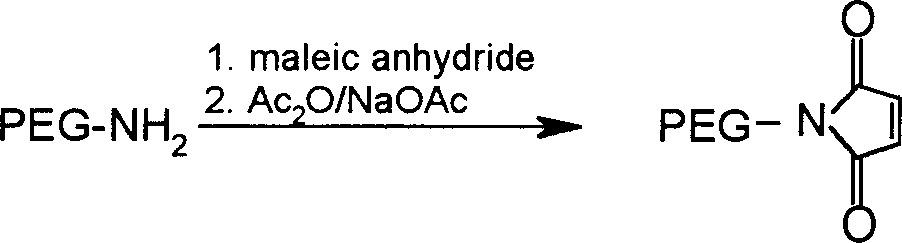
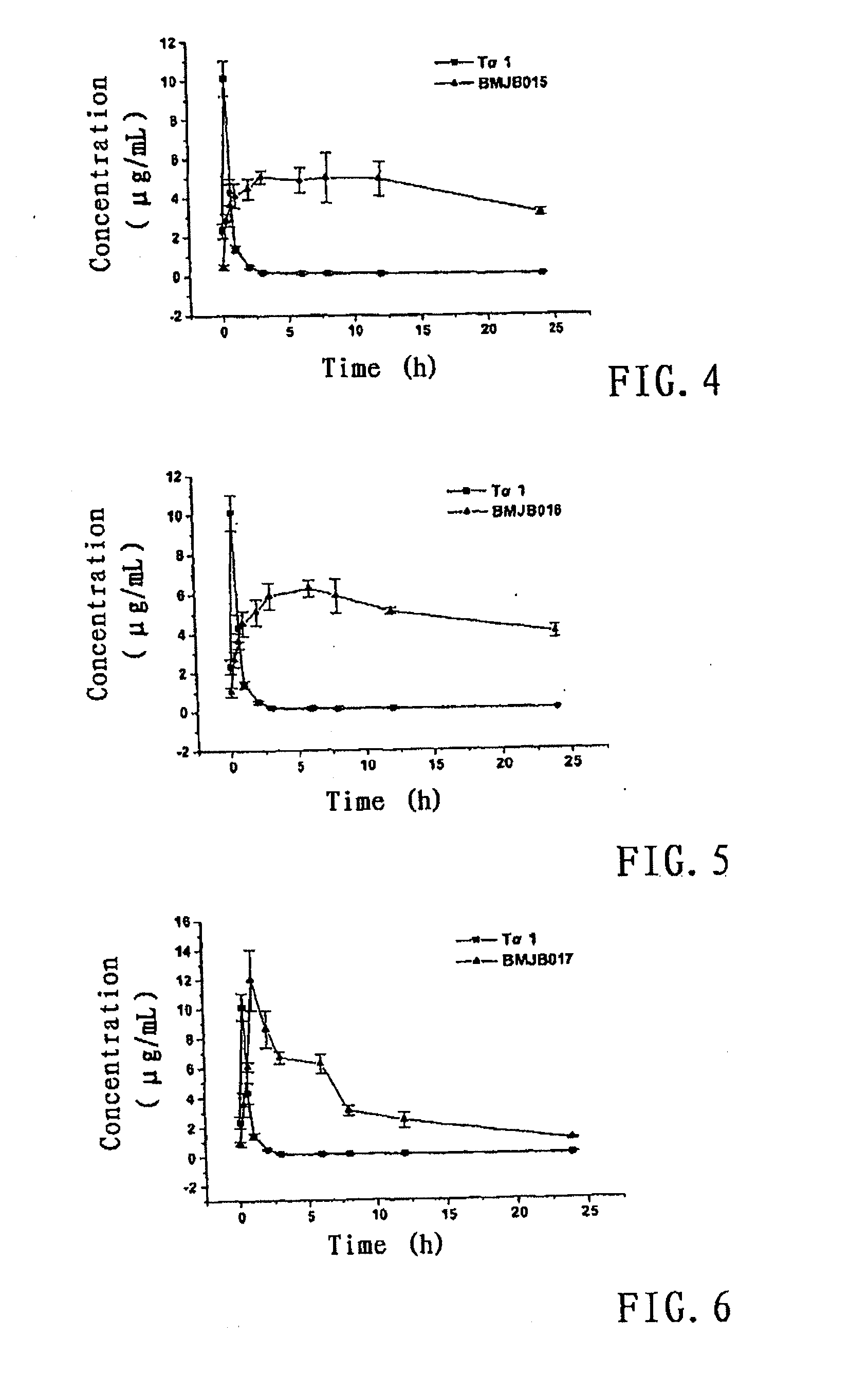
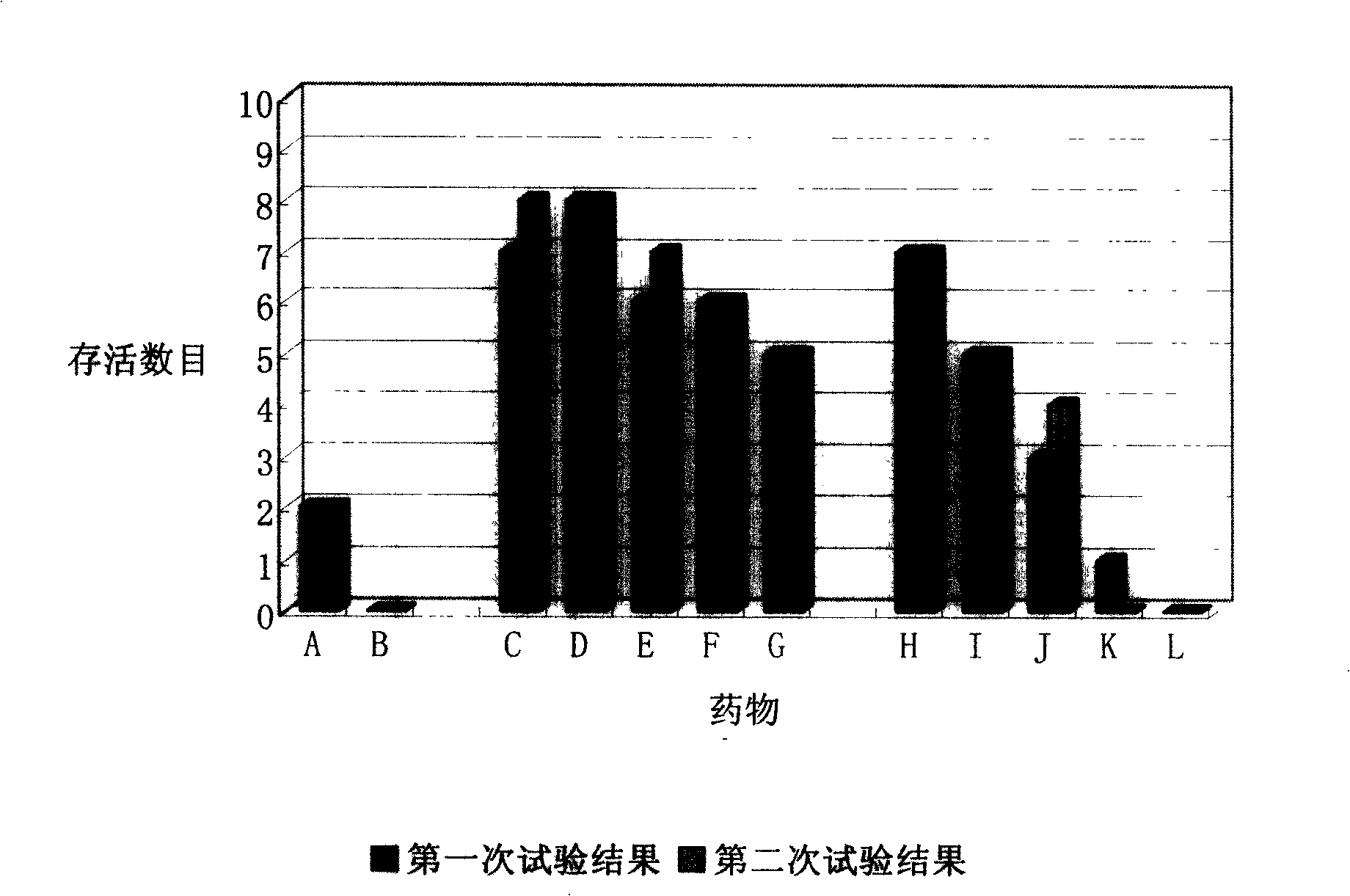
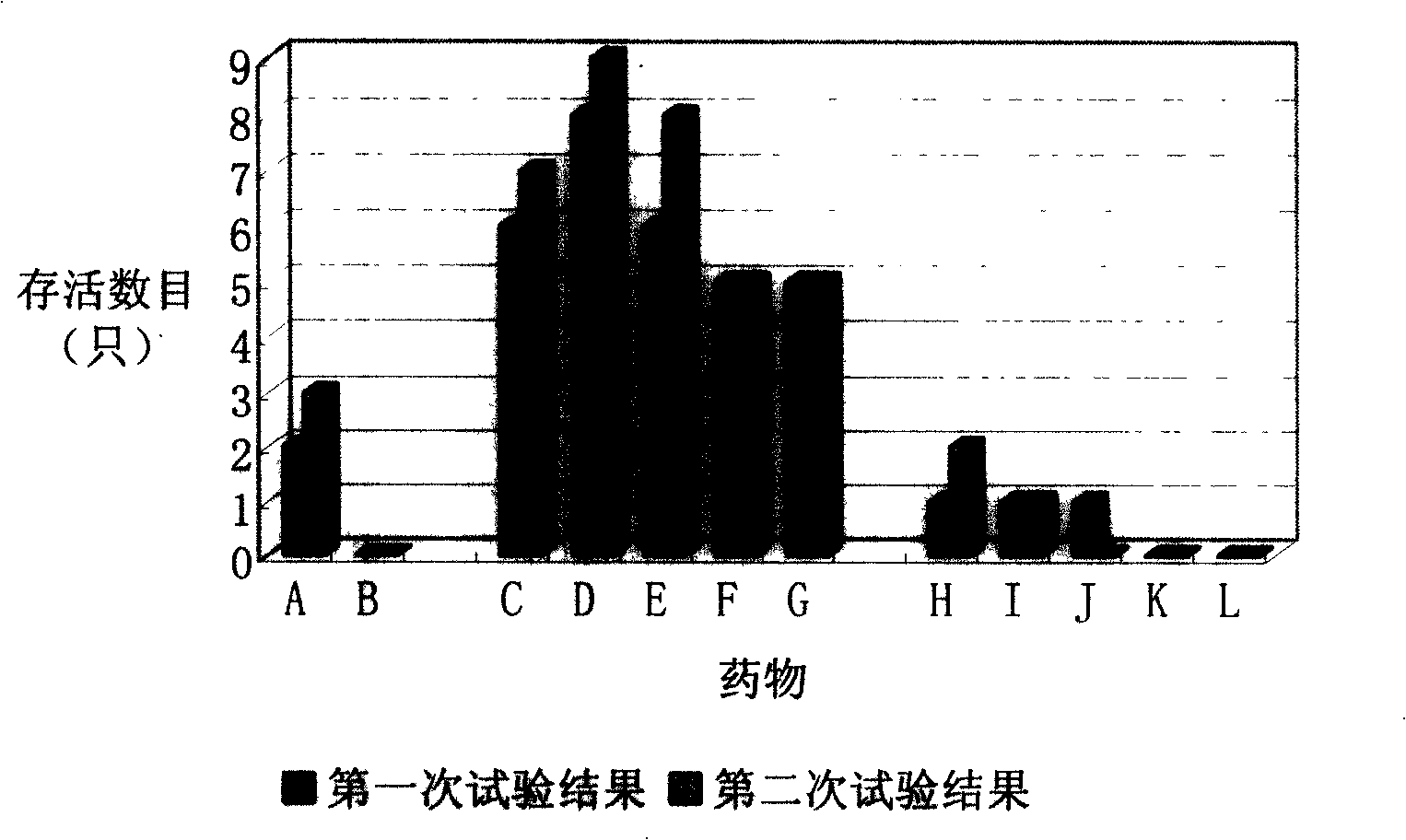
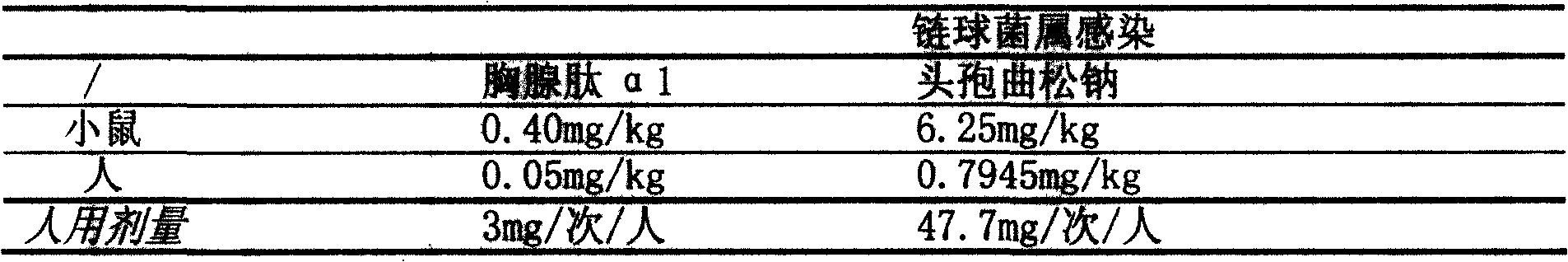
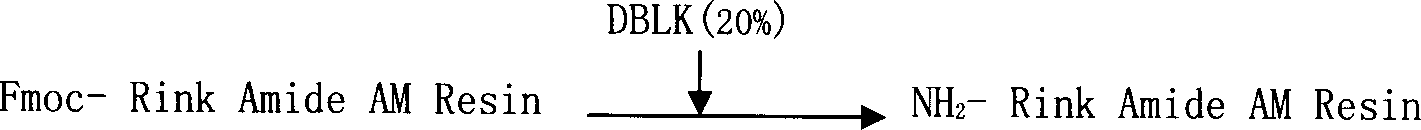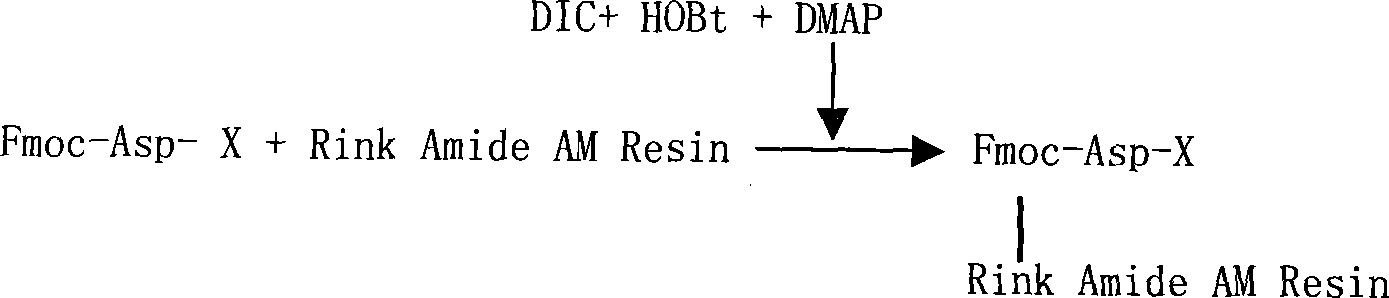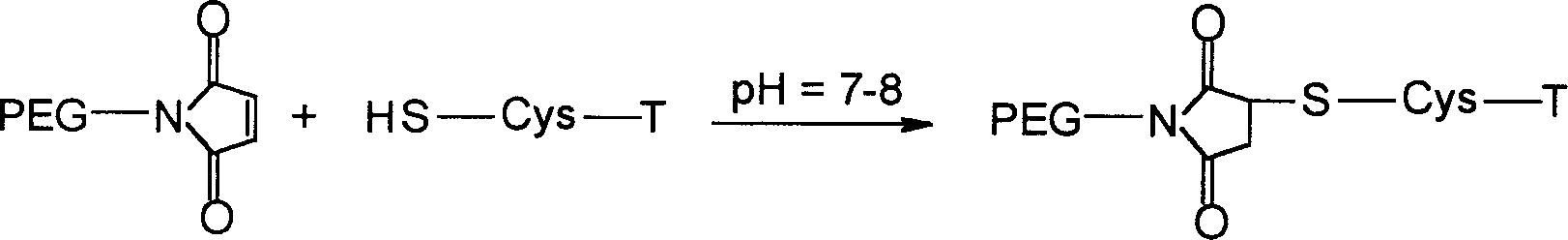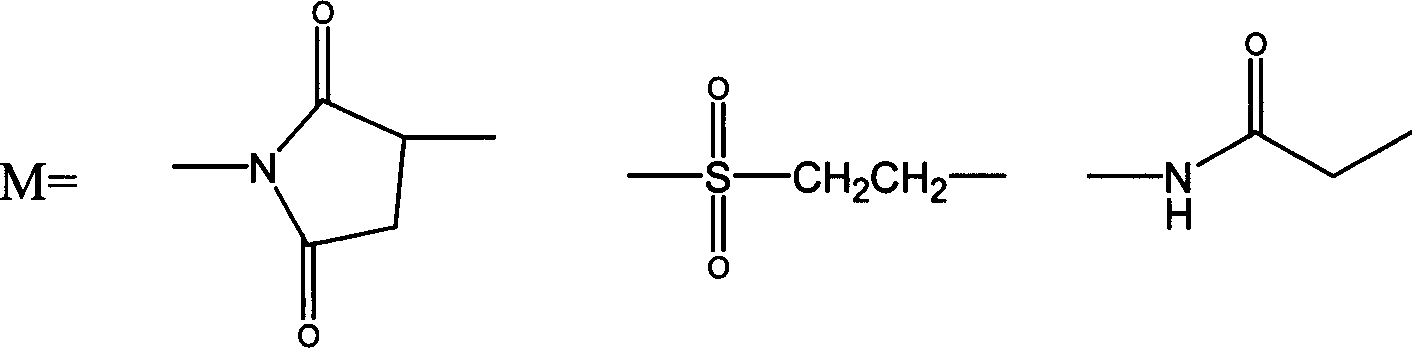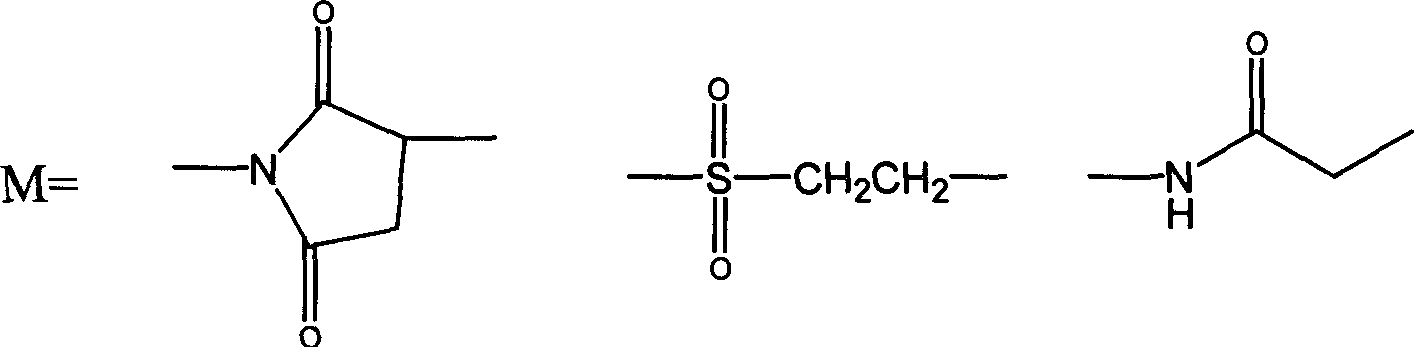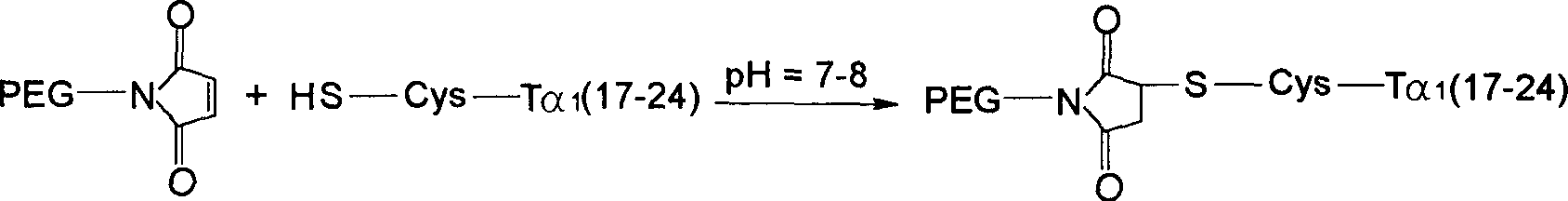Patents
Literature
102 results about "Thymosin alpha" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
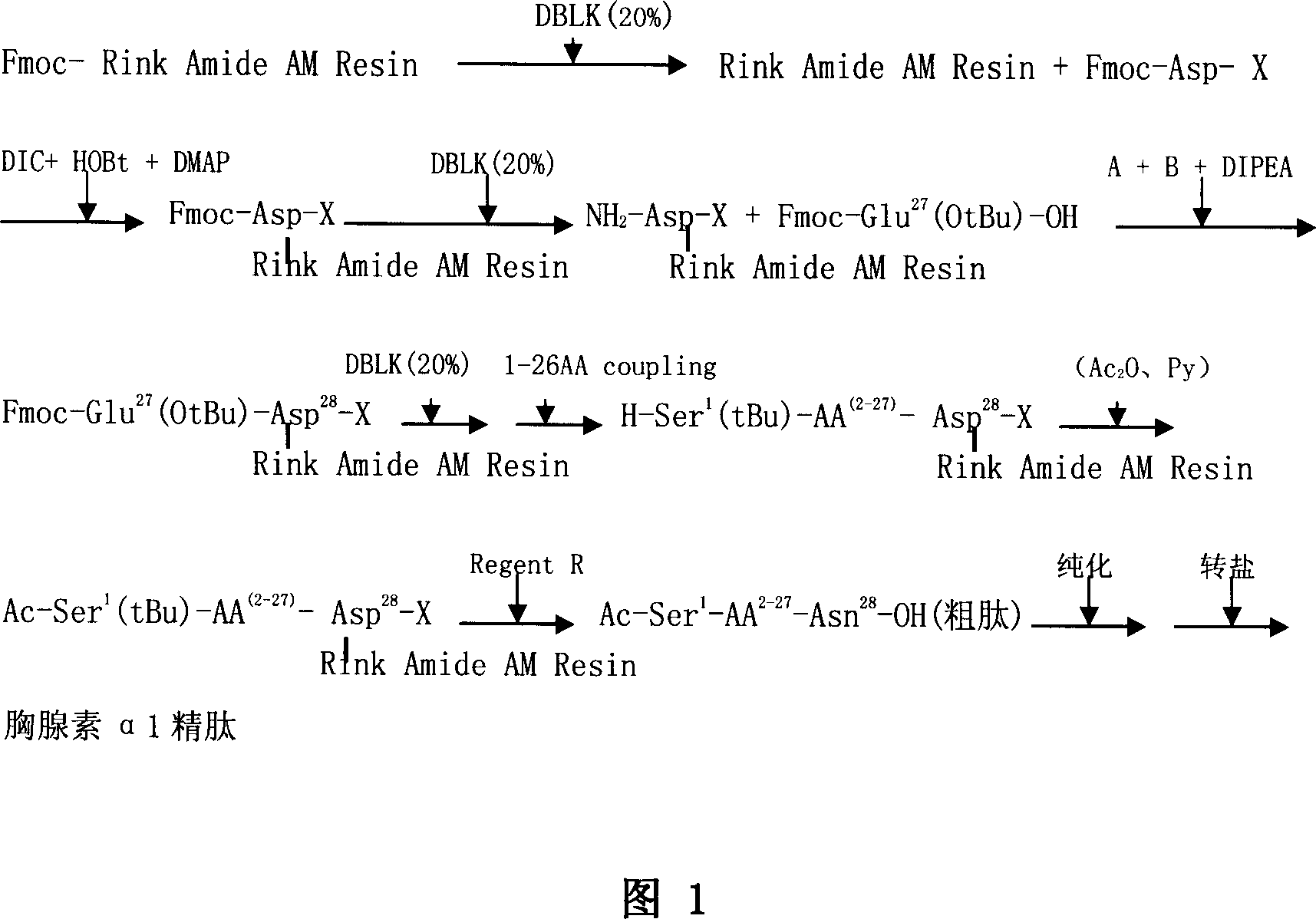
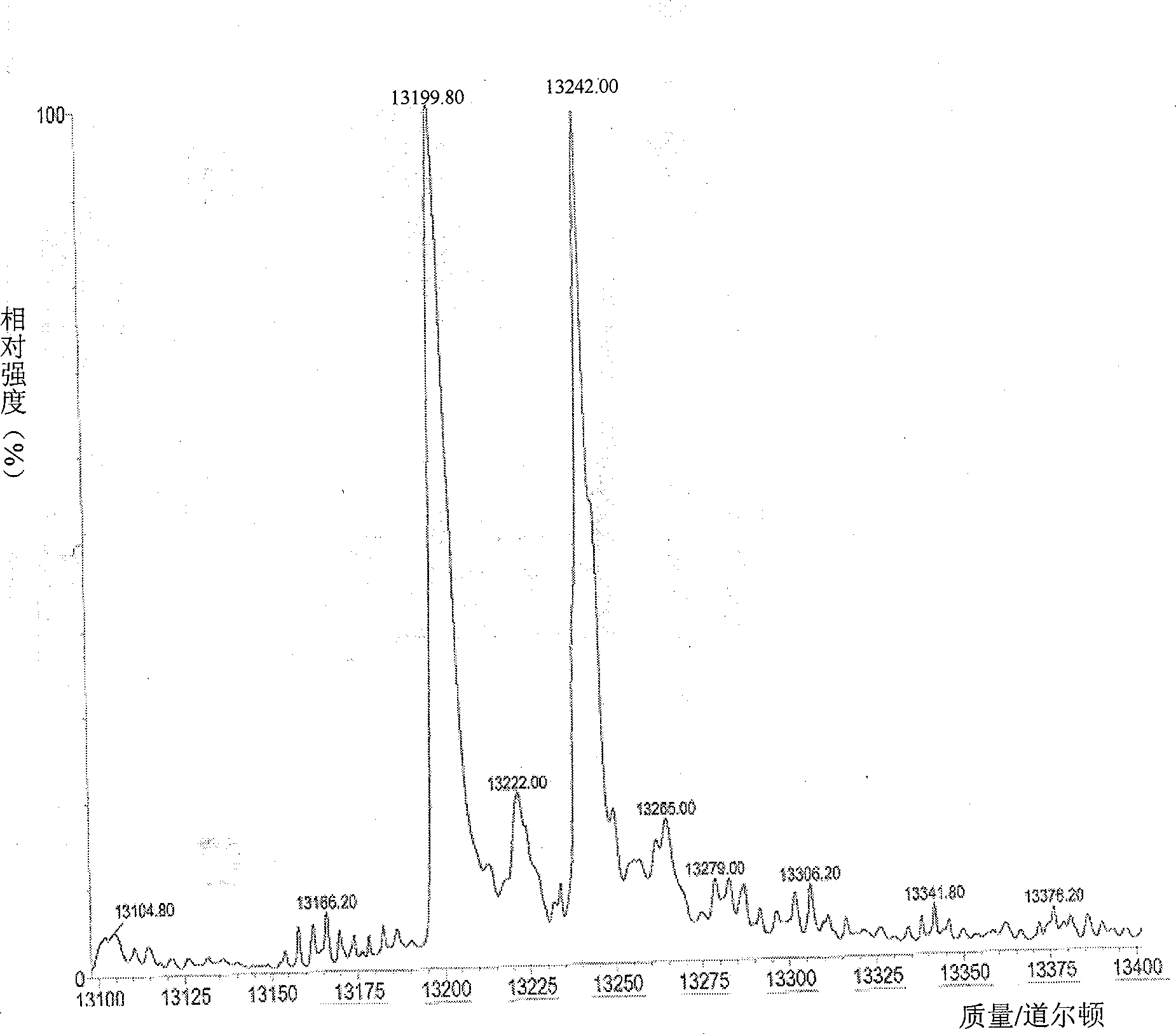
Solid phase synthetic technique for thymosin alpha1
ActiveCN101104638AAdvantages of solid phase synthesis processEasy to purifyThymopoietinsPeptide preparation methodsFluoroacetic acidAcetic anhydride
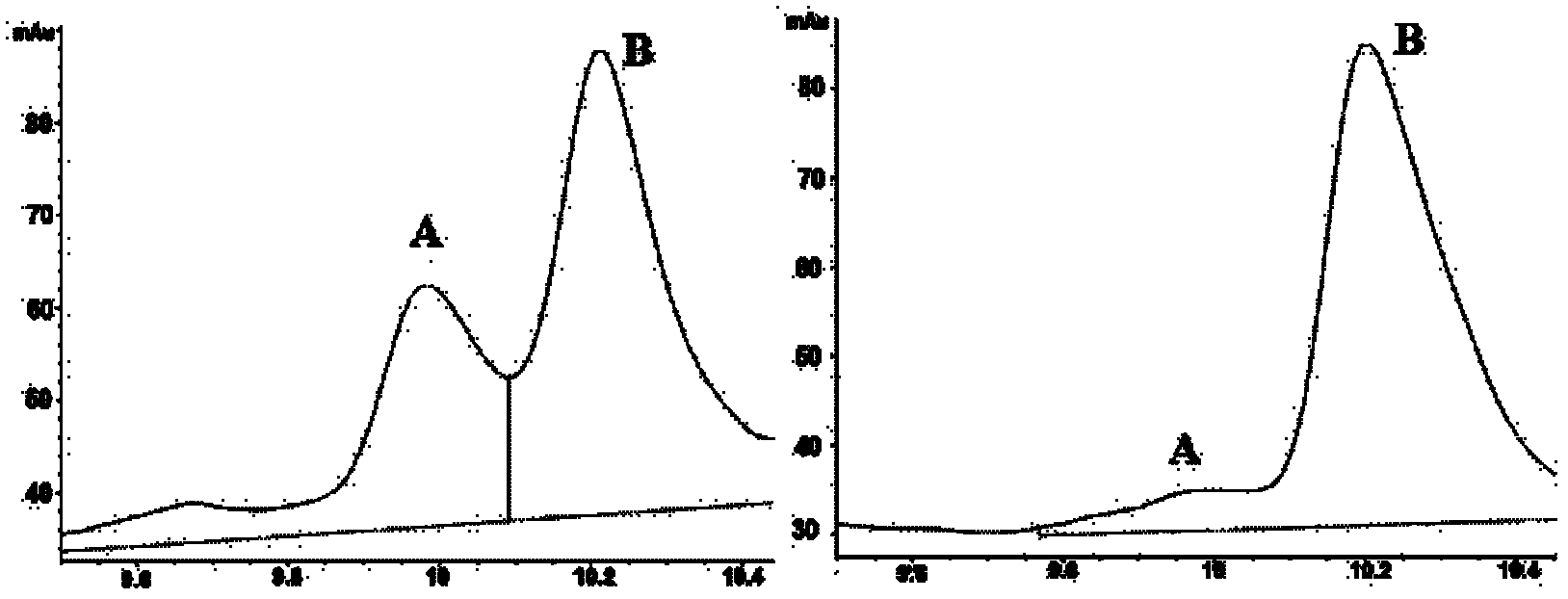
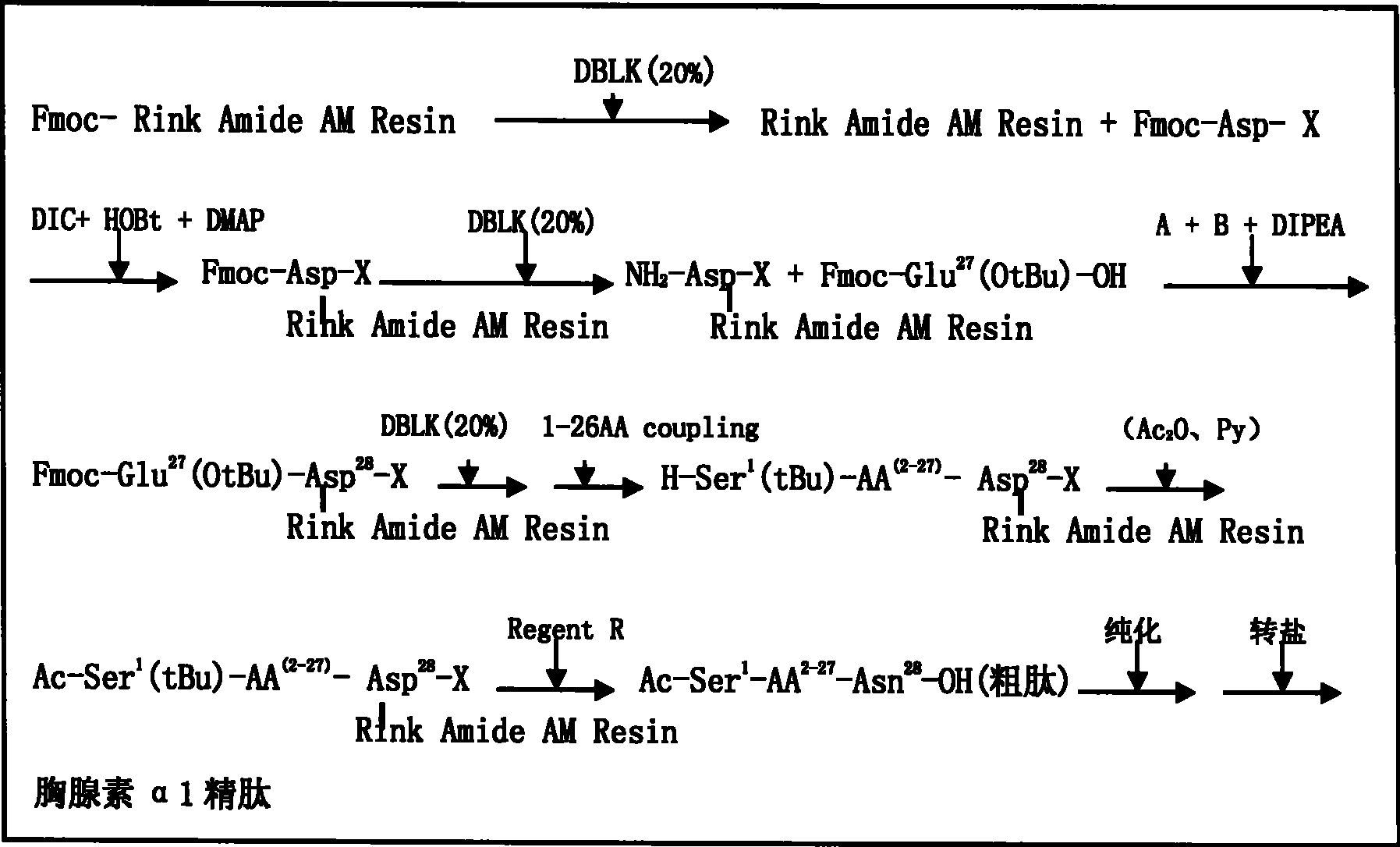
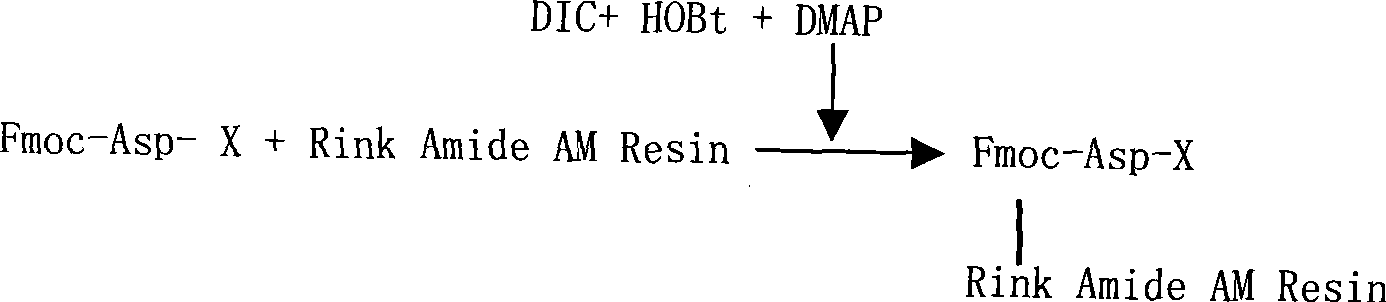
