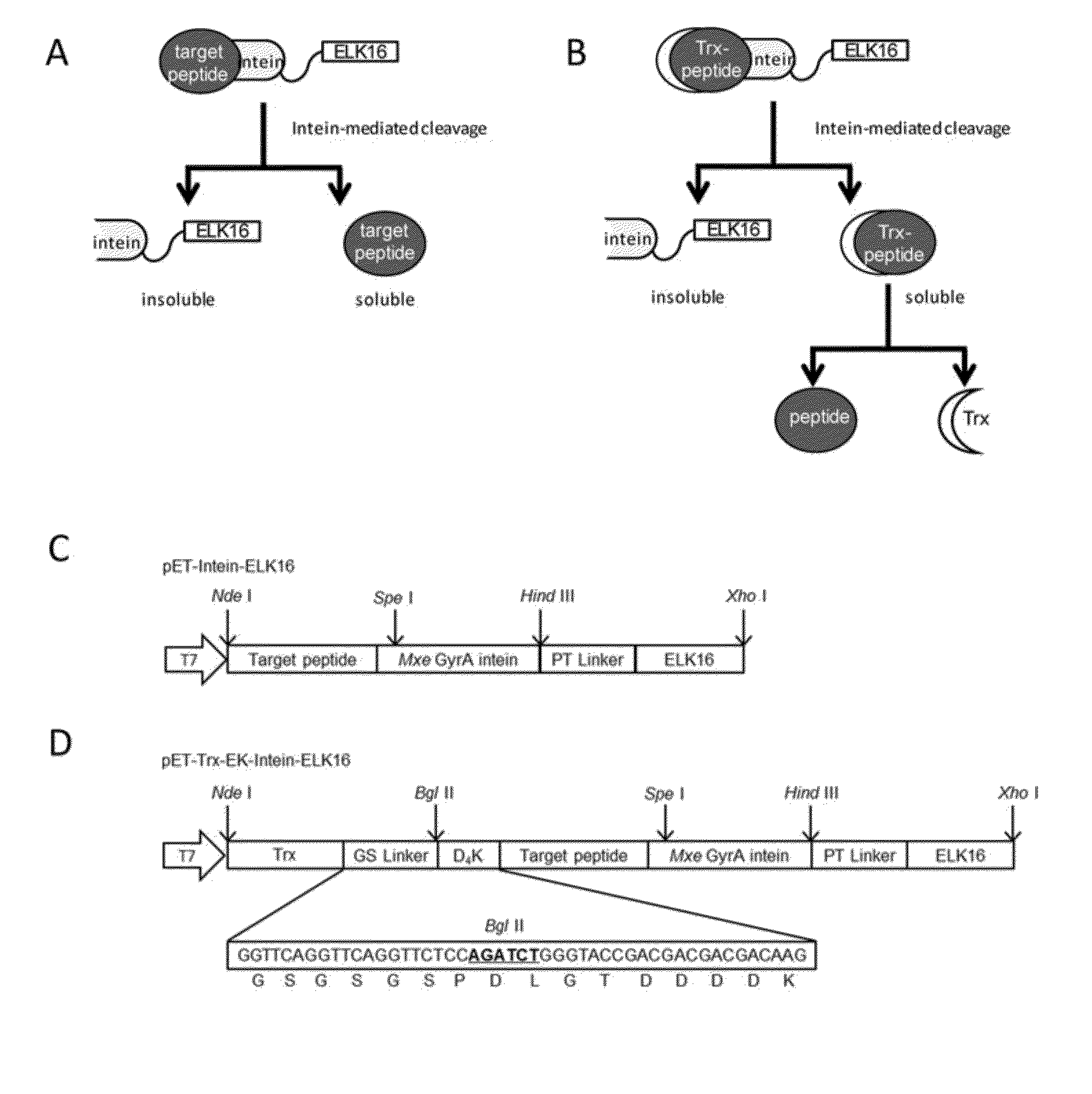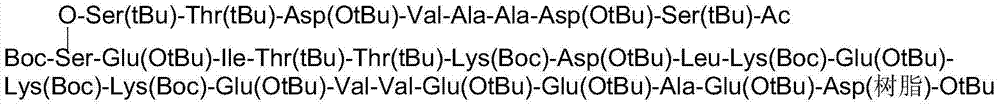Patents
Literature
113results about "Thymosin peptides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High purity peptides
InactiveUS20080287650A1High HPLC purityThymosin peptidesPeptide preparation methodsNesiritideHPLC measurement
Owner:TEVA PHARM USA INC
Method for improving stability of polypeptide active pharmaceutical ingredients
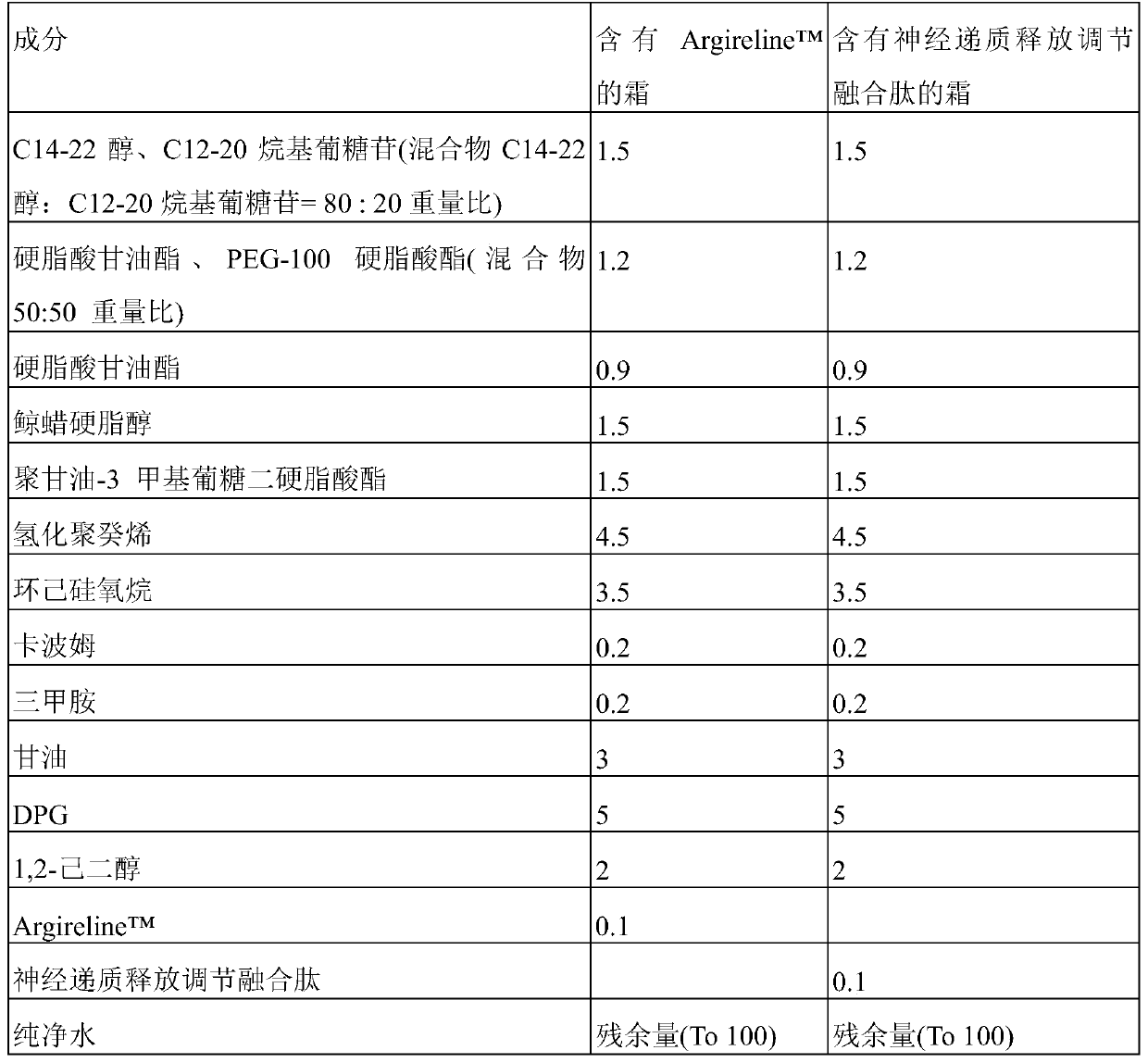
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Method for synthesizing thymalfasin
ActiveCN103497245AEase of mass productionEasy to purifyThymosin peptidesPeptide preparation methodsThymalfasinCombinatorial chemistry
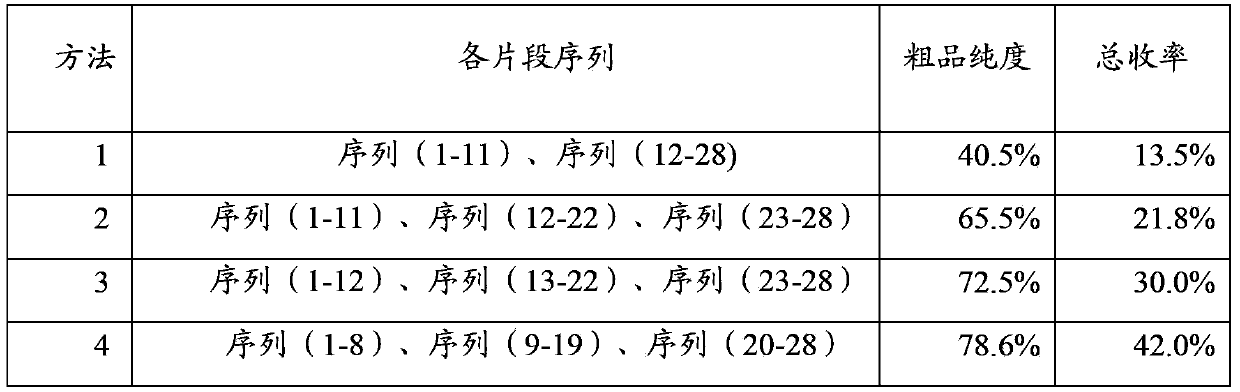
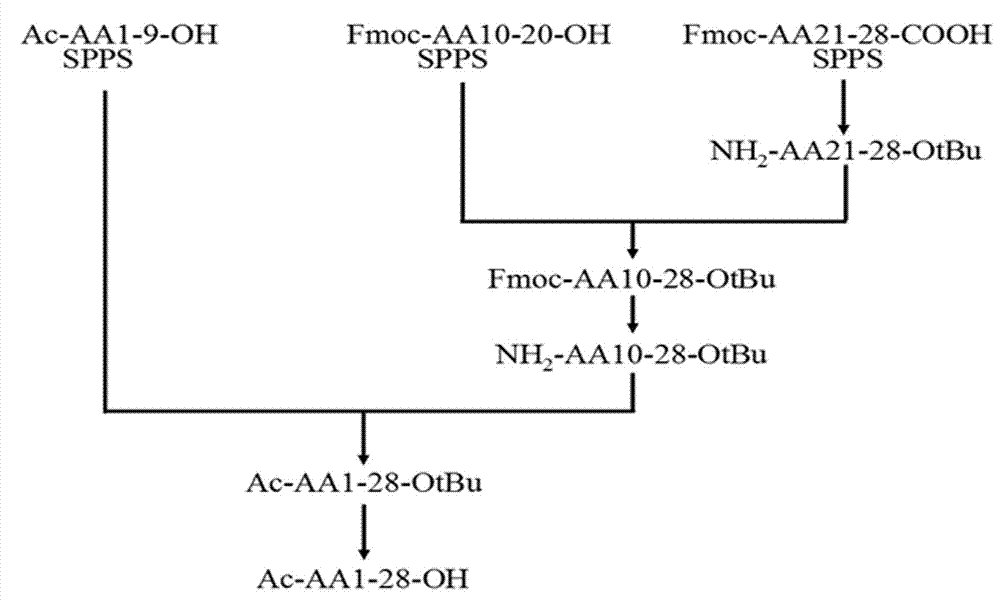
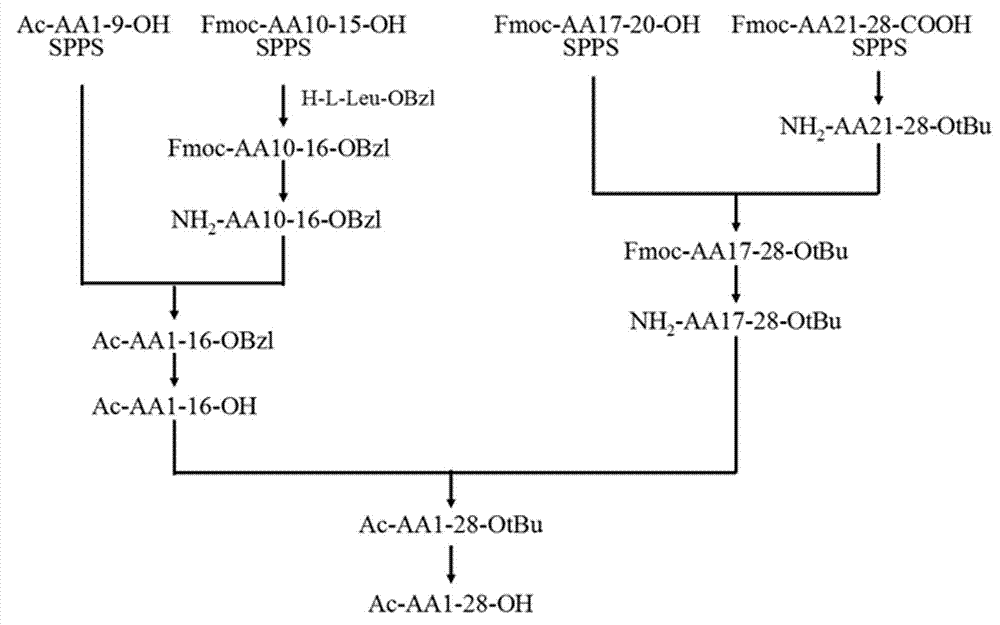
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing thymalfasin. According to the method of the invention, based on the amino acid sequence from the C terminal to the N terminal of the thymalfasin peptide chain, fragments of 1-8, 9-19 and 20-28 are synthesized, and the three polypeptide fragments are coupled to obtain thymalfasin. According to the invention, a plurality of fragments are synthesized simultaneously; the synthetic period is reduced by 2 / 3; intermediates are easy to purify; the cost is low; the purity of the finished products is high; by-products are few; the product yield is high; and the method facilitates large-scale production of thymalfasin.
