Trachinotus ovatus beta-thymosin and application thereof
A technology of oval pomfret and thymosin, which is applied in the field of molecular biology, can solve the problems of unclear function and application potential of beta-thymosin, and achieve the effect of improving disease resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
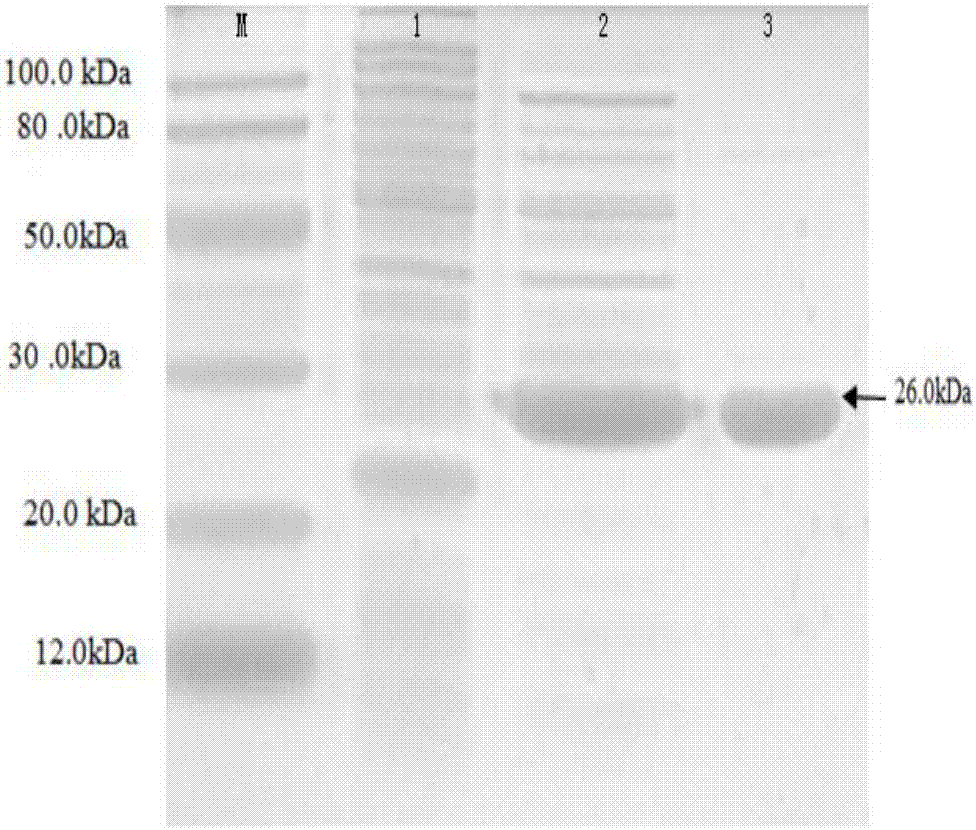
[0024] A kind of oval pomfret β-thymosin, the cDNA nucleotide sequence of the oval pomfret β-thymosin is as SEQ ID NO.1; The amino acid sequence of the oval pomfret β-thymosin is as SEQ ID NO.1 ID NO.2.
[0025] Extract the RNA of the ovoid pomfret, reverse transcribe the RNA into cDNA, then use the cDNA as a template, and use the following sequence as a primer to obtain the gene sequence of the β-thymosin disease resistance gene by PCR. The sequence of the primer is:
[0026] TroTβ-F: 5'-gatatcATGAGTGACAACAAGCCC-3';
[0027] TroTβ-R: 5'-gatatcTGATGACTCTGACTTCTCC-3'.
[0028] Reaction conditions: pre-denaturation at 94°C for 2 minutes, 30 cycles at 94°C for 30s, 52°C for 30s, and 72°C for 30s, a total of 35 cycles, and finally extension at 72°C for 5 minutes. The amplified fragments were recovered by gel, ligated with pMD19-T vector, transformed into Escherichia coli DH5α, and positive colonies were picked for PCR detection. After the detection is correct, the recombinant p...