The invention relates to a solid-phase synthesis process of a thymosin alpha 1, belonging to the polypeptide solid-phase synthesis technical field. The invention comprises the following steps: a. a Fmoc-Rink Amide AM resin or a Fmoc-Rink Amide MBHA resin is used as carrier, an H2N-Rink Amide AM resin or an H2N-Rink Amide MBHA resin is obtained after deprotection of the Fmoc; b. side chain carboxyl group of Fmoc-Asp-X is connected with resin amino by the method of solid-phase synthesis to obtain the Fmoc-Asp (resin)-X; c. the left amino acid in the sequence is synthesized in solid-phase with the Fmoc strategy; d. after the amino protection group Fmoc of N terminal amino acid is removed, the N terminal amino acid is acetylated by acetic anhydride and pyridine; e. then the acetylated N terminal amino acid is cut by a cracking agent (tri fluoroacetic acid / benzoylate sulfide / 1, 2- dithioglycol / Anisole) to obtain the thymosin alpha 1; f. crude product of the thymosin alpha 1 is prepared and separated by HPLC to obtain the pure thymosin alpha 1. The invention can increase significantly the yield of the thymosin alpha 1 and decrease the production cost, which is helpful for scale production and has better industrialization prospect.
Owner:苏州天马医药集团天吉生物制药有限公司
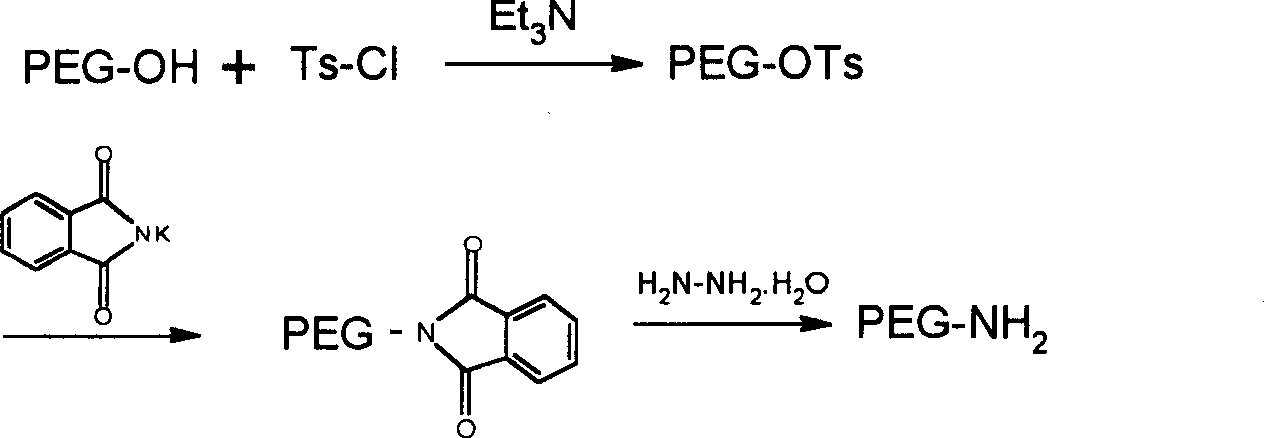
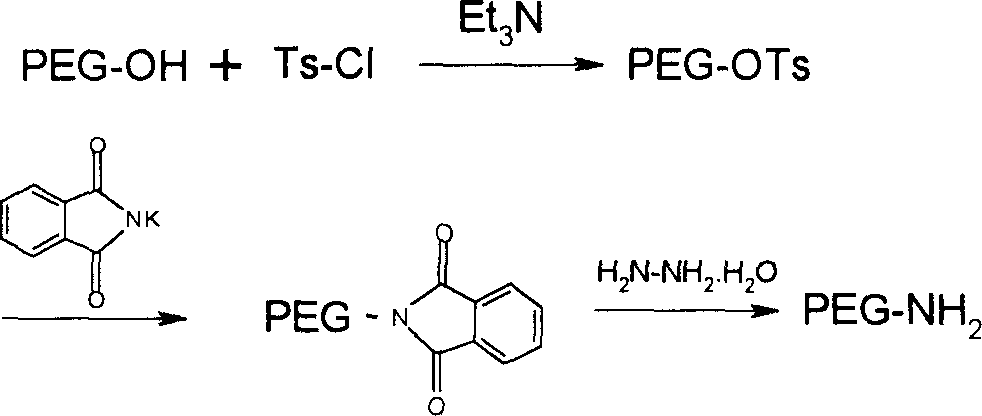
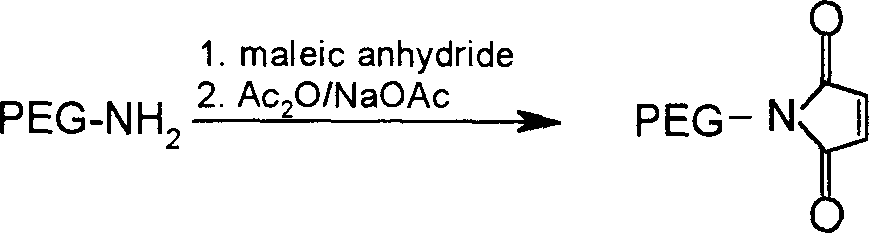
Polyethylene glycol derivatives of thymosin alphal
InactiveCN1532207AModified implementationThymopoietinsPeptide/protein ingredientsSmall-cell carcinomaMelanoma
The present invention relates to polyethylene glycol derivatives of thymosin alpha-1, their preparation process, the medicine composition containing them, and their application in the medicine for preventing and treating diseases related with immune deficiency and hypoimmunity, including hepatitis B, hepatitis C, malignant melanoma, non-small cell lung carcinoma, SARS, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Thymosin alpha 1 active segment cyclicpeptide analogue and its poly glycol derivative
The present invention relates to a kind of cyclopeptide derivative containing natural or artificial amino acid substituted active thymosin alpha-1 segemnt, and its preparation process, medicine composition and their medicines for treating or preventing diseases related to immune deficiency, hypoimmunity, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Separation and purification process for synthetic thymosin alpha 1
InactiveCN102477094AReduced risk of denaturation failureImprove concentration efficiencyHormone peptidesPeptide preparation methodsChemical synthesisDesalination
The invention relates to a purification process for thymosin alpha 1 after chemical synthesis. Specifically speaking, the conventional manner of combined ion exchange and vacuum condensation for desalination and condensation of thymosin alpha 1 obtained through chemical synthesis is changed into one-shot nanofiltration in the invention. The process provided in the invention has the advantages of simpleness, high efficiency, low energy consumption and suitability for industrial production.