Owner:HYBIO PHARMA
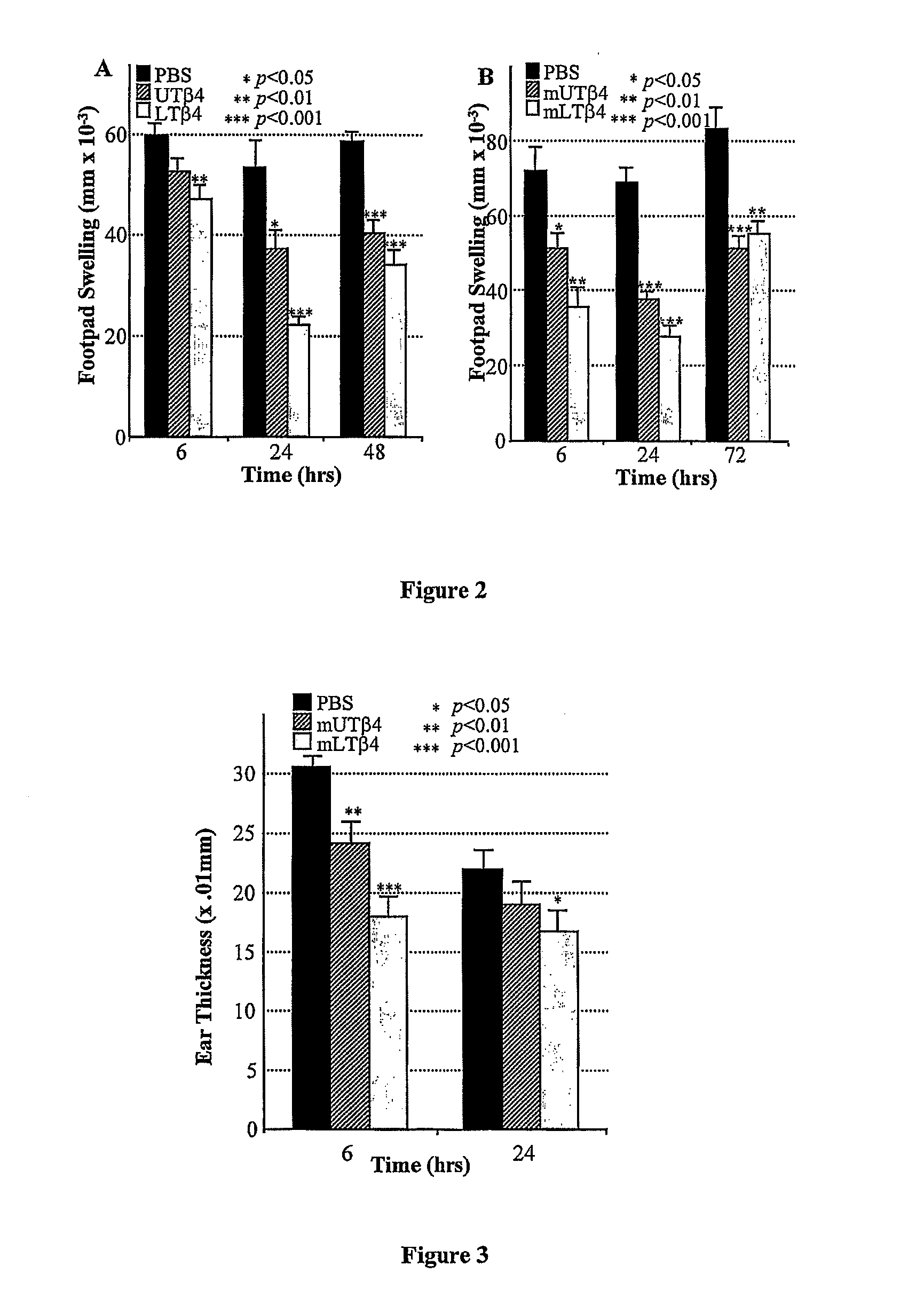
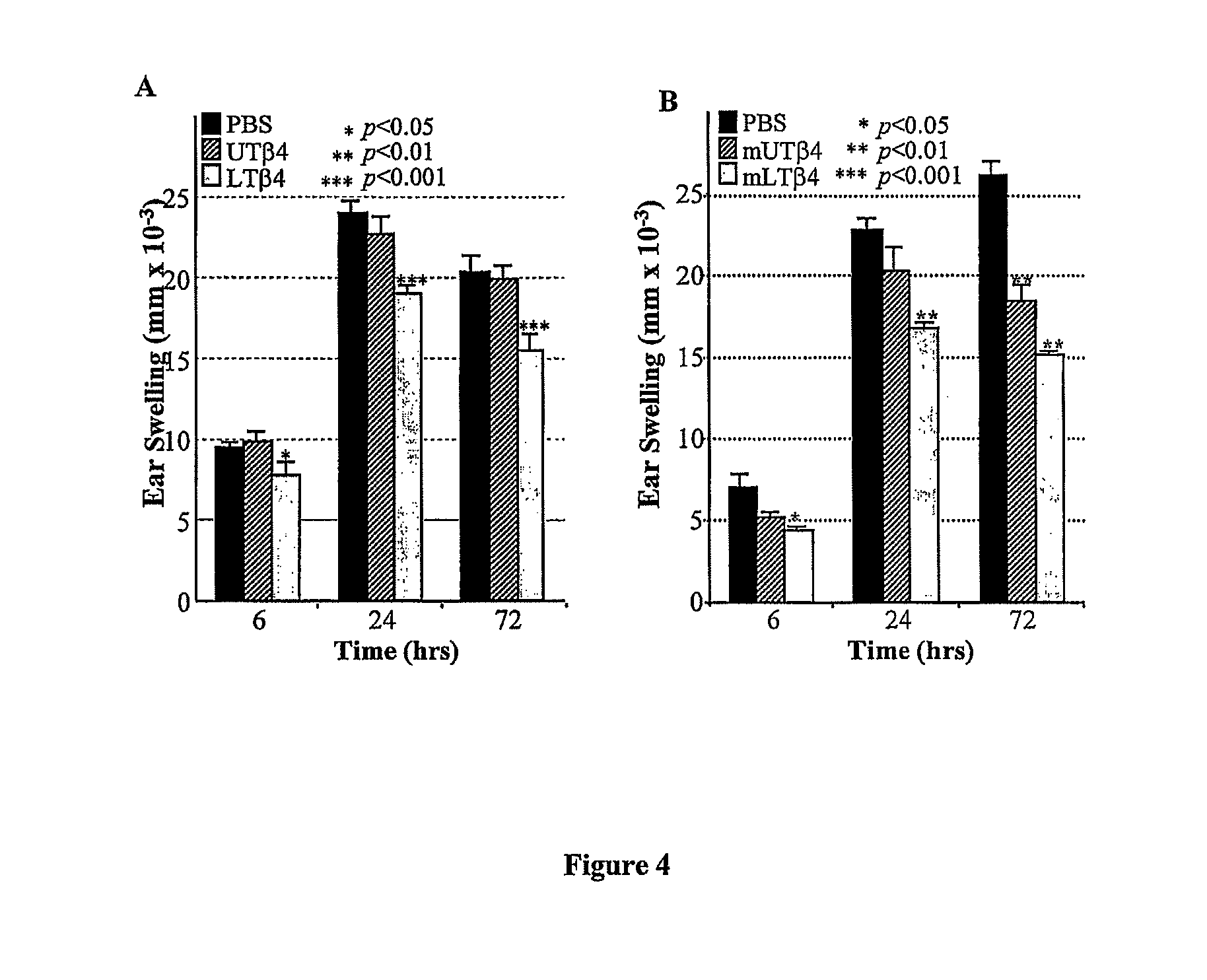
Anti-inflammatory and wound healing effects of lymphoid thymosin beta-4
The invention relates to a method of treating inflammatory conditions in a subject comprising administering to a subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant. The invention also provides a method of promoting wound healing in a subject comprising administering to the subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant. The invention also relates to methods of treating the above mentioned conditions in a subject comprising administering to the subject a nucleic acid encoding a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant. The invention also relates to pharmaceutical compositions comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4polypeptide variant, or salt thereof, and a pharmaceutically acceptable carrier.
Owner:KING'S COLLEGE LONDON +1
Method for preparing thymalfasin through dipeptide fragment liquid-solid bonding
InactiveCN104987382ASolve the problem of low coupling efficiencyAvoid formingThymosin peptidesPeptide preparation methodsDipeptideFluid phase
The invention belongs to the field of polypeptide synthesis, and relates to a method for preparing thymalfasin through dipeptide fragment liquid-solid bonding. By means of a liquid phase mode, a dipeptide fragment of continuous amino acid is synthesized, the dipeptide fragment is used for batch charging solid phase synthesis, and the problems that loci are difficult to couple, and a deletion peptide is prone to generation are solved. Meanwhile, purity and yield of crude peptides are increased. The technology is used for preparing the thymalfasin so that the purity of the crude peptides can be over 75%. Compared with the prior art, a synthetic route is simple, the problems that the difficult loci are not easy to couple and the deletion peptide is prone to generation are mainly solved, synthesis cost and purification cost are reduced, and industrial large-scale production is facilitated.
Owner:JINAN KANGHE MEDICAL TECH
Methods for production and purification of polypeptides
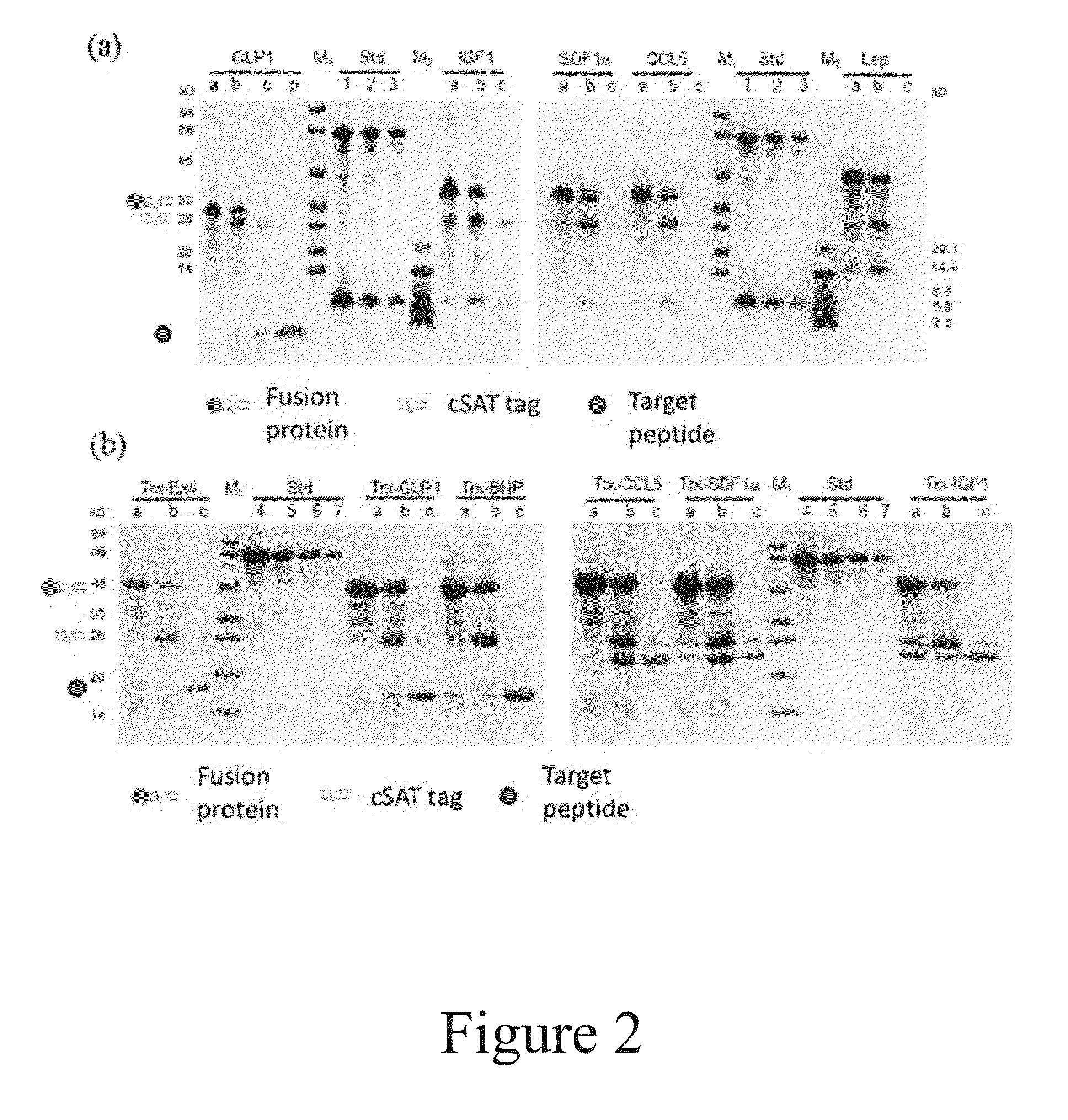
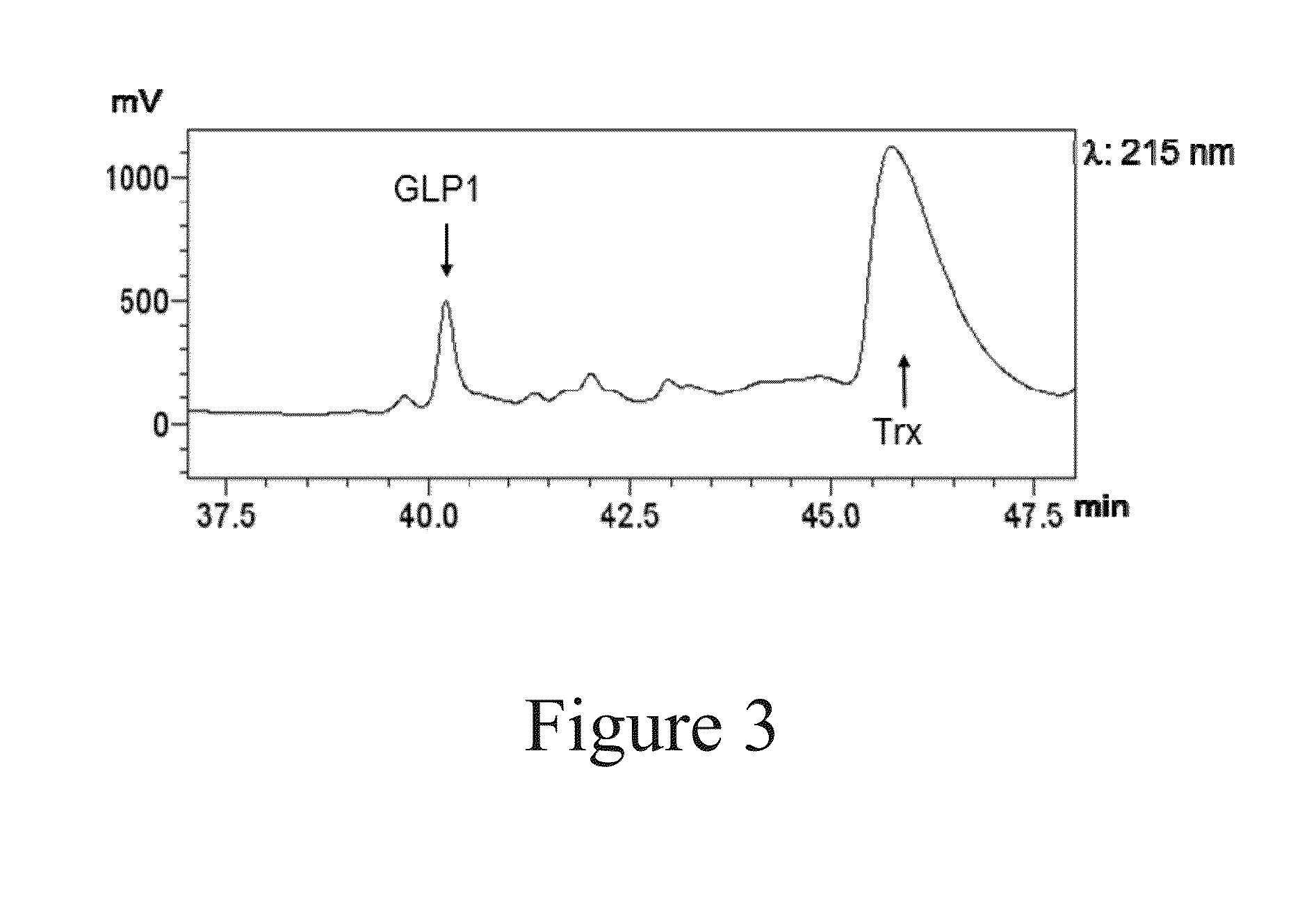
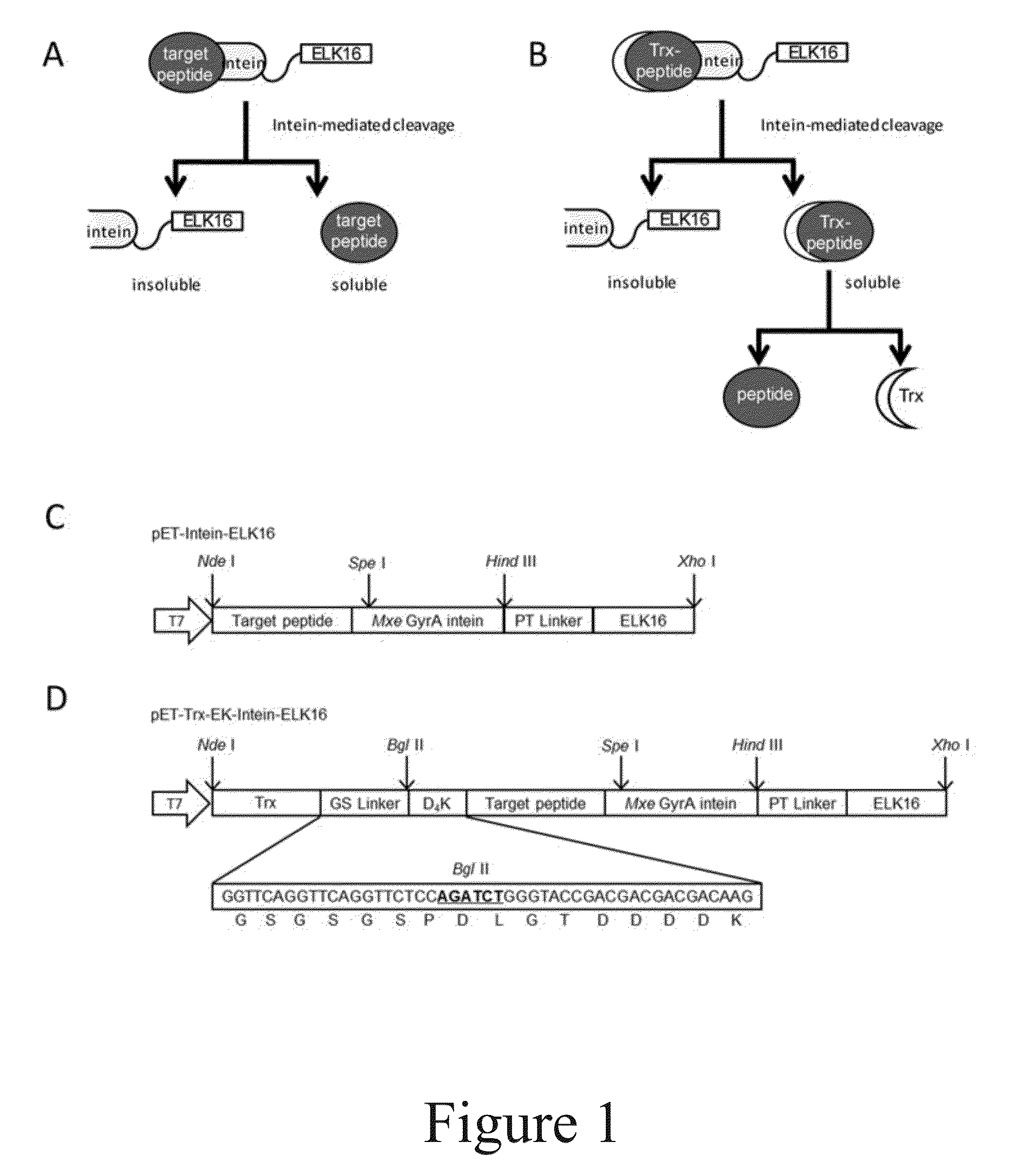
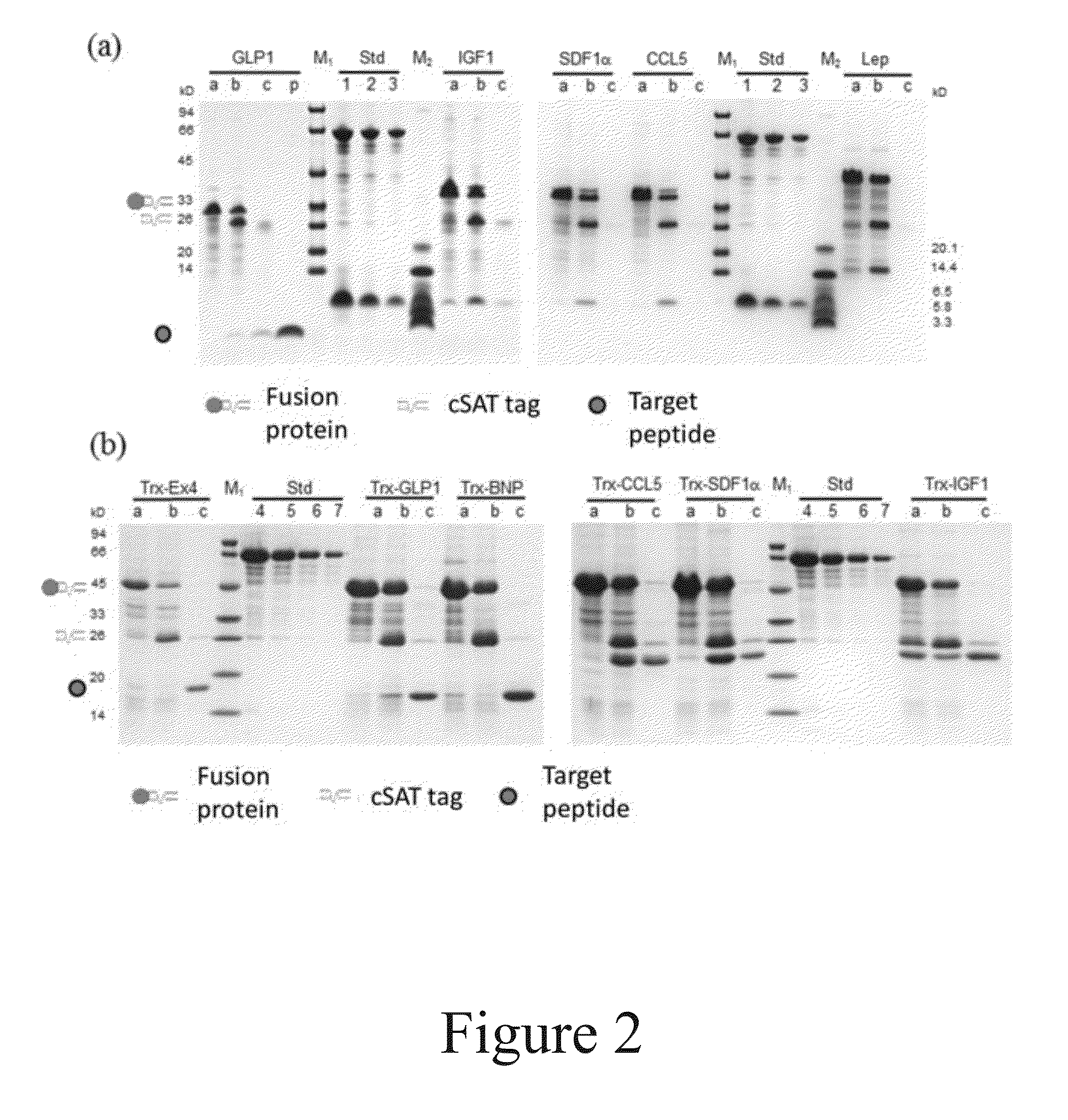
The present invention relates to a method for production and purification of polypeptides. In particular, the present invention relates to a fusion protein comprising a solubility-enhancing peptide tag moiety, a self-aggregating peptide moiety and a moiety of target peptide and to a method for production and purification of target peptides through expressing said fusion protein.