Embodiment 2
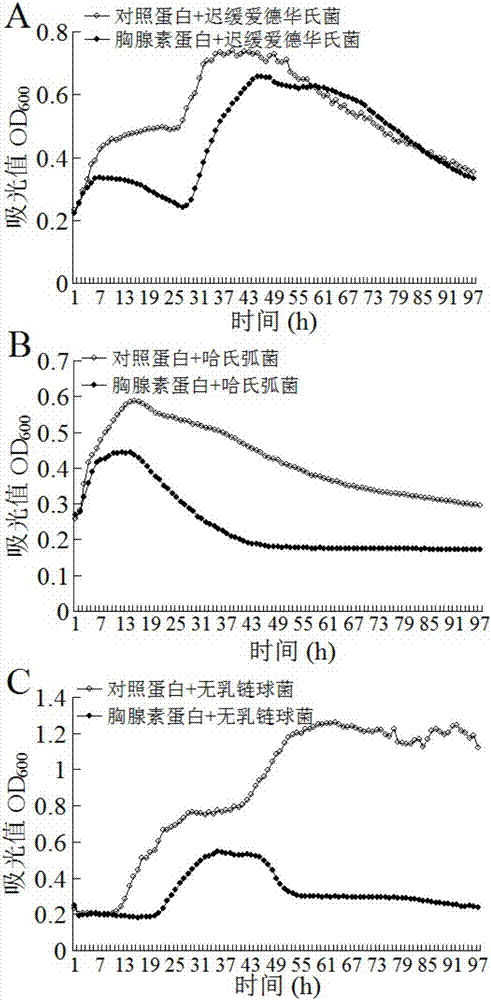
[0033] The bacteriostasis of embodiment 2β-thymosin
[0034]Dilute the purified β-thymosin protein described in Example 1 to 1 ug / ul in PBS. Edwardsiella tarda, Vibrio harveii and Streptococcus agalactiae were cultured in LB medium at 30°C with shaking at 200rpm / min until OD 600 About 0.6, respectively take 50ul bacterial liquid and 50ul β-thymosin diluent to mix, at 30°C, 250rpm speed, Bioscreen C of Growth Curves Company to measure, OD600 is measured once per hour, continuous measurement for 96 hours, β-thymus The protein can significantly inhibit the growth of Edwardsiella tardiformis, Vibrio harveii and Streptococcus agalactiae, the results are shown in figure 2 .
Embodiment 3
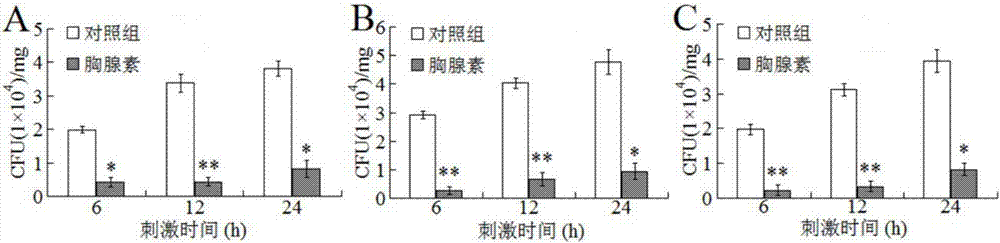
[0035] After embodiment 3 β-thymosin protein injects fish, the resistance to disease and infection of fish is significantly improved
[0036] Dilute the β-thymosin protein purified in Example 1 to 200ug / ml in PBS, which is the β-thymosin dilution. Thirty oval pomfrets (weighing about 15g) were randomly divided into 2 groups, 15 in each group. The two groups were named A and B respectively, each fish in group A was injected with 100ul beta-thymosin diluent, and group B was injected with 100ul PBS.
[0037] Cultivate Edwardsiella tarda in LB medium at 30°C with shaking at 200rpm / min to OD 600 About 0.6, estimated 1OD=5*10 8 CFU / ml, diluted to 10 in PBS 6 CFU / ml is the bacterial suspension of Edwardsiella tardigrade.
[0038] Attack infection
[0039] After injecting 100ul of β-thymosin dilution or 100ul of PBS to the experimental fish of groups A and B for 1 day, each fish was injected with 100ul of the bacterial suspension of Edwardsiella brachii prepared in the above step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com