Owner:BEIJING KAWIN TECH SHARE HLDG +1
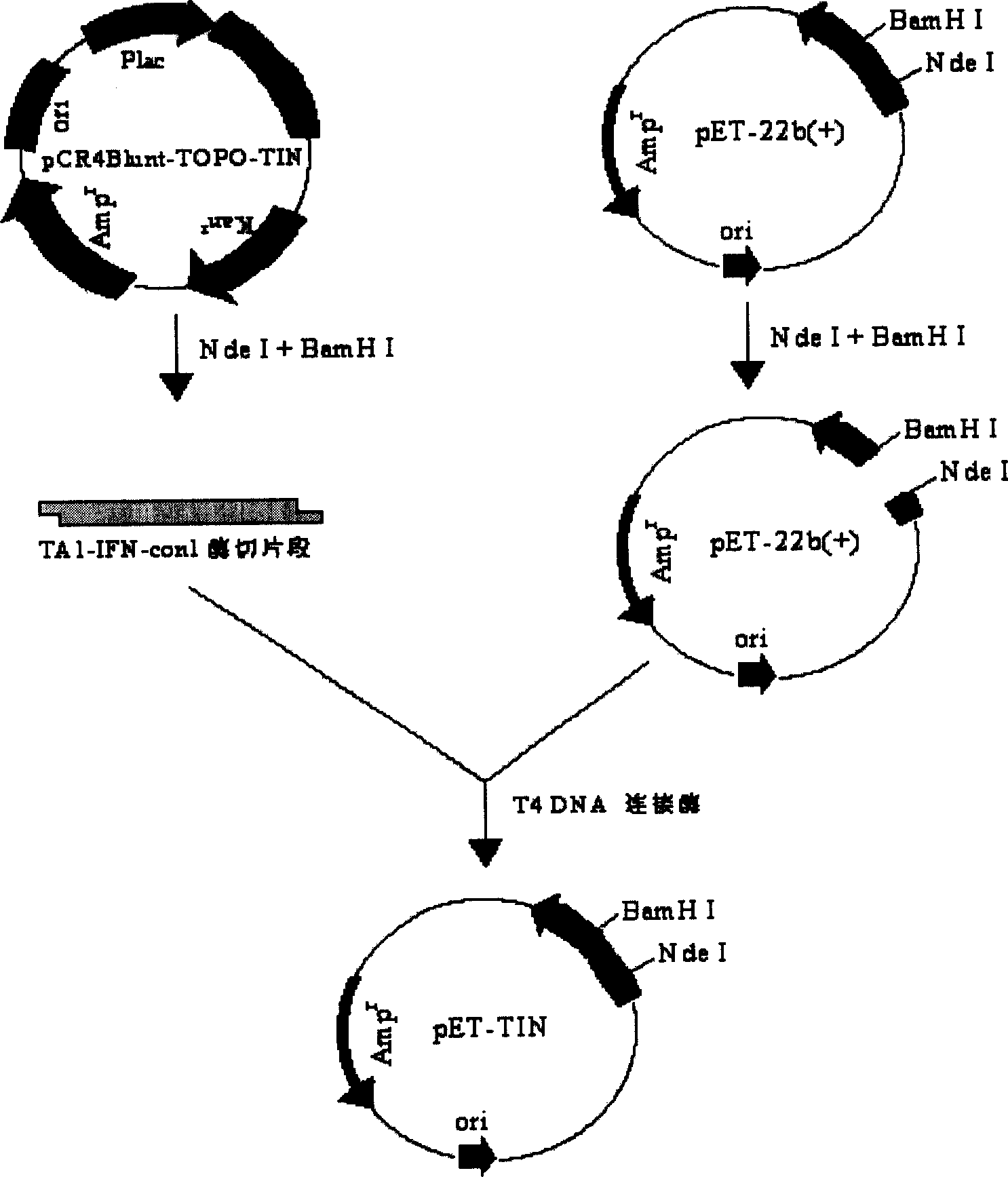
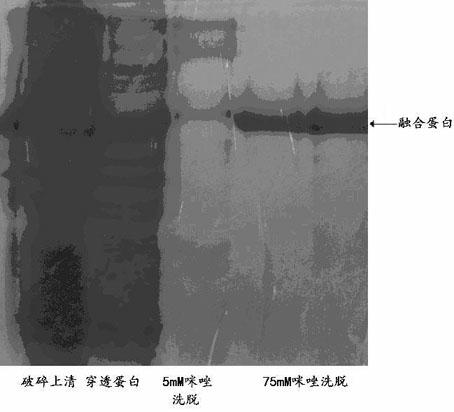
Fusion protein of human thymosin alpha1 and human composite interferon and preparation thereof
InactiveCN1523040ASimple purification processStrong antiviral activityPeptide/protein ingredientsAntiviralsPurification methodsVirus
The present invention relates to a fusion protein of human thymosin alpha 1and human compound interferon, and said protein expression and purification method and application of said protein which can be used as medicine for resisting virus and resisting tumor.
Owner:重庆康尔威药业股份有限公司
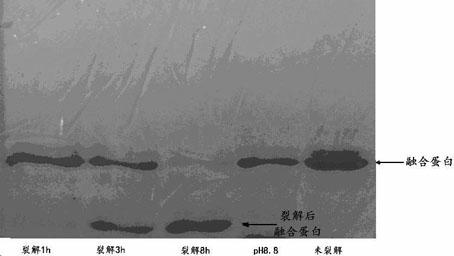
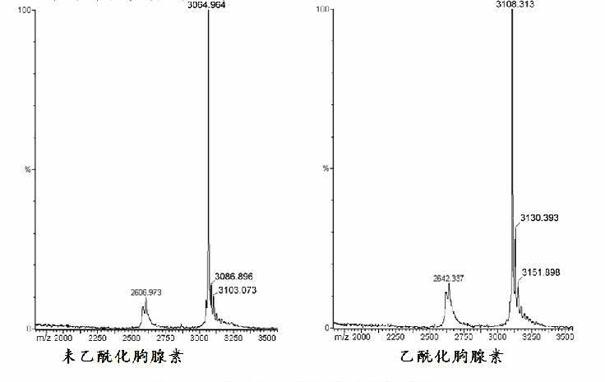
Method for preparing N-end acetylation modified thymosin alpha with recombined E. coli
The present invention discloses the preparation process of thynosin-alpha with acetylation modified N-end by using recombinant colibacillus. The preparation process includes obtaining thynosin-alpha gene, constructing recombinant expression vector containing thynosin-alpha, transforming prokaryotic cell, culturing prokaryotic cell and expressing thynosin-alpha, and separating and purifying thynosin-alpha with acetylation modified N-end. The present invention also discloses the process of utilizing recombinant colibacillus in preparing thynosin-alpha antigen with acetylation modified N-end and then cutting and separating to prepare thynosin-alpha-1 antigen with acetylation modified N-end, thynosin-alpha-2 antigen with acetylation modified N-end and similar matter. The thynosin-alpha with acetylation modified N-end has the functions of regulating immunity, cell proliferation, etc. and has wide application foreground in antivirus and antitumor.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Use of thymosin alpha 1 for preparing a medicament for the treatment of stage iv malignant melanoma
It is described the use of thymosin alpha in combination with dacarbazine and optionally with Interferon alpha, for preparing a medicament for the treatment of malignant melanoma on stage IV characterized by distant unresectable metastases.
Owner:SCICLONE PHARM INT LTD
Process of preparing calf thymus alphal
ActiveCN101033248AReduce usageWith large-scale production capacityPeptide preparation methodsAnimals/human peptidesAcetic anhydrideSide chain
The invention discloses a new technology to prepare calf thymus alpha 1 (natural existing in thymus cells of calf and other animals), which uses Fmoc-strategy solid phase method, including the following steps: a. taking any one of Rink Amide PEGA resin, Rink Amide AM resin, Rink Amide MBHA resin or Rink Amide PEGA as the starting material, using the amino acid protected by Fmoc as the monomer to hang resin, connecting amino acids in turn to get a protective 28-peptide resin according to the method of solid-phase synthesis, b. using acetic anhydride to close heads during the process in turn, c. removing Fmoc-protective group in turn, d. synchronizing the removal of side-chain protecting group and the cut of peptide to get a crude, e. purifying the crude with antiphase HPLC to get thymosin alpha 1.