Owner:TSINGHUA UNIV
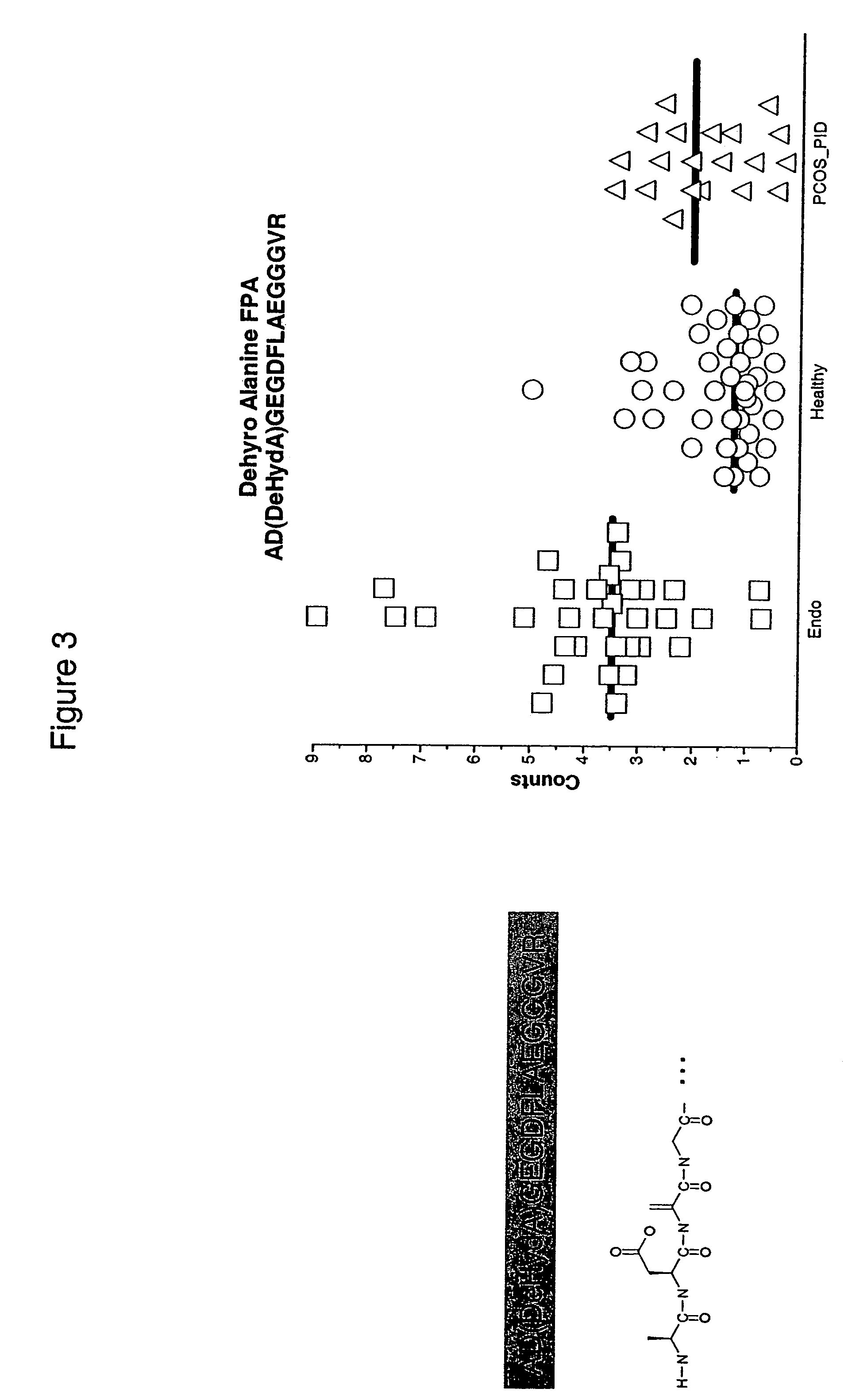
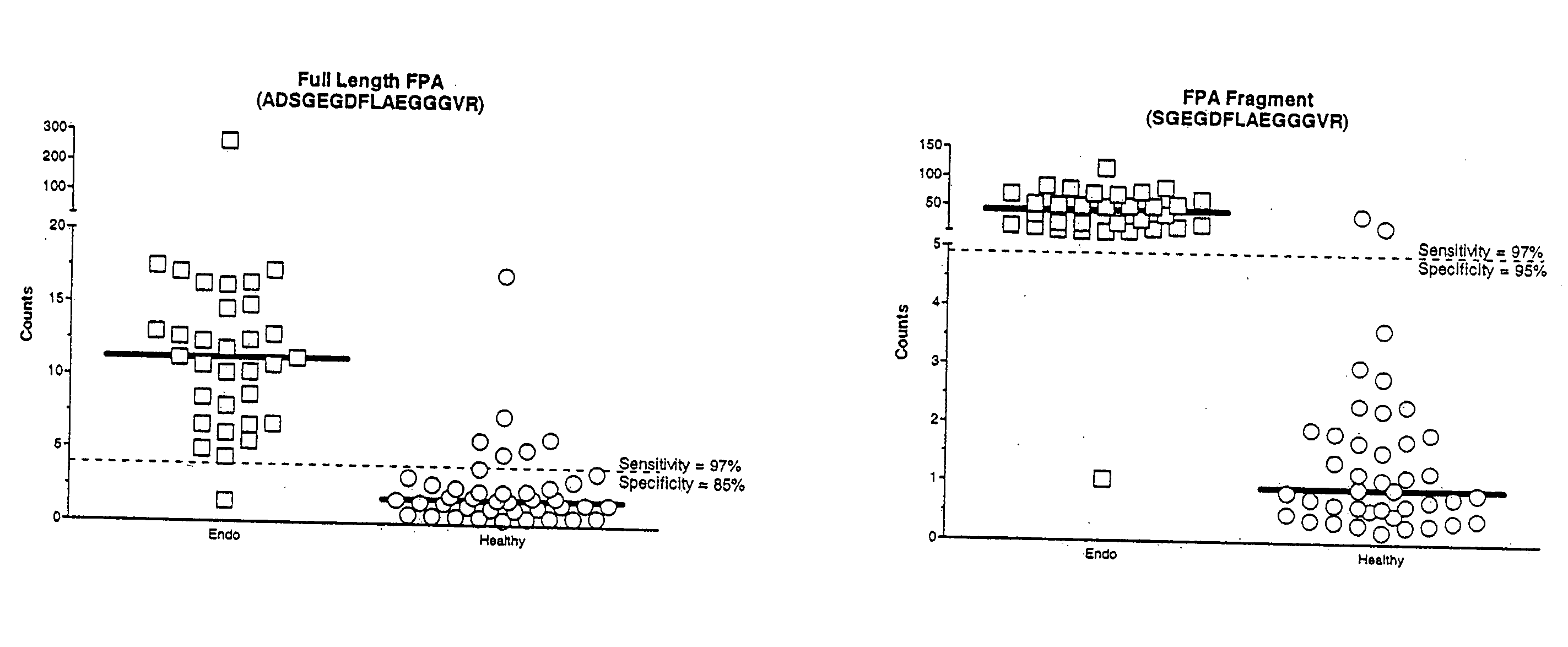
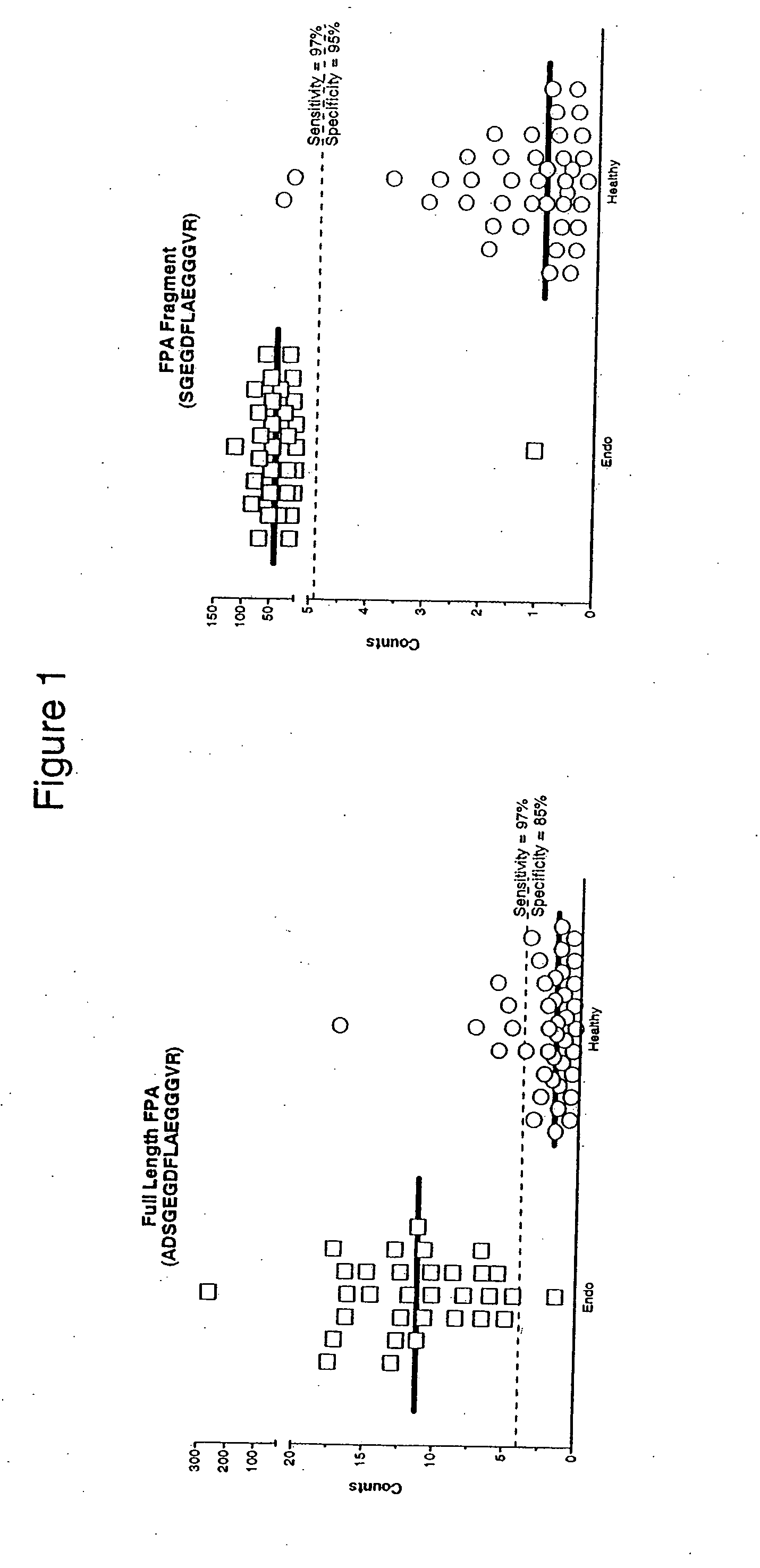
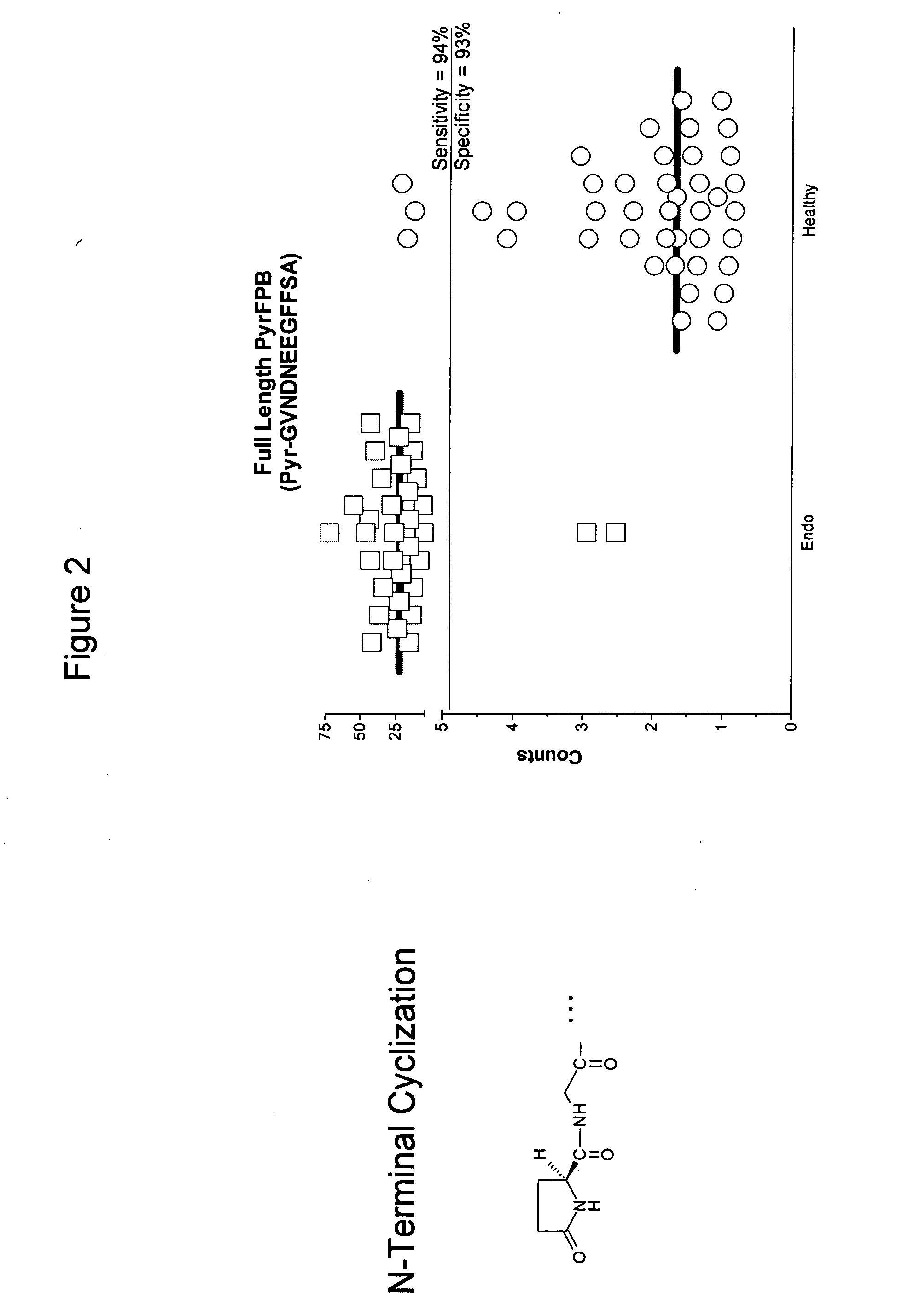
Compositions, kits, and methods for identification, assessment, prevention, and therapy of endometriosis
The invention relates to newly discovered marker polypeptides associated with endometriosis. Compositions, kits, and methods for detecting, characterizing, preventing, and treating endometriosis are provided.
