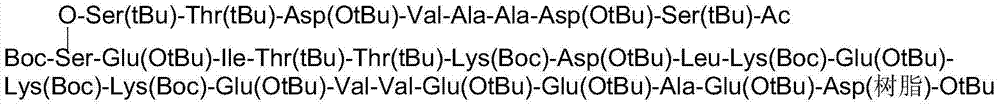Synthesis method of thymosin alpha1
A technique for synthesizing thymosin and solid-phase peptides, which is applied in the fields of peptide preparation methods, thymosin, chemical instruments and methods, etc., can solve problems such as the difficulty of synthesizing thymosin α, and achieve the effects of easy post-processing, good purity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a thymosin alpha 1 The nonlinear solid-phase synthesis method comprises the following steps:
[0036] (1) Using Rink Amide resin as a carrier, synthesize Fmoc-Asp(resin)-OtBu with Fmoc-Asp-OtBu, and then follow thymosin α 1The peptide sequence of the peptide sequence, using the solid-phase peptide synthesis method on the basis of Fmoc-Asp (resin)-OtBu to couple amino acids one by one from the C-terminal to the N-terminal, in this step, the last amino acid coupled is thymosin α 1 The serine at the 9th position of the N-terminus in the peptide sequence obtains the following peptide resin A:
[0037] Boc-Ser-Glu(OtBu)-Ile-Thr(tBu)-Thr(tBu)-Lys(Boc)-Asp(OtBu)-Leu-Lys(Boc)-Glu(OtBu)-Lys(Boc)-Lys( Boc)-Glu(OtBu)-Val-Val-Glu(OtBu)-Glu(OtBu)-Ala-Glu(OtBu)-Asp(resin)-OtBu;
[0038] (2) Use Fmoc-Ser(tBu)-OH to react with the free hydroxyl group of serine at the N-terminal of the above-mentioned peptide resin A to form an ester bond. thymosin alpha 1 Th...
Embodiment 1
[0057] Embodiment 1: the degree of substitution is the synthesis of Fmoc-Asp (resin)-OtBu of 0.2mmol / g
[0058] Weigh 500 g of Rink Amide resin with a substitution degree of 0.6 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes. The Fmoc protection was removed with DBLK (20% piperidine / DMF), followed by washing 4 times with DMF and 2 times with DCM. Weigh 51.4g Fmoc-Asp-OtBu (125mmol), 20.3g HOBt (150mmol) and dissolve in a mixed solution of DCM and DMF with a volume ratio of 1:1, add 23.4ml DIC (150mmol) under ice water bath for activation for 3min, then add solid phase reaction In the column, react at room temperature for 2 hours. Wash 3 times with DMF, add 1046.3ml of blocking solution (pyridine / acetic anhydride = 1:1, 6mol:6mol) to block for 8 hours (if the resin is not fully diffused, add DCM as a solvent). Wash 4 times with DMF, 4 times with DCM, shrink and dry with methanol to obtain Fmoc-Asp(resin)-Ot...
Embodiment 2
[0059] Embodiment 2: the degree of substitution is the synthesis of Fmoc-Asp (resin)-OtBu of 0.3mmol / g
[0060] Weigh 500 g of Rink Amide resin with a substitution degree of 0.6 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes. The Fmoc protection was removed with DBLK (20% piperidine / DMF), followed by washing 4 times with DMF and 2 times with DCM. Weigh 77.2g Fmoc-Asp-OtBu (187.5mmol), 30.4g HOBt (225mmol) and dissolve in the mixed solution of DCM and DMF with a volume ratio of 1:1, add 35.2ml DIC (225mmol) under ice water bath for activation for 3min, then add the solid phase In the reaction column, react at room temperature for 2 hours. Wash 3 times with DMF, add 1046.3ml of blocking solution (pyridine / acetic anhydride = 1:1, 6mol:6mol) to block for 8 hours (if the resin is not fully diffused, add DCM as a solvent). Wash 4 times with DMF, 4 times with DCM, shrink and dry with methanol to obtain Fmoc-Asp(r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com