Owner:SHANGHAI SOHO YIMING PHARMA
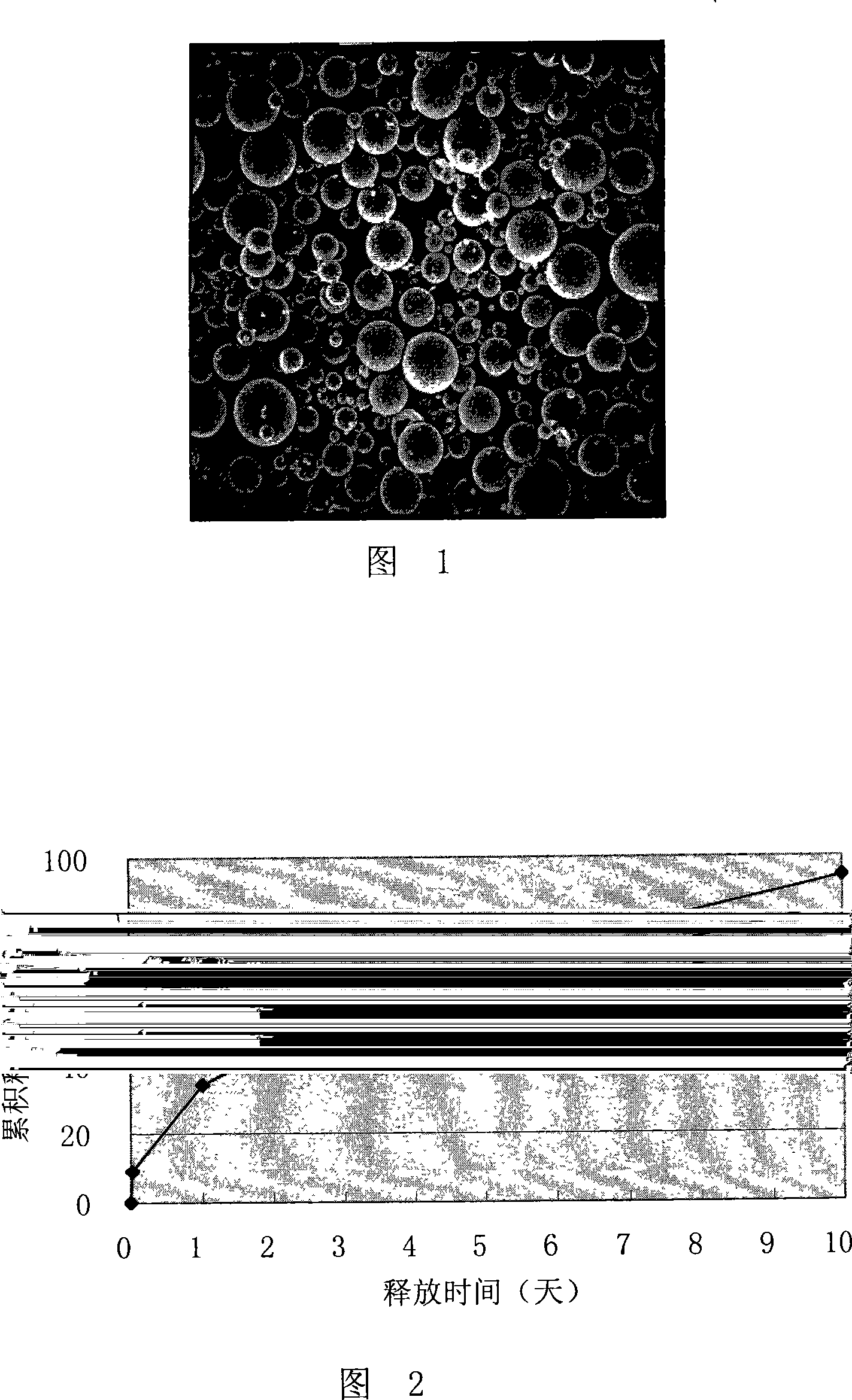
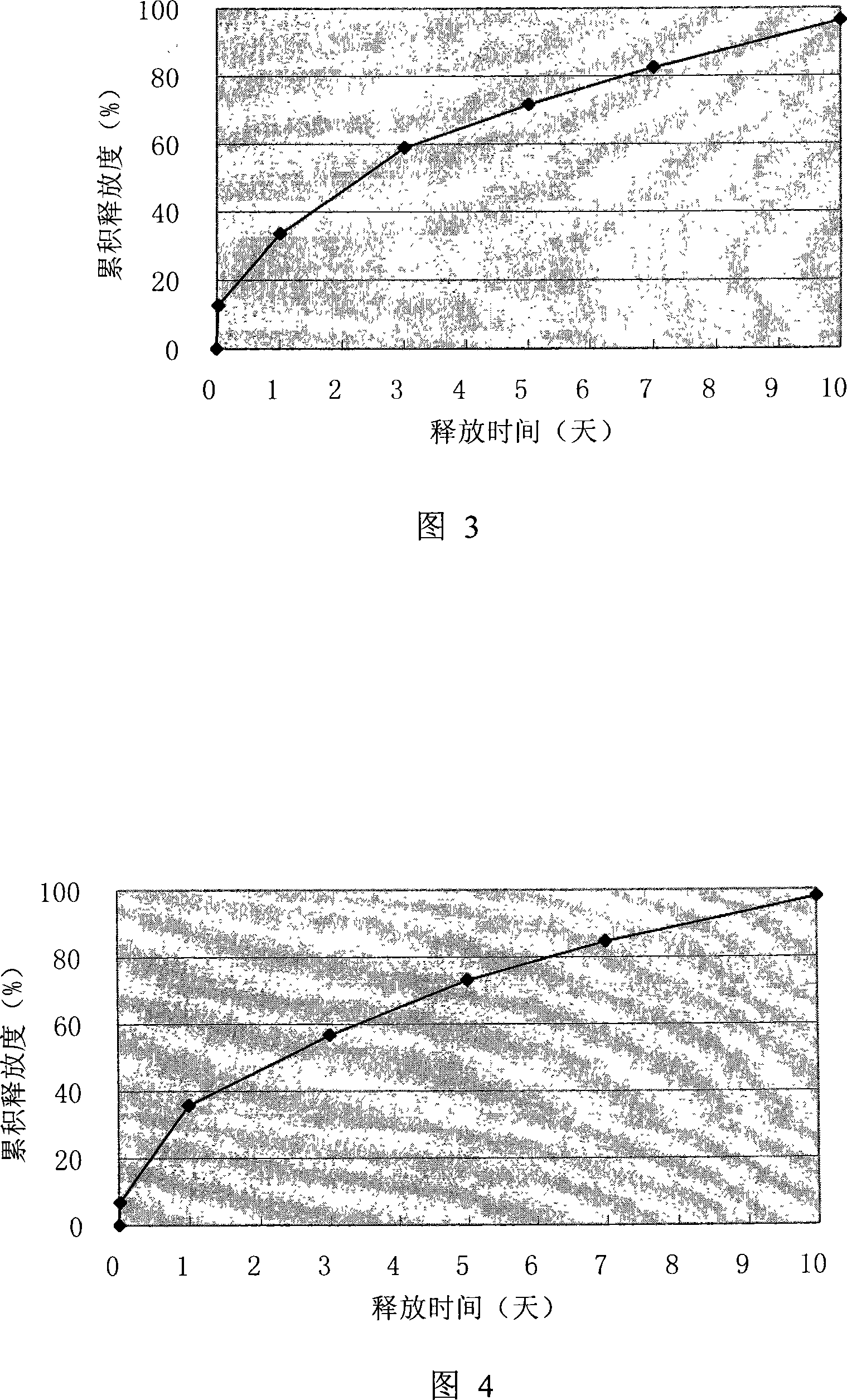
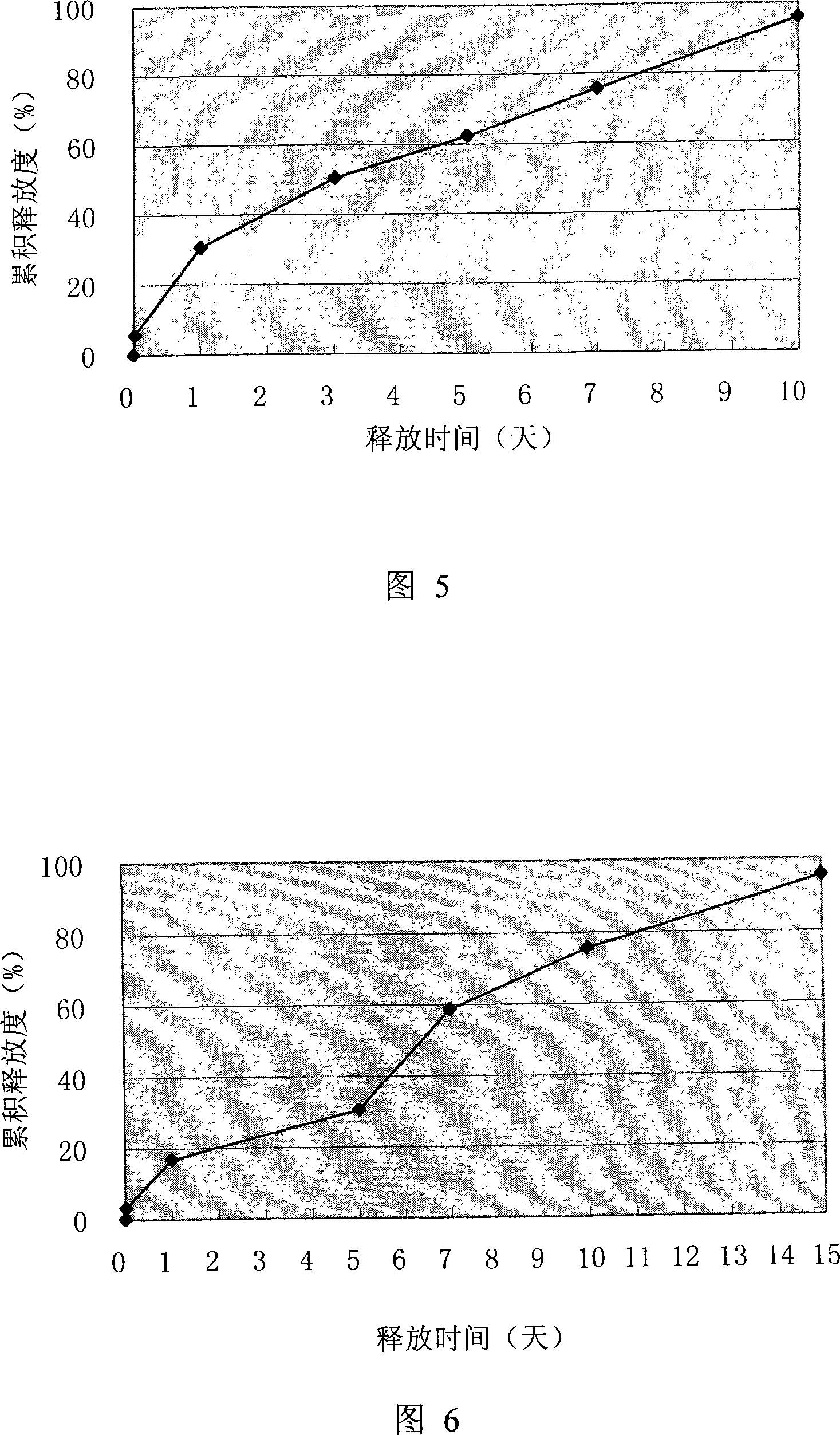
Thymus gland peptide alpha1sustained-release microsphere preparation for injection and preparation thereof
ActiveCN101244259AHigh encapsulation efficiencyRelease stabilityPeptide/protein ingredientsGranular deliverySide effectMicrosphere
The invention provides a slow release microsphere agents, comprising thymosin Alpha 1 of 0.5% to 10% of the microsphere weight, degradable pharmaceutical macromolecular accessories with a molecular weight of 5,000 to 500,000 Dalton and of 70% to 99.5% of the macroshpere weight, and 0% to 10% of other pharmaceutically acceptable accessories. The invention also provides the preparation method of the slow release microsphere agents. The slow release microsphere agent of thymosin Alpha 1 for injection has the advantages that the poison and side effect of thymosin Alpha 1 is reduced; the bioavailability is enhanced; times for drug taking is reduced and the drug can be conveniently taken by patients.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
Method for preparing N-terminated acetylated thymosin alpha 1 and special engineering bacteria therefor
InactiveCN101497863AImprove expression levelMicroorganismsMicroorganism based processesAcetylationGene engineering
The invention discloses a method for preparing N-terminal acetylation extrasin alpha1, and special engineering bacteria thereof. The engineering bacteria is obtained in such a way that encoding genes of the extrasin alpha1 and encoding genes of N-terminal acetyl transferase are introduced to host strains. The N-terminal acetyl transferase is the protein of the following a or b: a) protein is composed of amino acid sequences shown by a sequence 7 in a sequence table; and b) protein is derived from the a) and is provided with the N-terminal acetyl transferase through the replacement and / or deletion and / or adding of one or a plurality of amino acid residue of the amino acid sequences shown by the sequence 7 in the sequence table. Talpha1 obtained through the preparation of the engineering bacteria is all N-terminal acetylized. The invention overcomes the defects of no acetylization or only partial N-terminal acetylization in a traditional gene project technology, thoroughly realizes N-terminal acetylization Talpha1 through a gene engineering technology, and has strong practical purposes.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
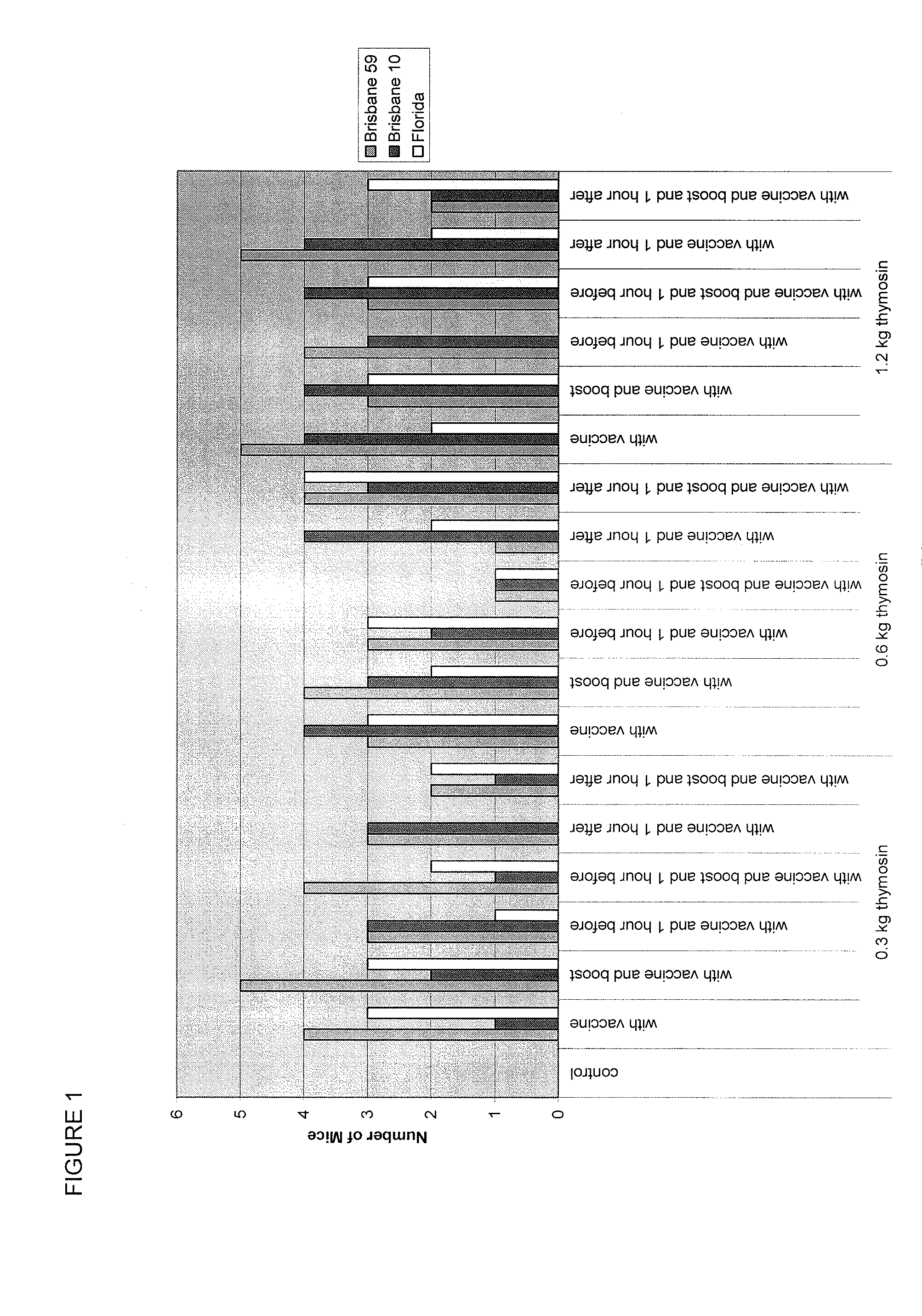
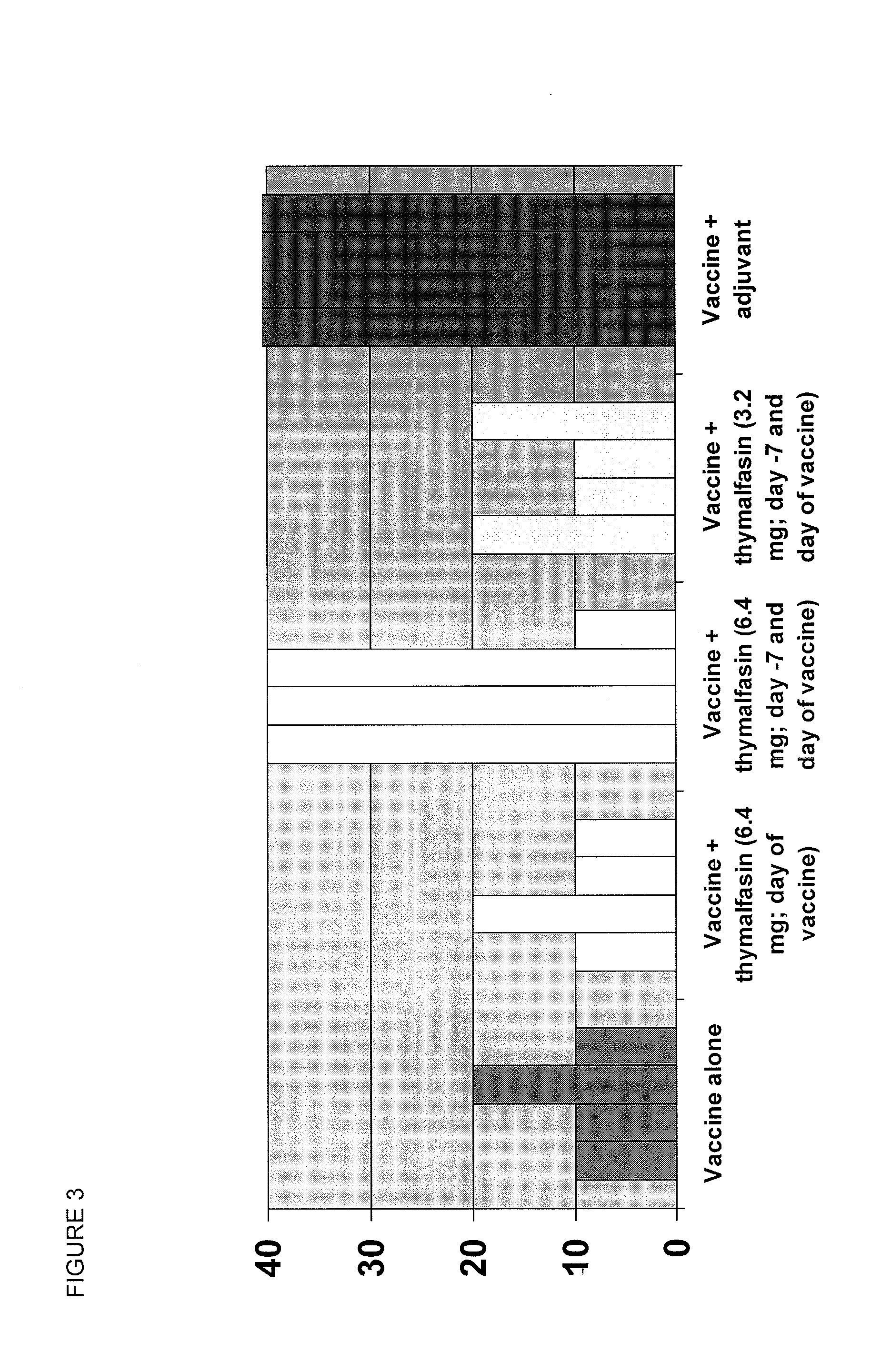
Alpha thymosin peptides as vaccine enhancers
ActiveUS20100285060A1Enhancing vaccine effectivenessEnhance vaccine effectivenessSsRNA viruses negative-senseBacterial antigen ingredientsRegimenImmunodeficiency