Owner:PRAECIS PHARM INC
Method for synthesizing thymalfasin
InactiveCN104098688AShort synthesis cycleHigh yieldThymosin peptidesPeptide preparation methodsSide chainCombinatorial chemistry
The invention relates to the field of pharmaceutical synthesis, in particular to a method for synthesizing thymalfasin, and aims to solve the technical problems of difficulty in separation and purification, low total yield and high production cost of a conventional method. According to the scheme, the method for synthesizing thymalfasin comprises the steps as follows: a, a polypeptide fragment 1 and a polypeptide fragment 2 provided with protecting groups on side chains are synthesized; b, a C terminal of the polypeptide fragment 1 and an N terminal of the polypeptide fragment 2 are coupled, and the protecting group at the N terminal is removed to obtain polypeptide resin I; c, according to an amino acid sequence of thymalfasin, amino acids from the eleventh to the first are sequentially coupled one by one according to the order from the C terminal to the N terminal, then the protecting group at the N terminal is removed, and acetylation is performed to obtain thymalfasin resin; and d, the thymalfasin resin is subjected to acidolysis to remove the C terminal resin and all protecting groups to obtain a coarse thymalfasin product, and thymalfasin is obtained after purification. With the adoption of the method, the product yield can be greatly improved, and the synthesis cycle is shortened.
Owner:CHENGDU SHENGNUO BIOPHARM
Compositions, kits, and methods for identification, assessment, prevention, and therapy of endometriosis
The invention relates to newly discovered marker polypeptides associated with endometriosis. Compositions, kits, and methods for detecting, characterizing, preventing, and treating endometriosis are provided.
Owner:PRAECIS PHARM INC
Method used for synthesizing thymalfasin
ActiveCN103980357AHigh yieldEasy to purifyThymosin peptidesPeptide preparation methodsCouplingFreeze-drying
The invention discloses a method used for solid-phase synthesis of thymalfasin, and relates to a novel technology used for preparing thymalfasin via Fmoc strategy solid-phase method. The method comprises following steps: (1) synthesis of Fmoc-Asp(RinkAmideMBHA resin)-OtBu is realized using Fmoc-Asp-OtBu and RinkAmideMBHA with an appropriate substitution degree; (2) Fmoc-Asp(RinkAmideMBHA resin)-OtBu is subjected to stepwise coupling or fragment coupling so as to obtain thymosin resin, wherein coupling of Glu18 and Lys19 is realized via fragment one-step peptide connection, and the rest amino acids are subjected to stepwise coupling, a mixed deprotection agent is used for deprotection in deprotection processes before coupling of Asp6, Thr12, Leu16, Lys17, Glu18-Lys19, and an special acylation reagent is used for terminal blocking of thymosin resin before Asp2 coupling; and (3) thymosin resin is subjected to cracking so as to obtain a crude peptide, and thymalfasin is obtained via RP-HPLC purification, salt transport, and freeze-drying. The invention provides the solid-phase synthesis method of thymalfasin with high product purity and convenient for purification.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Cosmetic composition containing fusion protein with skin penetration enhancing peptide conjugated thereto for skin improvement
PendingCN110997696AImprove permeabilityImprove wrinklesCosmetic preparationsThymosin peptidesSkin penetrationHair loss
The present invention relates to a cosmetic composition containing a fusion protein with a skin penetration enhancing peptide conjugated thereto for skin improvement and, more specifically, to a fusion protein in which PDGFa is conjugated to a skin penetration enhancing peptide, a cosmetic composition containing the fusion protein for skin improvement, a functional cosmetic product for skin improvement, a cosmetic composition for hair loss relief, and a quasi-drug product composition containing the fusion protein.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
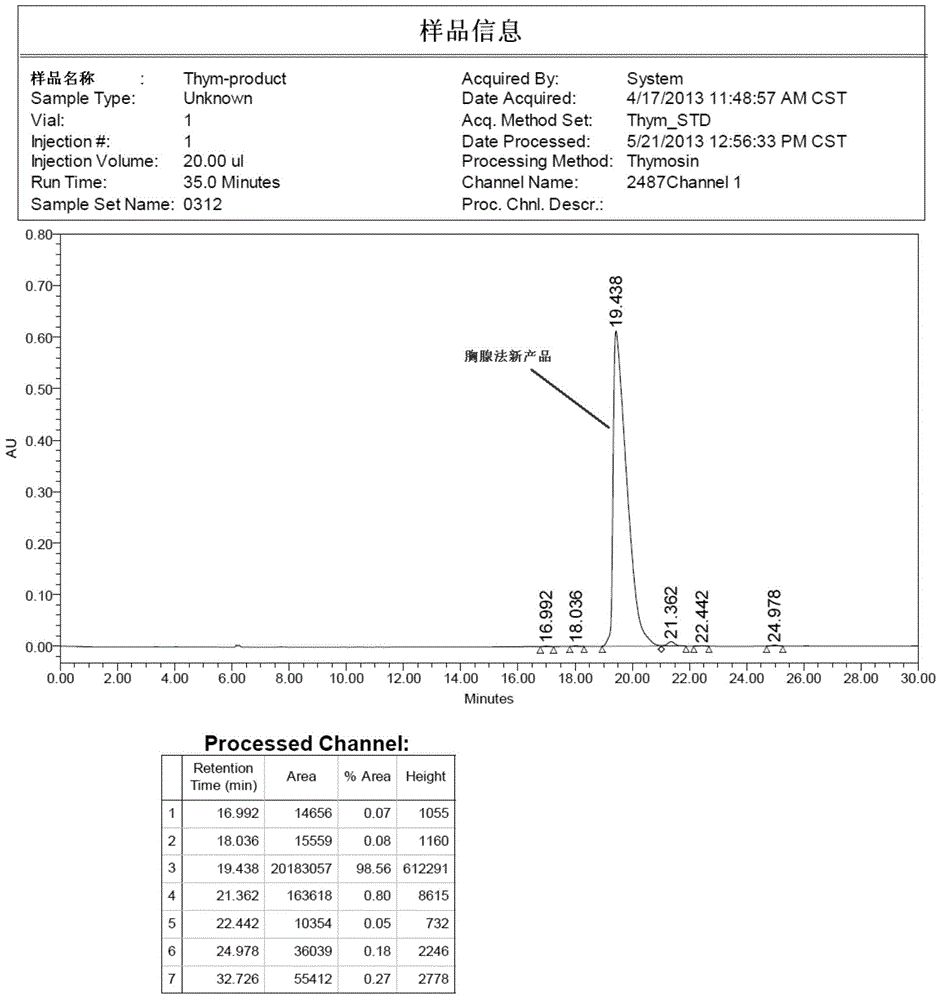
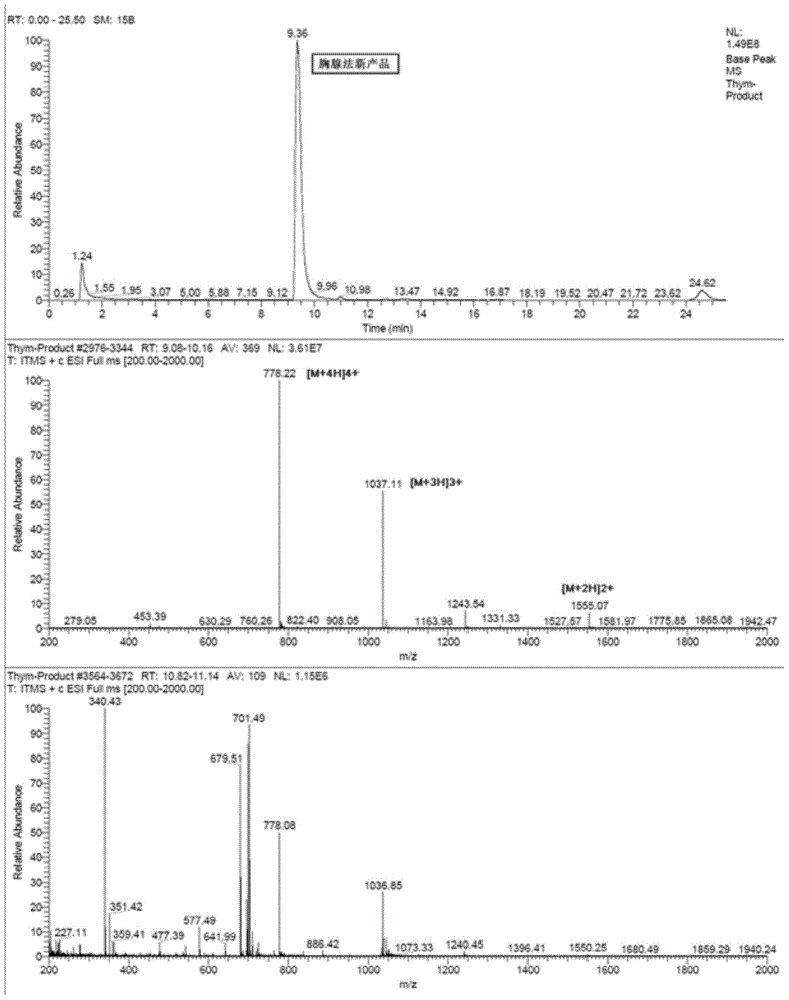
Method for preparing high-purity thymalfasin
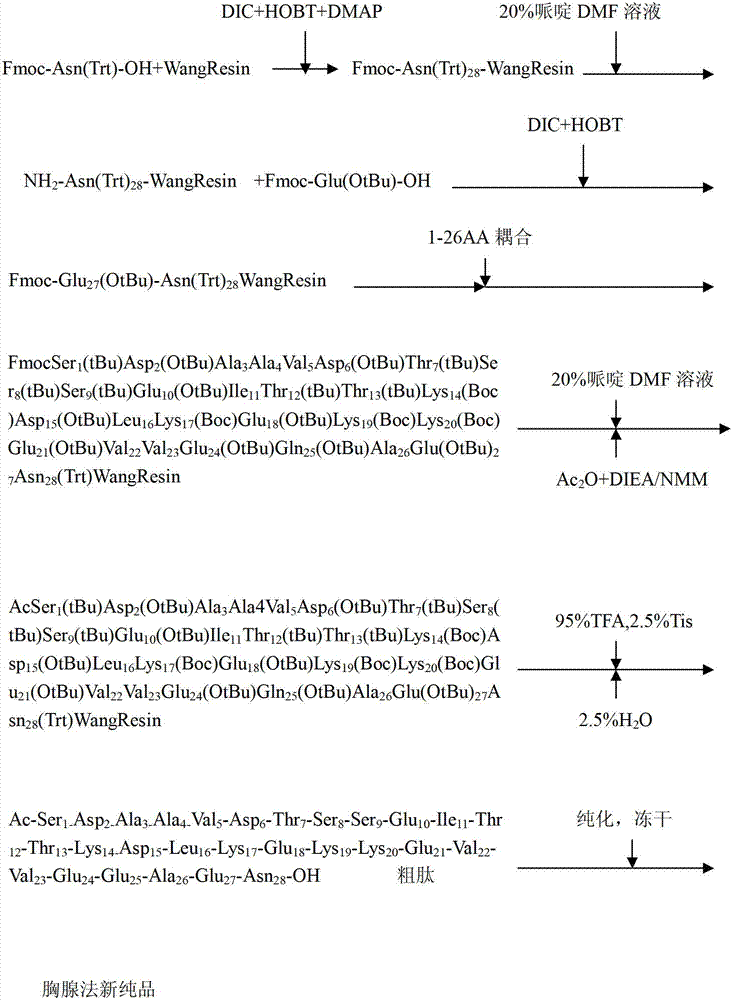
ActiveCN103880945AExtended reaction timeShort reaction timeThymosin peptidesPeptide preparation methodsWang resinThymalfasin
The invention discloses a method for preparing high-purity thymalfasin. The preparation method comprises: firstly preparing a first resin polypeptide fragment, a second resin polypeptide fragment, a third resin polypeptide fragment, a fourth resin polypeptide fragment and a fifth resin polypeptide fragment respectively; cleaving the second resin polypeptide fragment, the third resin polypeptide fragment, the fourth resin polypeptide fragment and the fifth resin polypeptide fragment to obtain crude peptide fragments; purifying the obtained second, third, fourth and fifth crude polypeptide fragments respectively; linking each purified polypeptide fragment to the first resin polypeptide fragment; carrying out an acetylation reaction on resin polypeptide fragments to obtain thymalfasin wang resin; cleaving to obtain the thymalfasin crude product; then purifying the obtained thymalfasin crude product twice; and collecting the mobile phase containing thymalfasin in a collection and purification process, evaporating to dryness under reduced pressure and centrifuging to obtain thymalfasin, and vacuum drying to obtain thymalfasin products. The thymalfasin is prepared by the method disclosed by the invention, the reaction time is effectively shortened, the reaction yield and the quality of the final product are improved; and the purity of thymalfasin prepared is more than 99%.
Owner:郑州大明药物科技有限公司
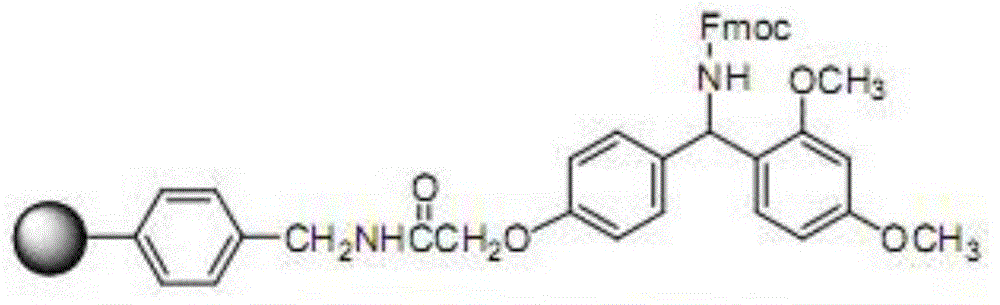
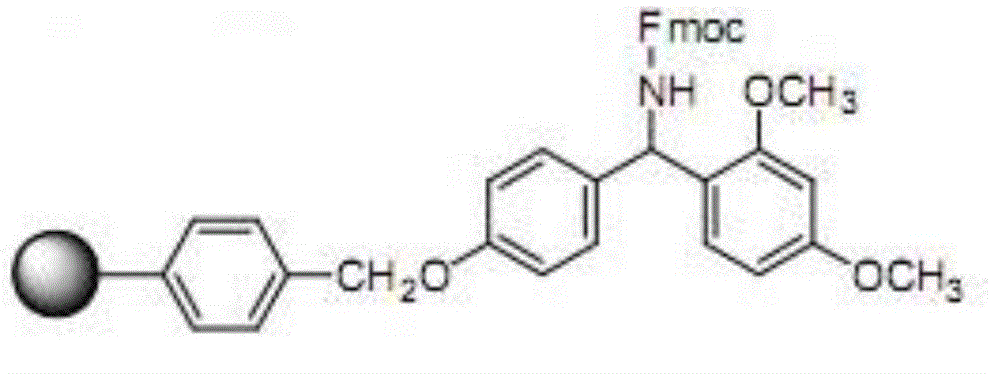
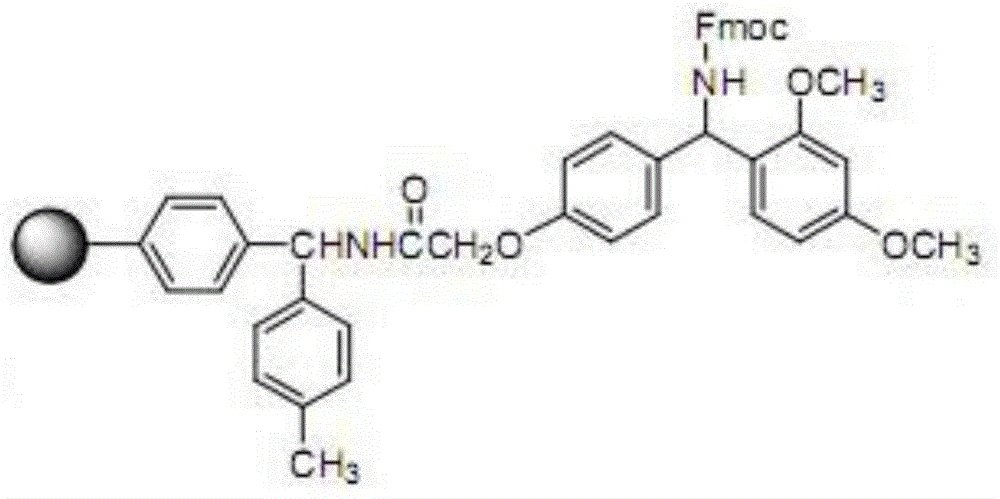
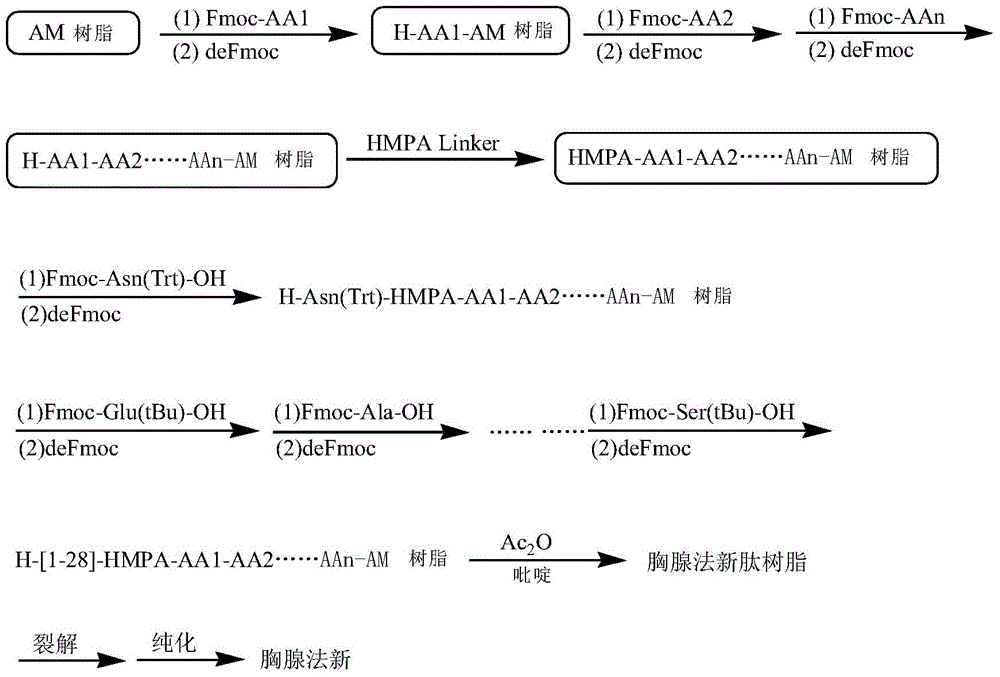
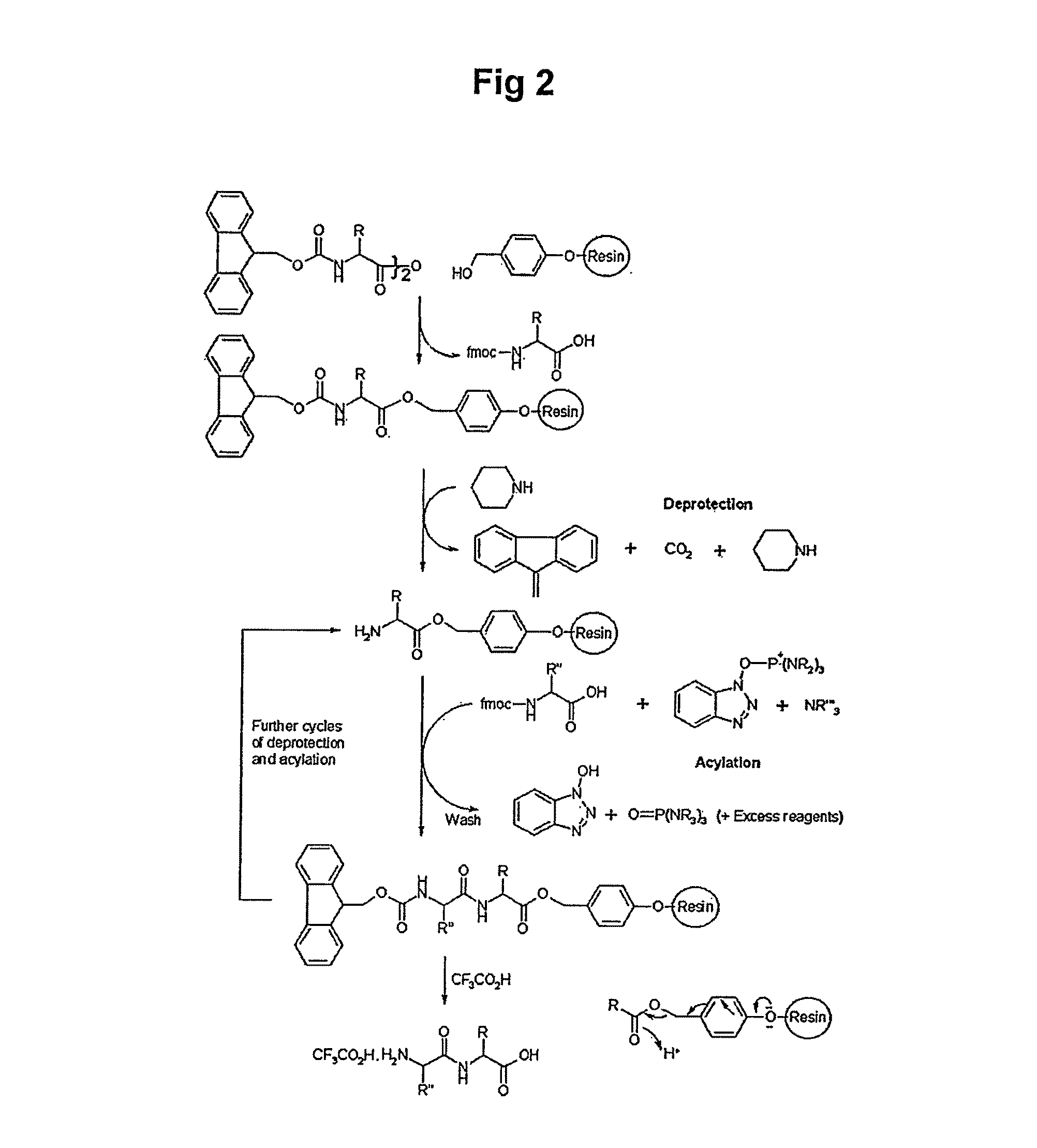
Solid-phase resin and its preparation method and use
The invention discloses solid-phase resin and its preparation method and use. The solid-phase resin has a structure shown in the formula I of HMPA-AAn-AM resin I. In the formula I, AA represents the same or different side chain-protection amino acids such as Arg, Lys, Asn, Gln, Asp, Glu, Pro and Gly, and n represents an integer of 0-8. The solid-phase resin can be used for thymalfasin solid-phase synthesis.
Owner:HAINAN SHUANGCHENG PHARMA
Anti-inflammatory and wound healing effects of lymphoid thymosin b-4
InactiveUS20090317457A1Powder deliveryOrganic active ingredientsWound healingAnti-inflammatory analgesics
The invention relates to a method of treating inflammatory conditions in a subject comprising administering to a subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant. The invention also provides a method of promoting wound healing in a subject comprising administering to the subject a composition comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant. The invention also relates to methods of treating the above mentioned conditions in a subject comprising administering to the subject a nucleic acid encoding a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant. The invention also relates to pharmaceutical compositions comprising a lymphoid thymosin-β4 polypeptide or a functional lymphoid thymosin-β4 polypeptide variant, or salt thereof, and a pharmaceutically acceptable carrier.
Owner:YALE UNIV +1
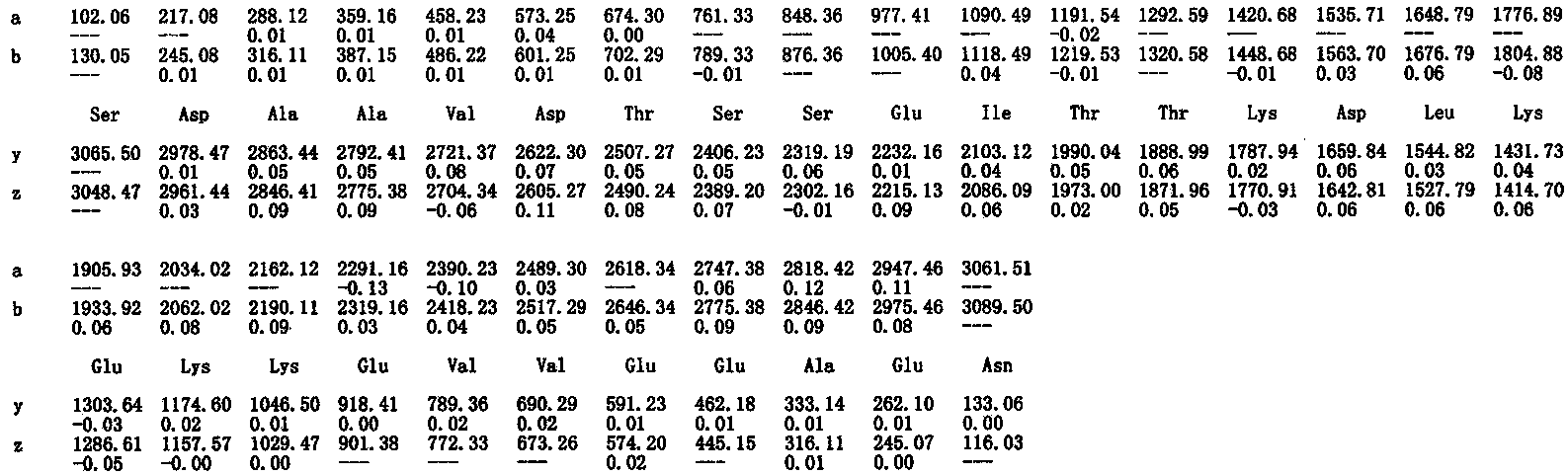
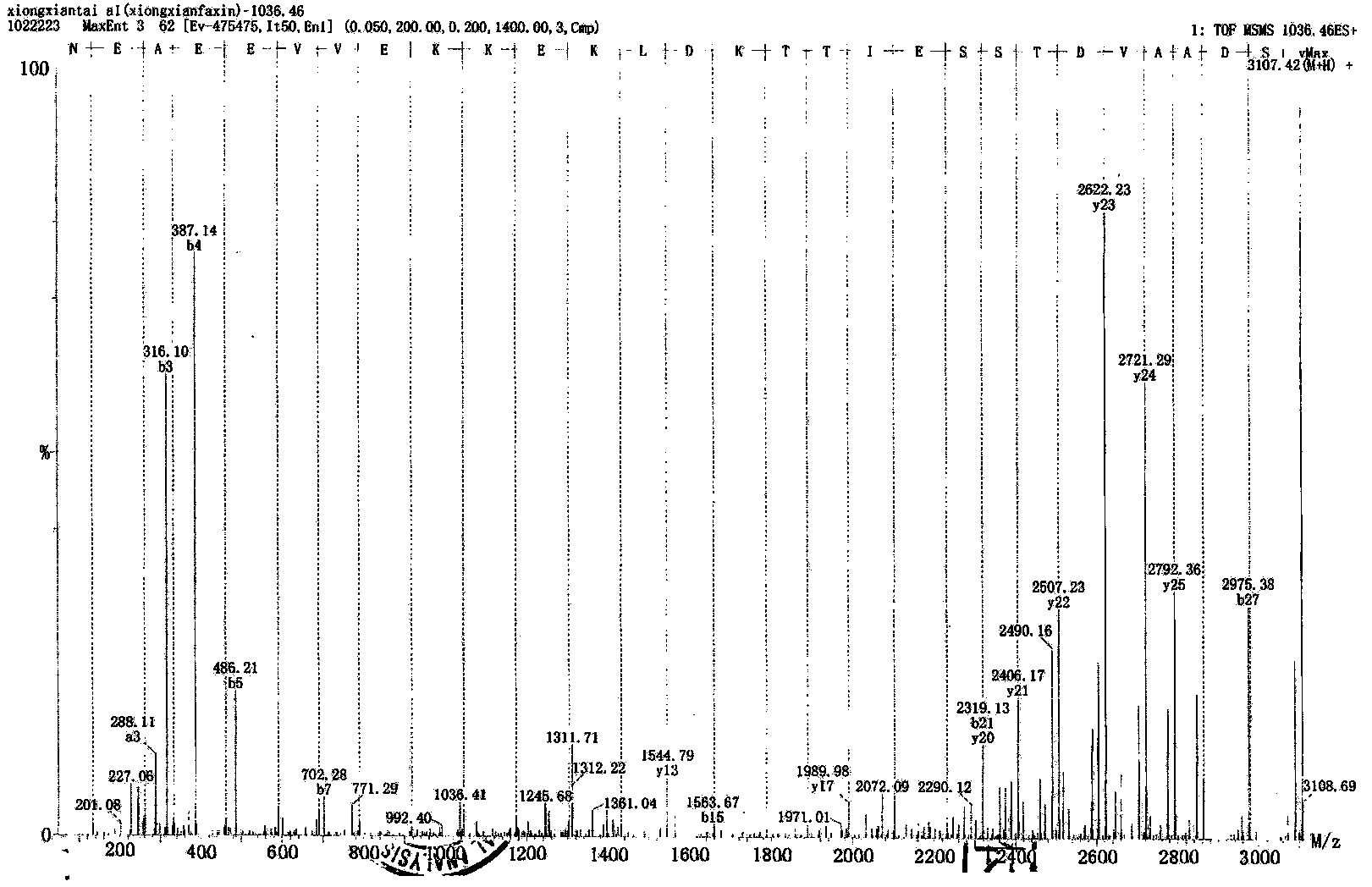
Method for preparing thymosin alpha 1 by liquid phase fragment condensation
ActiveCN103665144AHigh purityHigh yieldThymosin peptidesPeptide preparation methodsFluid phaseTarget peptide
The invention provides a method for preparing thymosin alpha 1 by liquid phase fragment condensation, belonging to the technical field of biochemistry. According to the method, high capacity value (not less than 0.8mmol / g) resin is used as a starting material, firstly, a standard solid phase polypeptide synthesis (SPPS) technology is adopted for synthesizing a high-purity peptide fragments with selected structures, then, a liquid phase condensation technology is adopted for connecting the peptide fragments, and thus, a high-purity (more than 99%) target peptide is obtained. Compared with the solid phase thymosin alpha 1 synthesis technology, the method provided by the invention avoids the problem of low coupling ratio of amino acids behind the 12th position, and greatly improves yield (up to 25-30%) of the thymosin alpha 1; meanwhile, the peptide fragment formed by solid phase synthesis is free from purification, post-treatment technology is simplified, finally, the thymosin alpha 1 is purified by means of high performance liquid chromatography, preparation difficulty is reduced, preparation times is decreased, synthesis cost of the thymosin alpha 1 is lowered, and implementation of large-scale and industrialized production is facilitated.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Hybrid peptide with functions of regulating immunity, resisting oxidation and inflammation and detoxifying body, and preparation method and application thereof