The present invention provides methods of vaccination as well as pharmaceutical combinations and kits for enhancing vaccine effectiveness, including for immunodeficient or immunecompromised patients, including non-responders and low-responders to vaccination. As disclosed herein, the invention relates to administering a vaccine and a regimen of thymosin alpha peptide so as to provide higher antibody titers, speed the development of such antibody titers, and / or to provide for a longer duration of such antibody titers, thereby providing a greater protective effect. In another aspect, the invention allows for reducing a vaccine dose, such as an influenza vaccine dose, by administration of a thymosin peptide regimen.
Owner:SCICLONE PHARM INT LTD
Method of utilizing biosynthesis and chemical modification technology in producing thymic hormone al monomer
InactiveCN1388133AOvercome the problem of difficult synthesis in vivoSimple processHormone peptidesChemical synthesisChemical modification
The present invention belongs to the field of biosynthesis and chemical modification technology. By means of bioengineering process, several thymosin genes are recombined and the recombinant gene is expressed in host cell to obtain high-expression thymosin fusion body. The fusion body is cracked and modified chemically to form thymosin alpha 1 monomer finally. The production process can produce thymosin alpha 1 identical with that produced chemically and is simple, low in cost and suitable for production in large scale. The present invention also provides the production process of several thymosin alpha 1 derivatives.
Owner:吴建中
Method for preparing thymosin polypeptide by using interin
InactiveCN102676534ASimple processLow costHormone peptidesPeptide preparation methodsAcetylationMonomer
The invention discloses a method for preparing thymosin polypeptide by using interin. The method comprises the following steps of: 1, synthesizing a thymosin alpha-1 gene fragment; 2, fusing a synthesized thymosin alpha-1 gene into a purification tag gene and an interin gene by using nucleate endonuclease and ligase to obtain a fusion protein recombinant gene and introducing into a host cell; 3, fermenting the host cell to obtain thymosin fusion protein; 4, purifying the fusion protein obtained by the step 3 by using a purification tag; 5, cutting the fusion protein obtained by the step 4 to obtain a thymosin 28 peptide monomer; 6, acetylating the 28 peptide monomer to obtain thymosin alpha-1 with a natural structure; and 7, purifying the thymosin alpha-1 with the natural structure. According to the method for preparing thymosin polypeptide provided by the invention, polypeptide can be easily expressed in the host cell; and the method has the advantages of simple process, low cost, easiness in operation and industrialization and no environmental pollution.