ActiveCN110128548AHigh expressionImprove antioxidant capacityThymosin peptidesPeptide/protein ingredientsAnti-inflammatoryImmunosuppression
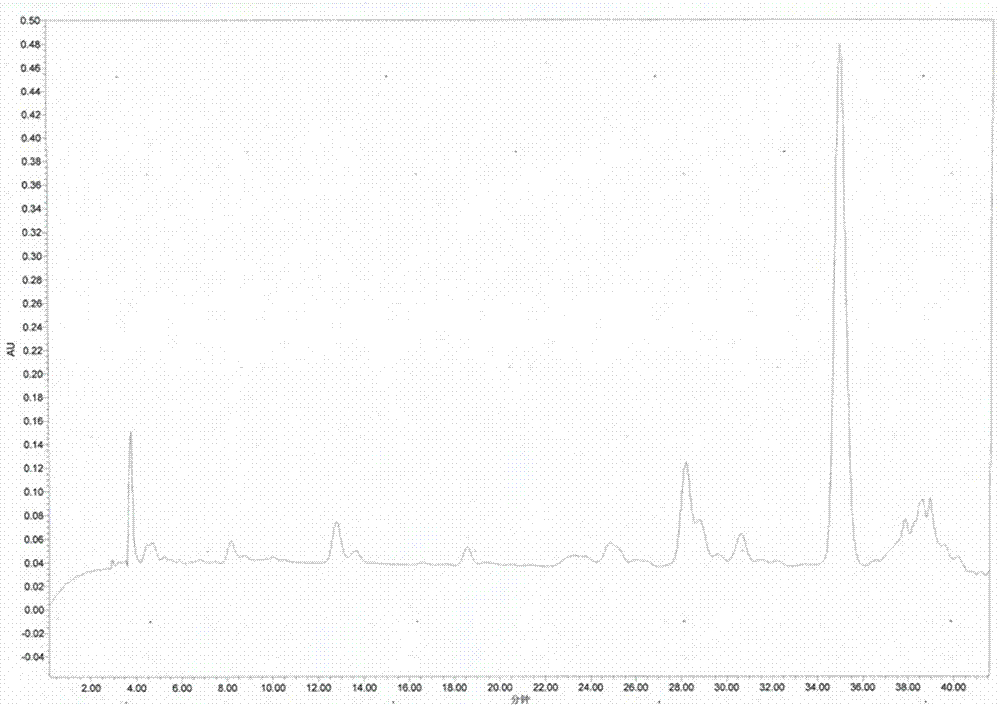
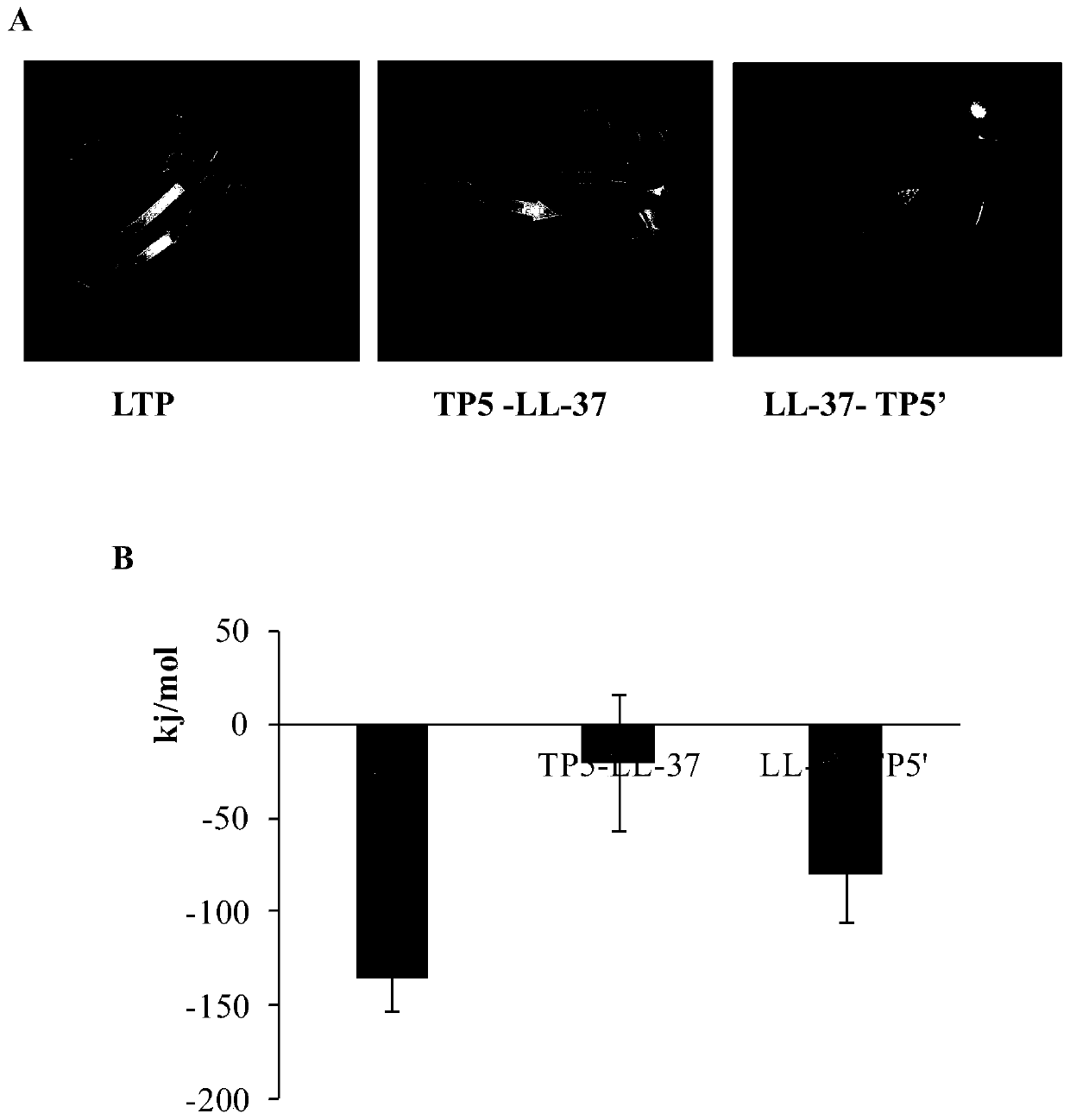
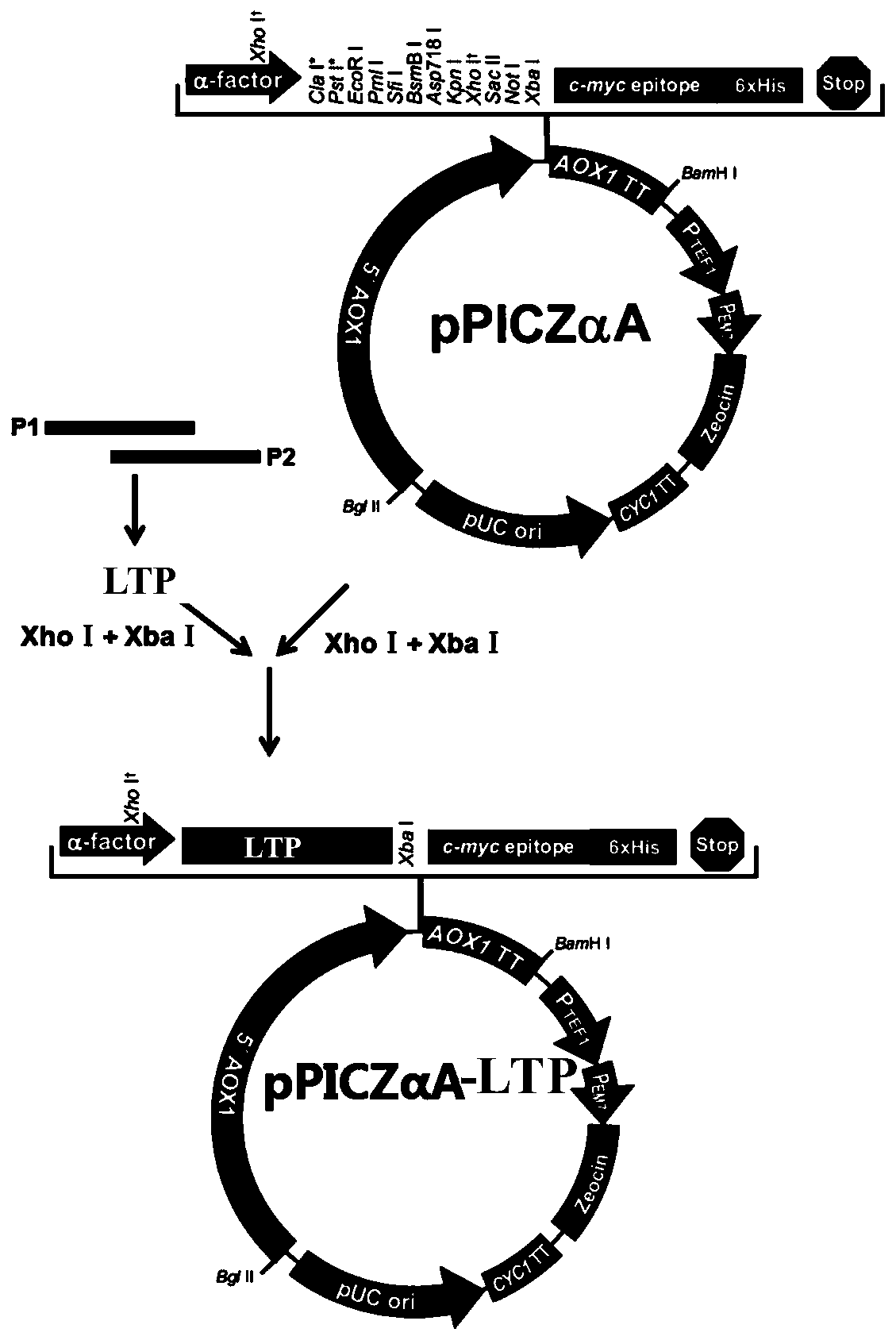
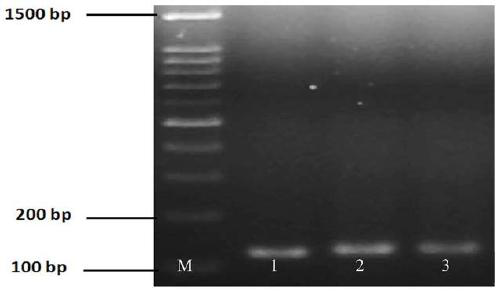
The invention relates to a hybrid peptide with functions of regulating immunity, resisting oxidation and inflammation and detoxifying the body, and a preparation method and application thereof. The hybrid peptide named LTP is obtained through design by a protein engineering computer, heterozygosis, optimization, and in vitro and in vivo rescreening of antibacterial peptide LL-37 and thymopentin TP5, wherein the amino acid sequence is represented by SEQ ID NO.1. The hybrid peptide LTP has high bidirectional immunomodulatory activity, can enhance the immune function of the body under a normal orimmunosuppressed state, and can protect the body from injuries caused by immunosuppression; in an inflammatory state, the LTP can neutralize and eliminate endotoxin, inhibit oxidation reaction and inflammatory reaction of the body and alleviate injuries to tissues by the inflammatory reaction, has the advantages of low cytotoxicity, high safety, simplicity in preparation, low cost and the like, and can be used as an ideal immune, anti-oxidative and anti-inflammatory regulator or an immune anti-oxidative detoxification peptide, thereby having high application potential and value.
Owner:CHINA AGRI UNIV
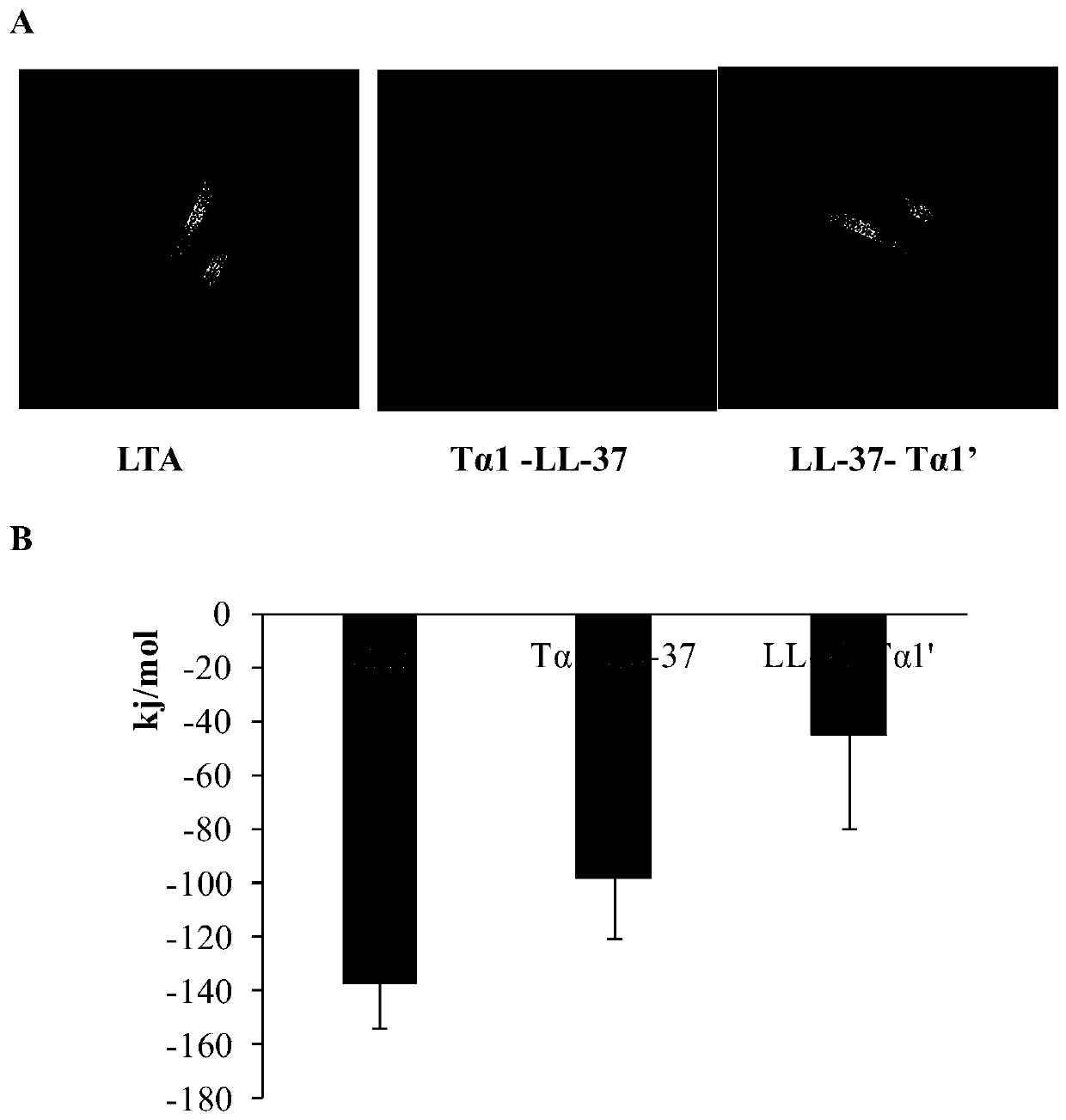
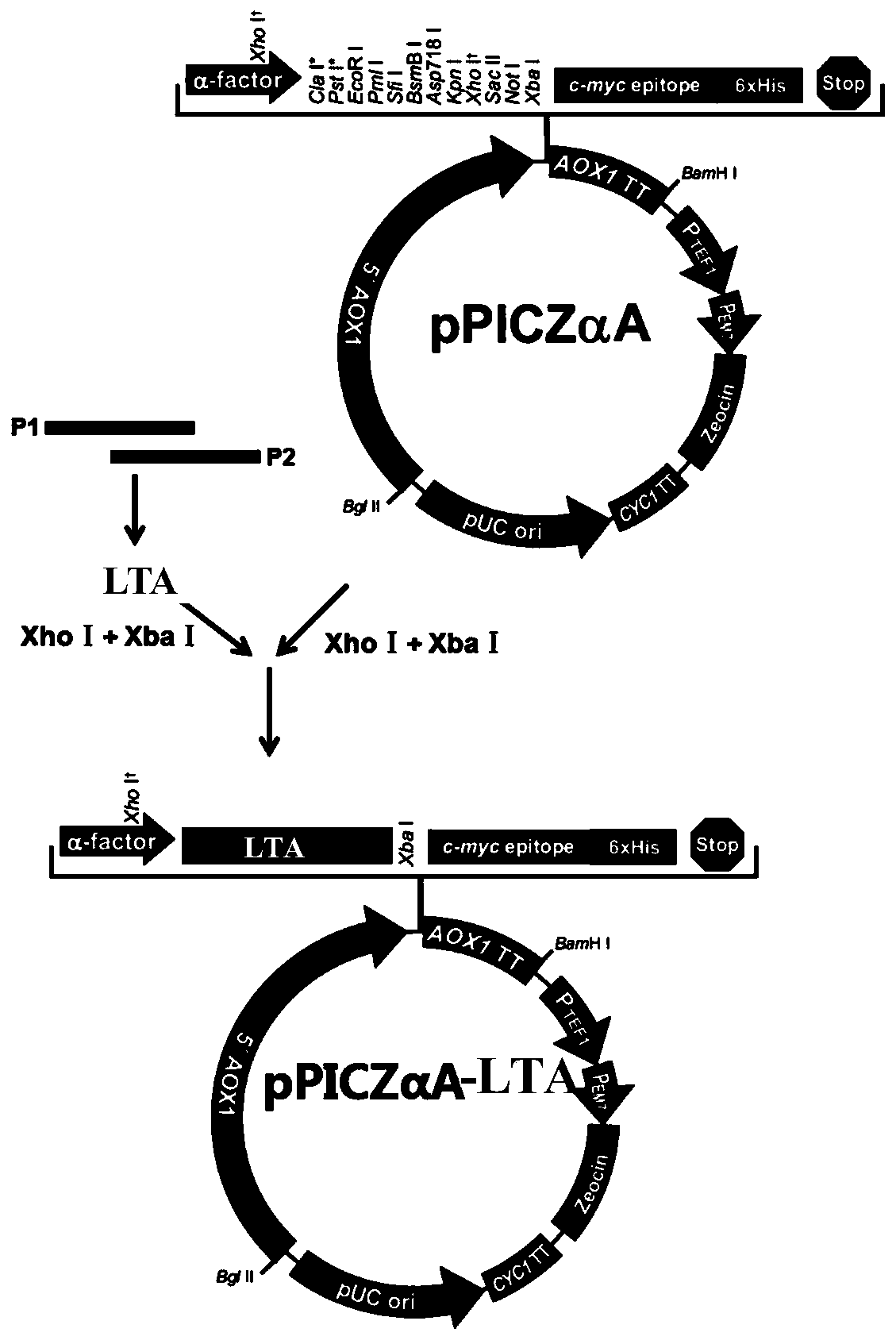
Hybrid peptide with functions of regulating immunity, neutralizing and digesting endotoxin and resisting inflammation, and preparation method and application thereof
The invention relates to the technical field of gene engineering and biological agents, in particular to a hybrid peptide with functions of regulating immunity, neutralizing and digesting endotoxin and resisting inflammation, and a preparation method and application thereof. The hybrid peptide is obtained from antibacterial peptide LL-37 and thymosin T alpha 1 through designing a protein engineering computer, optimizing heterozygosity and re-screening in vivo and in vitro (abbreviated as LTA), wherein the amino acid sequence is shown as SEQ ID NO. 1. The LTA has higher bidirectional immunoregulation activity, can enhance the immunologic function of an organism under the normal or immunosuppression state and protect the damage to the organism caused by immunosuppression; in an inflammatorystate, the LTA can also neutralize endotoxin, inhibit the inflammatory reaction of organisms, relieve the damage of the inflammatory reaction to tissues, has the advantages of low cytotoxicity, high safety, convenient preparation, low cost and the like, can be used as an ideal immune anti-inflammatory regulator, and has good application potential and value.
Owner:CHINA AGRI UNIV
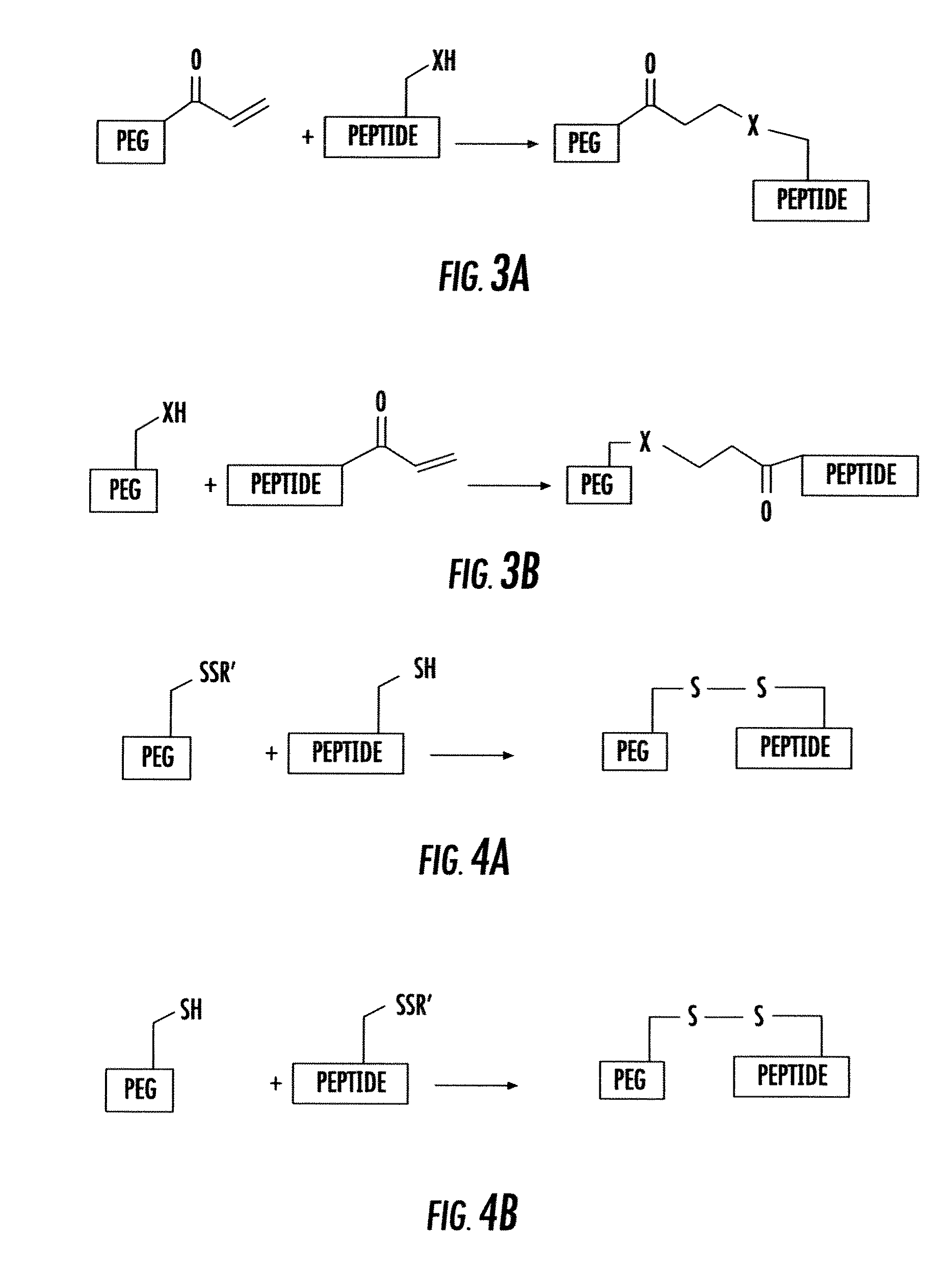
Polyethlene glycol modifications of thymosin alpha-1
Polyethylene glycol modifications of thymosin alpha 1 (T&agr; 1-PEGs), their preparation process, the medicine composition containing them, and their application in the medicine for preventing and treating diseases related with immune deficiency and hypoimmunity, including hepatitis B, hepatitis C, hepatoma, malignant melanoma, non-small cell lung cancer, SARS, and AIDS etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
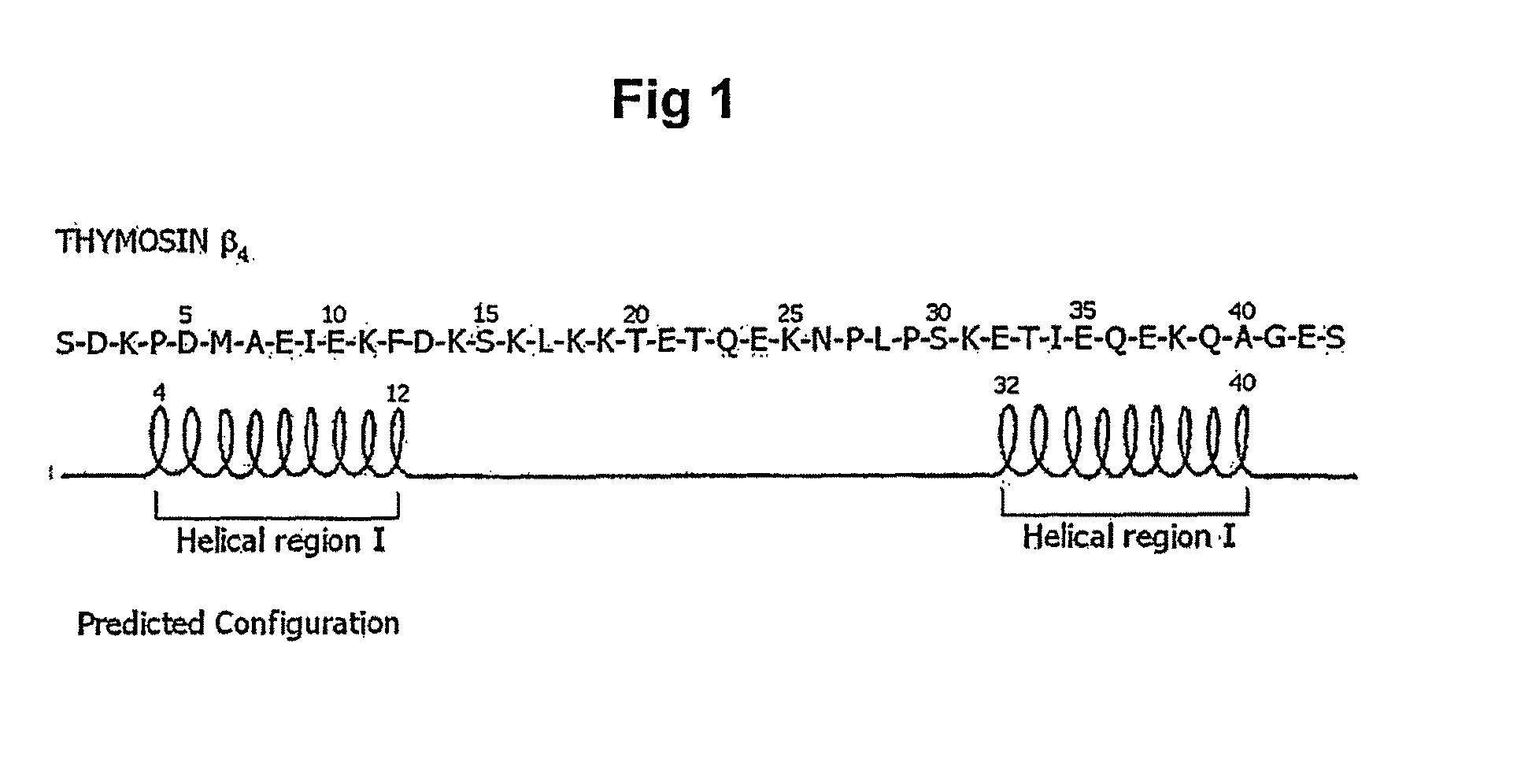
THYMOSIN Beta4 PEPTIDES PROMOTE TISSUE REGENERATION
The present invention relates to thymosin β-4 peptides and analogs thereof that can promote tissues regeneration, particularly cardiac tissue.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Recombinant thymosin protein PaTHY1 of Periplaneta americana and expression method thereof
ActiveCN107759684ASolve the problem of extremely low content and difficult purificationThymosin peptidesMicroorganism based processesProtein targetChemical composition
The invention discloses a recombinant thymosin protein PaTHY1 of Periplaneta americana and an expression method thereof, belonging to the field of biotechnology. The expression method comprises the following steps: (1) extracting RNAs from the tissue of Periplaneta americana and carrying out inverse transcription on the RNAs to obtain cDNAs; (2) synthesizing primers; (3) with the cDNAs as a template, carrying out PCR to obtain a coding gene fragment of the thymosin protein of Periplaneta americana; (4) cloning the coding gene fragment into a prokaryotic expression vector for transformation ofhost bacterium, carrying out culturing on an obtained recombinant engineering bacterium and inducing the recombinant engineering bacterium to express the target protein; and (5) purifying the target protein by using affinity chromatography so as to obtain the recombinant thymosin protein PaTHY1 of Periplaneta americana. The recombinant protein is obtained through genetic engineering for the firsttime, overcomes the problems of low content and difficult purification of thymosin in complex chemical components of Periplaneta americana, and lays a foundation for research on the pharmacodynamic functions of thymosin from Periplaneta americana.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Hybrid peptide with immunomodulatory and anti-inflammatory functions and preparation method and application thereof
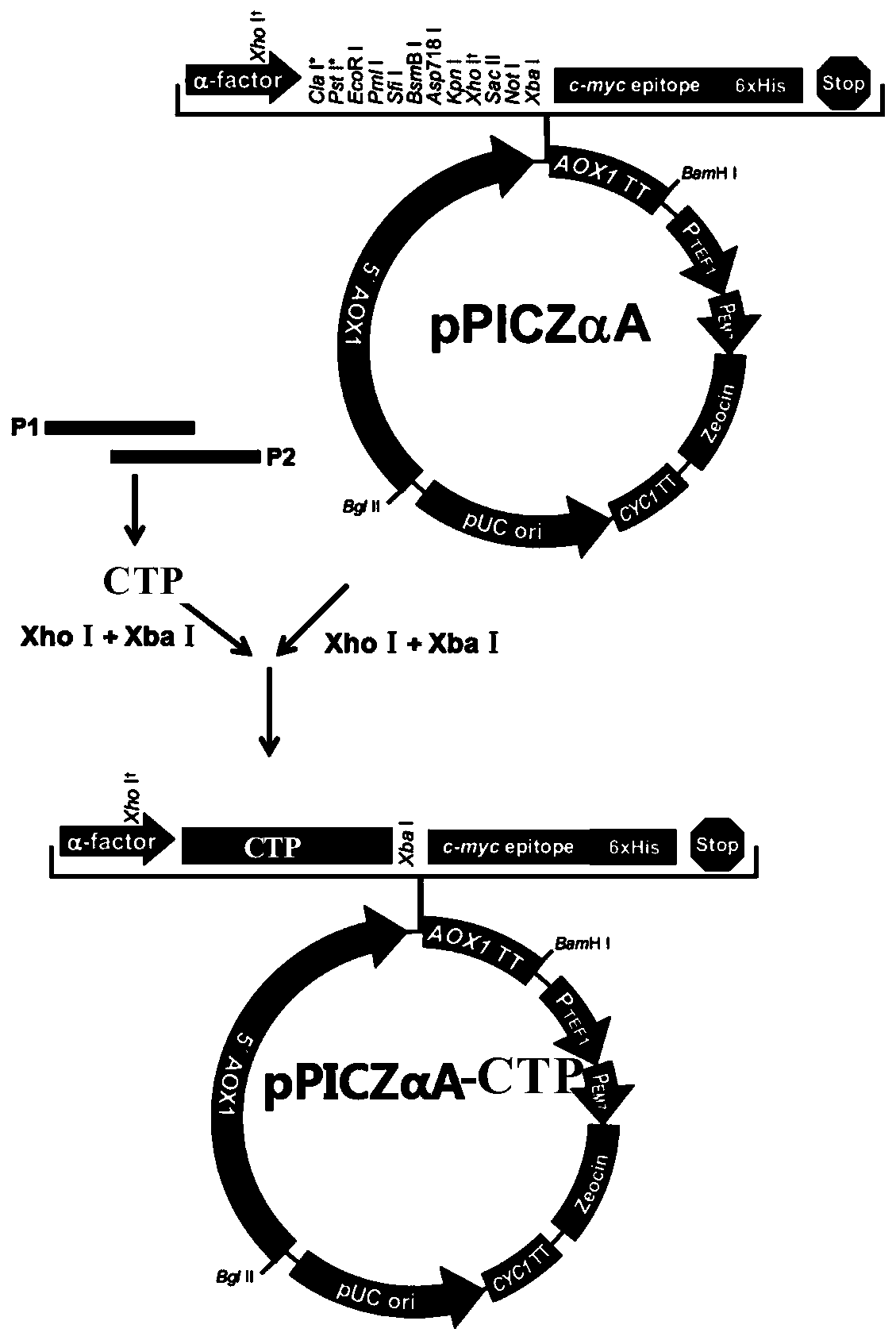
ActiveCN110128544AHigh expressionReduce expressionThymosin peptidesPeptide/protein ingredientsCytotoxicityGenetic engineering
The invention relates to the technical field of genetic engineering and biological preparation, in particular to a hybrid peptide having immunomodulating and anti-inflammatory functions, and a preparation method and an application thereof. The invention provides an immuno-anti-inflammatory hybrid peptide CTP, which is obtained by hybridization, optimization and screening of antibacterial peptide CATH-2 and thymosin TP5, and an amino acid sequence is shown as SEQ ID NO. 1. The hybrid peptide CTP has high bidirectional immunomodulatory activity, can enhance the immune function of the body undernormal or immunosuppressed state, and protects the body from damage caused by immunosuppression; in the inflammatory state, CTP can also inhibit the body's inflammatory response, alleviates the damageof an inflammatory reaction on the tissue, and has the advantages of low cytotoxicity, high safety, simple preparation method and low cost, can be used as an ideal immuno-anti-inflammatory regulator,and has good application potential and value.
Owner:CHINA AGRI UNIV
Methods for production and purification of polypeptides
ActiveUS9200306B2Polypeptide with localisation/targeting motifThymosin peptidesSolubilityTarget peptide
The present invention relates to a method for production and purification of polypeptides. In particular, the present invention relates to a fusion protein comprising a solubility-enhancing peptide tag moiety, a self-aggregating peptide moiety and a moiety of target peptide and to a method for production and purification of target peptides through expressing said fusion protein.
Owner:TSINGHUA UNIV
Synthesis method of thymosin alpha1
ActiveCN103936848ASimple and fast operationPost-processing is simpleThymosin peptidesPeptide preparation methodsSide chainEnd-group
The invention relates to a nonlinear solid phase synthesis method of thymosin alpha1. The method is characterized in that a coupling site is a side chain hydroxy group not a routine N amino end group of Ser during coupling of Ser-Ser fragments; and after a peptide chain is synthesized, ester bonds recombine under appropriate conditions to form an amide structure in order to obtain thymosin alpha1. Compared with traditional linear solid phase synthesis methods, the method provided by the invention has the advantages of maintenance of simple operation and easy post-treatment, and realization of high yield and god purity brought by reduction of the coupling difficulty due to beta-folding, alleviation of the steric hindrance, and provides a brand new idea for the large scale production of thymosin alpha1.
Owner:HYBIO PHARMA
Trachinotus ovatus beta-thymosin and application thereof
ActiveCN107082804AGrowth inhibitionImprove disease resistanceAntibacterial agentsThymosin peptidesBiotechnologyDisease
The invention provides trachinotus ovatus beta-thymosin and application thereof, and relates to the field of molecular biology. The cDNA nucleotide sequence of the trachinotus ovatus beta-thymosin is shown as SEQ ID NO. 1, and the amino acid sequence is shown as SEQ ID NO. 2. The invention also provides application of the trachinotus ovatus beta-thymosin to anti-bacterial disease medicine, and a recombinant protein expression method of the trachinotus ovatus beta-thymosin. The beta-thymosin provided by the invention can obviously inhibit the growth of pathogenic bacteria; after the injection into the fish is finished, the fish disease-resistant capability can be obviously improved.
Owner:HAINAN UNIVERSITY
Method for desalinating thymopeptide alpha 1
InactiveCN105254746AEfficient removalReduce pollutionThymosin peptidesPeptide preparation methodsFreeze-dryingIon exchange
The invention discloses a method for desalinating thymopeptide alpha 1 through reversed-phase high-performance liquid chromatography and mainly solves the technical problems that much time is consumed in a method for desalination through iron exchange resin, pretreatment is troublesome, only a few of samples are treated each time, the work efficiency is low, reproducibility is poor, and the method is not suitable for large-scale industrial production. According to the technical scheme, the method comprises steps as follows: 1), the pH (potential of hydrogen) of a purified solution of thymopeptide alpha 1 is adjusted to range from 6 to 8, the purified solution is filtered with a filter membrane with the specification of 0.45 mu m for standby application, gradient elution for purification is performed through a reverse phase silica gel column with octadecyl silane as a fixed phase, a water solution as an A phase and a methanol solution as a B phase, and a peptide solution reaching a target peak value is collected; the peptide solution obtained finally is subjected to rotary evaporation, concentration and freeze drying under reduced pressure, and the finished powdery peptide is obtained. The method is used for large-scale and industrial desalination of thymopeptide alpha 1.
Owner:GL BIOCHEM SHANGHAI
Preparation method of thymosin
InactiveCN109457007ASatisfy taste requirementsGreat tasteThymosin peptidesPeptide preparation methodsFreeze-dryingNanofiltration
The invention relates to the technical field of deep processing of animal thymuses, in particular to a preparation method of thymosin. According to the preparation method, fresh livestock thymuses areadopted as a main raw material, through pretreatment, enzymolysis, solid-liquid separation, degreasing purification, nanofiltration and concentration purification, a concentrated solution of the thymosin is obtained, and the concentrated solution can be freeze-dried to obtain a freeze-dried product. The preparation method is short in production cycle, low in cost and free of environment pollution, the obtained product is high in safety, high in purity, high in product activity and controllable in molecular weight, and can be widely used in the fields such as food, special medical food, healthcare products and medicines.
Owner:河北肽都生物科技集团有限公司
Derivates of Polyethylene Glycol Modified Thymosin Alpha 1
Pharmaceutical compositions that include thymosin alpha 1 peptide derivatives modified at the C-terminal of the peptide chain with polyethylene glycol, and their pharmaceutical acceptable salts, are generally disclosed. Also, new methods used to prepare these thymosin alpha 1 peptide derivatives modified at the C-terminal of the peptide chain with polyethylene glycol are generally provided. The presently disclosed compounds and their salts can be prepared administered to humans to treat immune disease and can also be used in adjuvant treatment.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing thymosin injection
PendingCN110496216AOvercome viscosityOvercoming flocsThymosin peptidesPeptide/protein ingredientsFreeze thawingFiltration
The invention belongs to the technical field of biological product preparation, and particularly relates to a method for preparing a thymosin injection. A calf thymus which passes inspection is removed of inner and outer packaging, and after pretreatment, primary mincing is conducted for 1 to 3 times; the primarily minced calf thymus is subjected to fine mincing and grinding for 3 to 8 minutes tobe prepared into homogenate; the homogenate is subjected to supersonic decomposing in an ultrasonic crushing device, the pH value of a lysate solution is adjusted with an HCl solution to 2.0 to 2.5; the lysed slurry is placed in a cold storage at -20 DEG C for freeze thawing for 1 to 2 times; after the last freeze thawing, heating is conducted to 80 DEG C + / - 2 DEG C, the temperature is kept for 10 to 20 minutes, a filter cloth is used for filtering, and an extraction solution is prepared; the extraction solution is subjected to centrifugal separation, filtration, and ultra filtration to obtain a semi-finished product; and water for injection is added to the qualified semi-finished product to obtain a finished product. The method for preparing the thymosin injection has the advantages thatthe technology is simple, the cost is low, the production cycle can be greatly shortened, manpower and material resources are saved, the production efficiency of thymosin injection is improved, and the application prospect is wider.
Owner:SHANDONG SINDER TECH
Solid-phase synthesis method of thymalfasin
InactiveCN103923210AGood effectDifficult sequence to overcomeThymosin peptidesPeptide preparation methodsCombinatorial chemistrySolvent
The invention relates to a solid-phase synthesis method of thymalfasin. The solid-phase synthesis method comprises the process steps: solid-phase linear synthesis of linear thymalfasin is carried out, a DMF solution of a salt composed of bulky anions and monovalence cations is used as a reaction solvent in a difficult sequence, hydrogen bond formation is interfered, and a beta structure of a peptide chain is destroyed. The process can overcome the polypeptide synthesized difficult sequence, improves the coupling rate of the difficult sequence so as to improve the yield of thymalfasin, reduces the production cost, and is beneficial for industrialized production.
Owner:SHANDONG NEWTIME PHARMA
Peptides for promoting hair growth and improving wrinkle and cosmetic compositions comprising the same
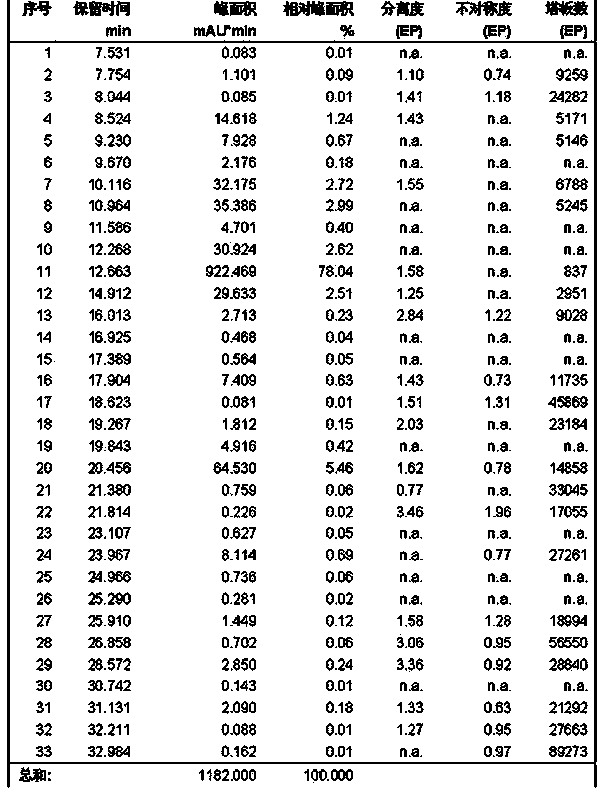
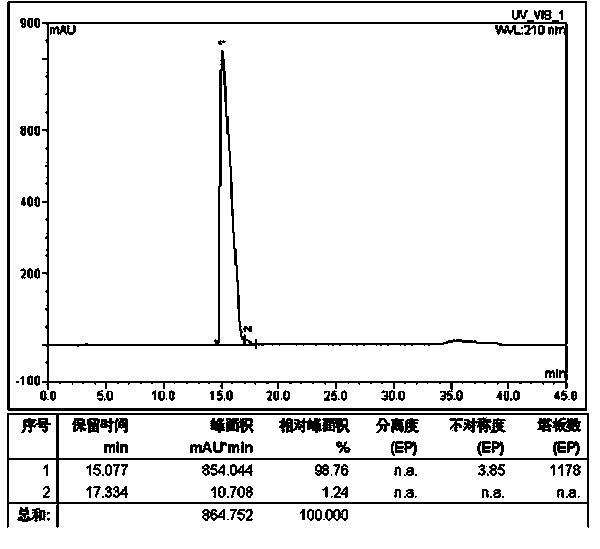
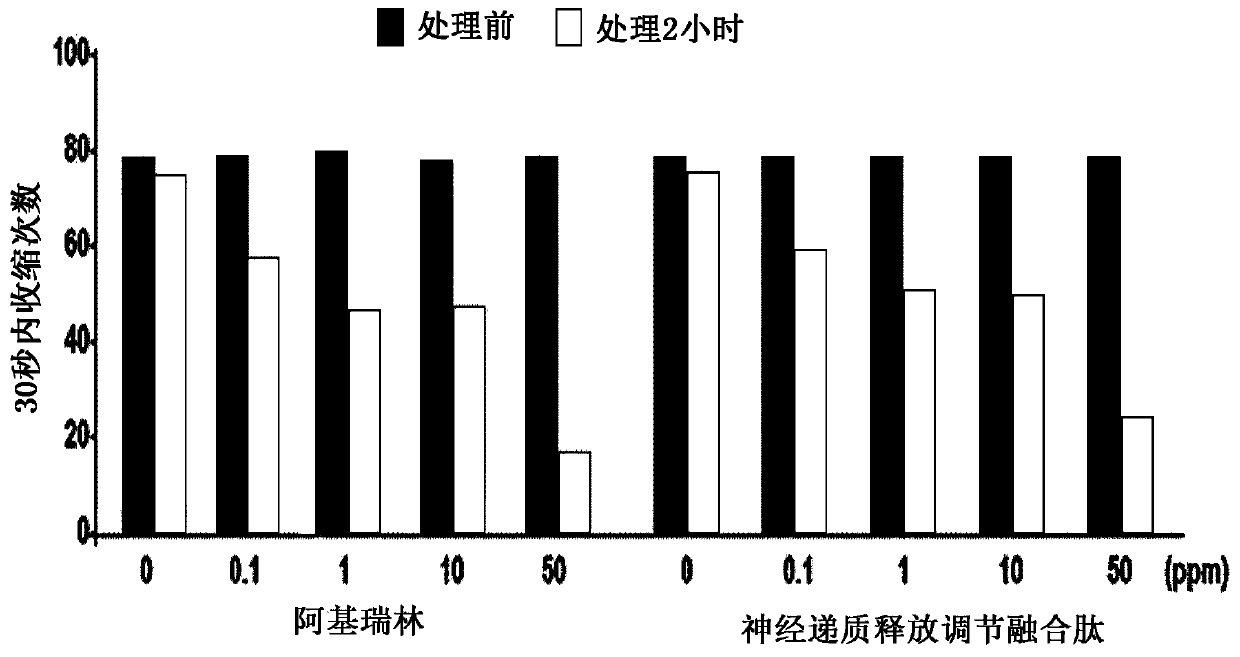
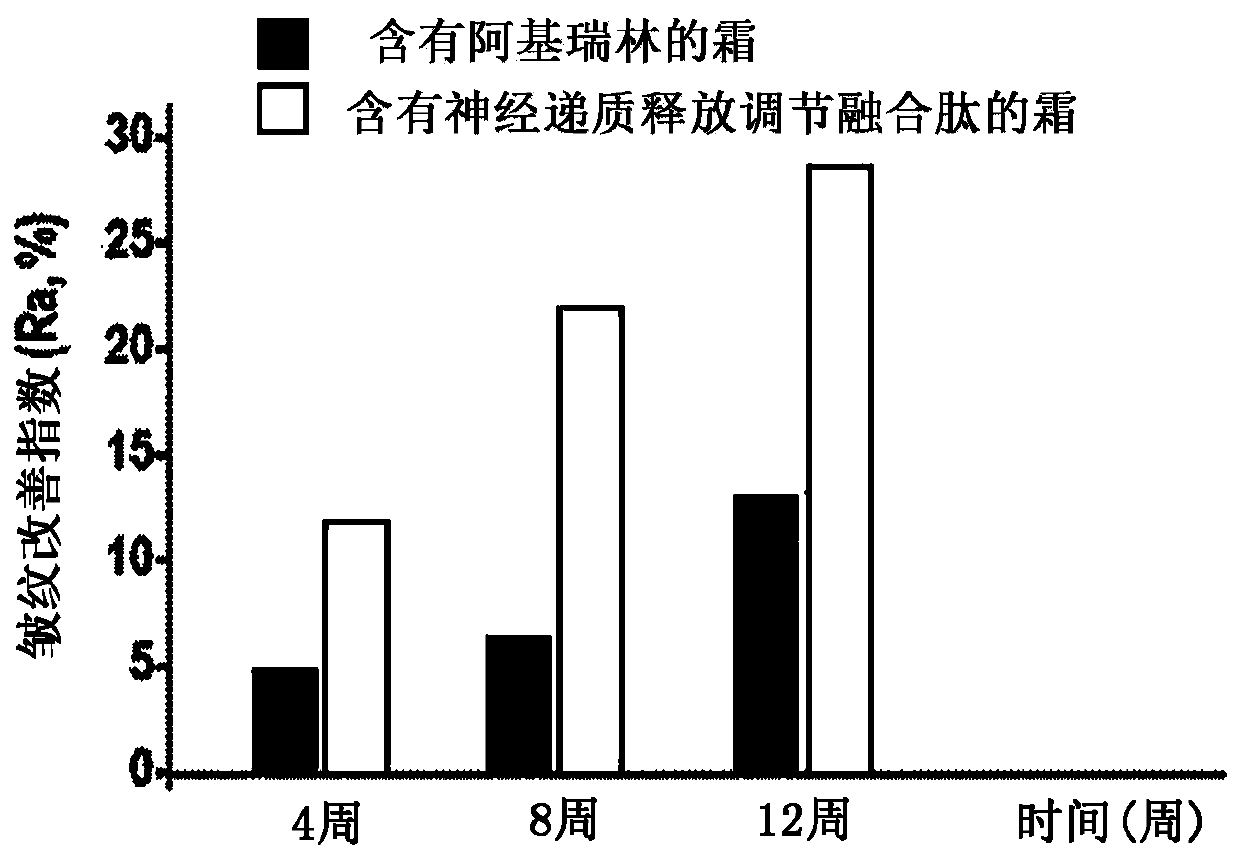
ActiveUS8106017B2Good activity and stabilityThymosin peptidesPeptide/protein ingredientsToothpasteHair streams
The present invention relates to a peptide comprising a specific amino acid sequence possessing human thymosin β-4 (Tβ4) activities and its uses. The peptide of this invention has identical or similar functions or actions to human Tβ4 and its biological activity is almost identical to natural-occurring Tβ4. In addition, the peptide of this invention exhibits much higher stability and skin permeation than natural-occurring Tβ4. In these connections, the composition comprising the peptides of this invention can exhibit excellent efficacies on improvement in thymosin β-4-effective disorders or conditions. In addition, the peptide of this invention can be advantageously applied to drugs, cosmetics, toothpaste and compositions for mouth cleaning and caring, most preferably, cosmetics. Specifically, the peptide of this invention is advantageously applied to cosmetics for promoting hair growth.
Owner:CAREGEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com