Owner:SHANGHAI ENTS BIOTECH
Active fragment of thymosin alphal and its polyethylene glycol derivatives
The present invention relates to active fragment of natural or artificial amino acid substituted thymosin alpha-1 and its polyethylene glycol derivatives, their preparation process, the medicine composition containing them, and their application in the medicine for preventing and treating diseases related with immune deficiency and hypoimmunity, including hepatitis B, hepatitis C, malignant melanoma, non-small cell lung carcinoma, SARS, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
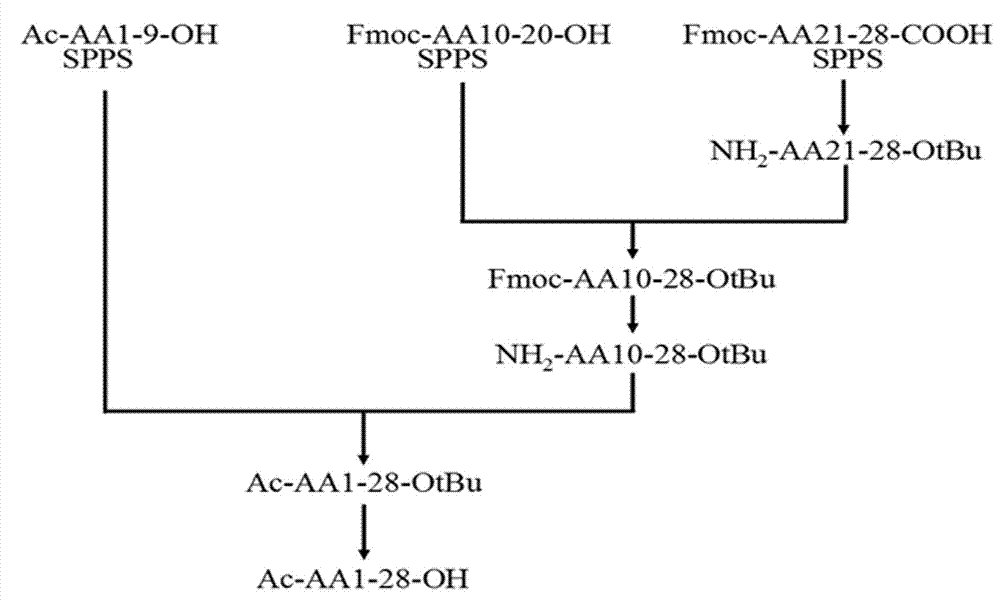
Method for preparing thymosin alpha 1 by liquid phase fragment condensation
ActiveCN103665144AHigh purityHigh yieldThymosin peptidesPeptide preparation methodsFluid phaseTarget peptide
The invention provides a method for preparing thymosin alpha 1 by liquid phase fragment condensation, belonging to the technical field of biochemistry. According to the method, high capacity value (not less than 0.8mmol / g) resin is used as a starting material, firstly, a standard solid phase polypeptide synthesis (SPPS) technology is adopted for synthesizing a high-purity peptide fragments with selected structures, then, a liquid phase condensation technology is adopted for connecting the peptide fragments, and thus, a high-purity (more than 99%) target peptide is obtained. Compared with the solid phase thymosin alpha 1 synthesis technology, the method provided by the invention avoids the problem of low coupling ratio of amino acids behind the 12th position, and greatly improves yield (up to 25-30%) of the thymosin alpha 1; meanwhile, the peptide fragment formed by solid phase synthesis is free from purification, post-treatment technology is simplified, finally, the thymosin alpha 1 is purified by means of high performance liquid chromatography, preparation difficulty is reduced, preparation times is decreased, synthesis cost of the thymosin alpha 1 is lowered, and implementation of large-scale and industrialized production is facilitated.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Polyethlene glycol modifications of thymosin alpha-1
Polyethylene glycol modifications of thymosin alpha 1 (T&agr; 1-PEGs), their preparation process, the medicine composition containing them, and their application in the medicine for preventing and treating diseases related with immune deficiency and hypoimmunity, including hepatitis B, hepatitis C, hepatoma, malignant melanoma, non-small cell lung cancer, SARS, and AIDS etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Treatment of aspergillus infections with alpha thymosin peptides
A method for treating a human infected with Aspergillus by using thymosin alpha 1 as an immuno-stimulator in activating dendritic cells. The method is particularly useful in preventing an infection by Aspergillus in an immuno-compromised host being treated with a bone marrow transplantation.
Owner:SCICLONE PHARM INT LTD
Treatment of hepatitis B infection with thymosin alpha 1 and lamivudine
InactiveCN1320041AEffective treatmentCombined securityPeptide/protein ingredientsPharmaceutical delivery mechanismDrug regimenFamciclovir
A method of treatment of hepatitis B virus (HBV) infection in a patient by administering to the patient a drug regimen including an antiviral-effective amount of thymosin alpha 1 (Talpha1), an antiviral-effective amount of lamivudine, and optionally an antiviral-effective amount of famciclovir is disclosed.
Owner:SCICLONE PHARMACEUTICAL INC
Antiretroviral compositions comprising thymosin alpha peptides and protease inhibitors
The present invention provides a method and a pharmaceutical combination for treating a human infected with HIV utilizing an HIV, treatment-effective amount of a Tα1 peptide, and an HIV, inhibitarialy-effective amount of at least one pratease inhibitor compound.
Owner:SCICLONE PHARMACEUTICAL INC
Medicament combination preparation for treating bacterial infection
ActiveCN101244262APromote recoveryMprove ideasAntibacterial agentsPeptide/protein ingredientsCurative effectTherapeutic effect
The invention provides a drug combination package, comprising thymosin Alpha 1 and antibiotic with a certain ratio; the drug combination package has the advantages that the optimal combination between drugs is realized within the range of the ratio; the using dosage of the antibiotic and the thymosin Alpha 1 can be greatly reduced as well as ensuring the therapeutic effect, thus being propitious to control the generation of the drug resistance for bacteria, which has very important significance for alleviating increasingly serious drug resistance; on the other hand, due to the reduced using dosage, the treatment cost for the sufferer is lowered, which is propitious to resolve the ever increasing social medical burden problem.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
Interferon-thymosin broad-spectrum antiviral pharmaceutical preparation for pigs and preparation method thereof
InactiveCN101721686ASimple production processReduce manufacturing costPeptide/protein ingredientsAntiviralsEscherichia coliEukaryotic plasmids
The invention discloses an interferon-thymosin broad-spectrum antiviral pharmaceutical preparation for pigs and a preparation method thereof. According to the molecular structure characteristics of interferon alpha 1 (IFN-alpha 1) and thymosin alpha 1 (THY-alpha 1) and the preference of Escherichia coli codon, primers are designed; the PCR method is used for acquiring the fusion gene of the IFN-alpha 1 and the THY-alpha 1; the gene is cloned to a pGEX4T-2 prokaryotic expression vector to construct pGEX4T-IFN alpha 1-THY alpha 1 prokaryotic expression plasmids; and the recombinant plasmids are converted into the BL21 (DE3) recipient bacterium to acquire IFN alpha 1-THY alpha 1 fusion protein through IPTG induction, expression and purification. The biological activity of the protein is detected through cytopathy inhibiting experiment and E-rosette forming experiment, and the result shows that the IFN alpha 1-THY alpha 1 fusion protein has double biological activity.
Owner:JILIN UNIV
Derivates of Polyethylene Glycol Modified Thymosin Alpha 1
Pharmaceutical compositions that include thymosin alpha 1 peptide derivatives modified at the C-terminal of the peptide chain with polyethylene glycol, and their pharmaceutical acceptable salts, are generally disclosed. Also, new methods used to prepare these thymosin alpha 1 peptide derivatives modified at the C-terminal of the peptide chain with polyethylene glycol are generally provided. The presently disclosed compounds and their salts can be prepared administered to humans to treat immune disease and can also be used in adjuvant treatment.
Owner:JIANGSU HANSOH PHARMA CO LTD
New application of recombinant human thymosin alpha collagens
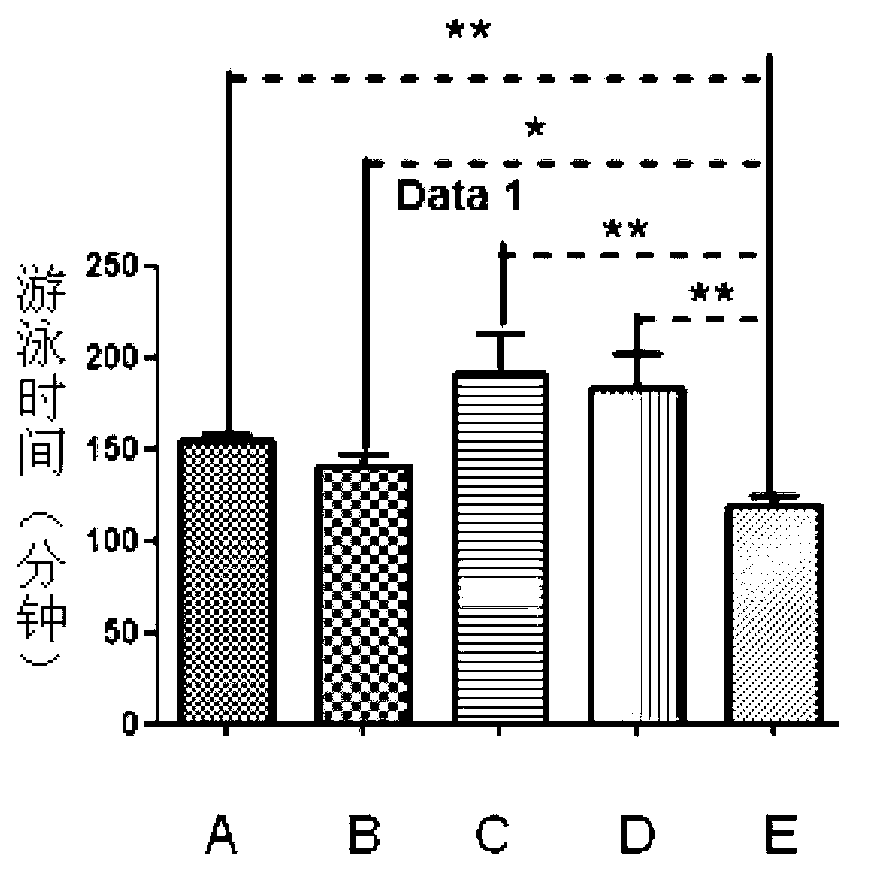
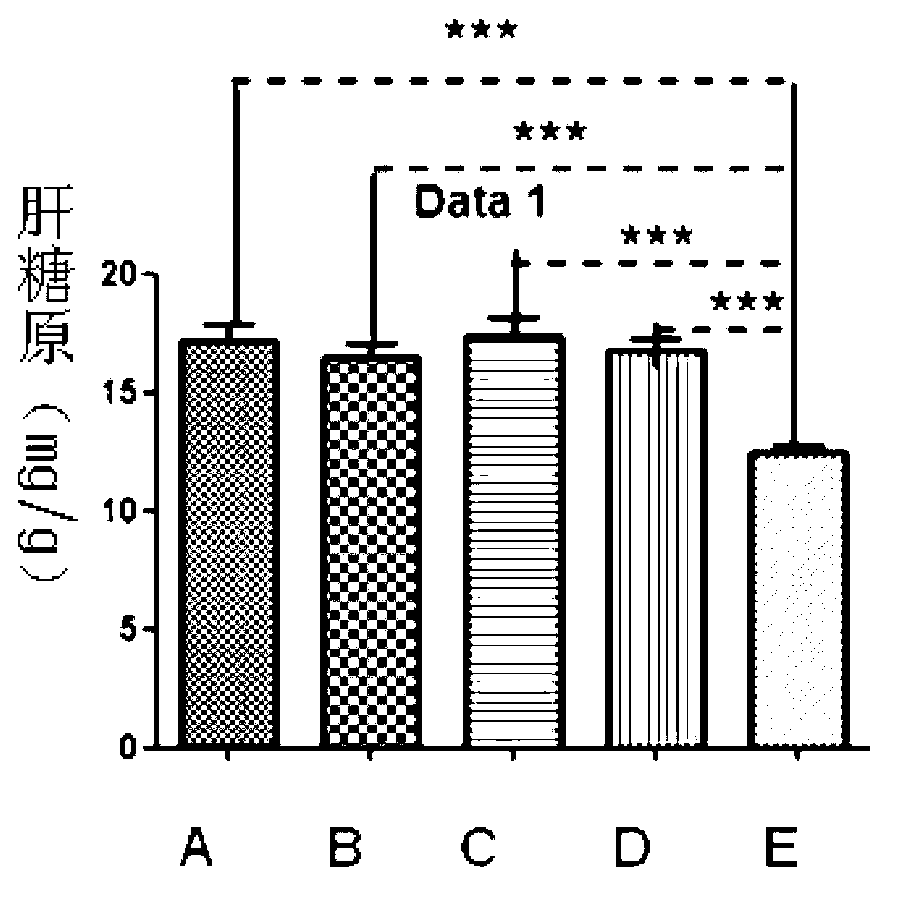
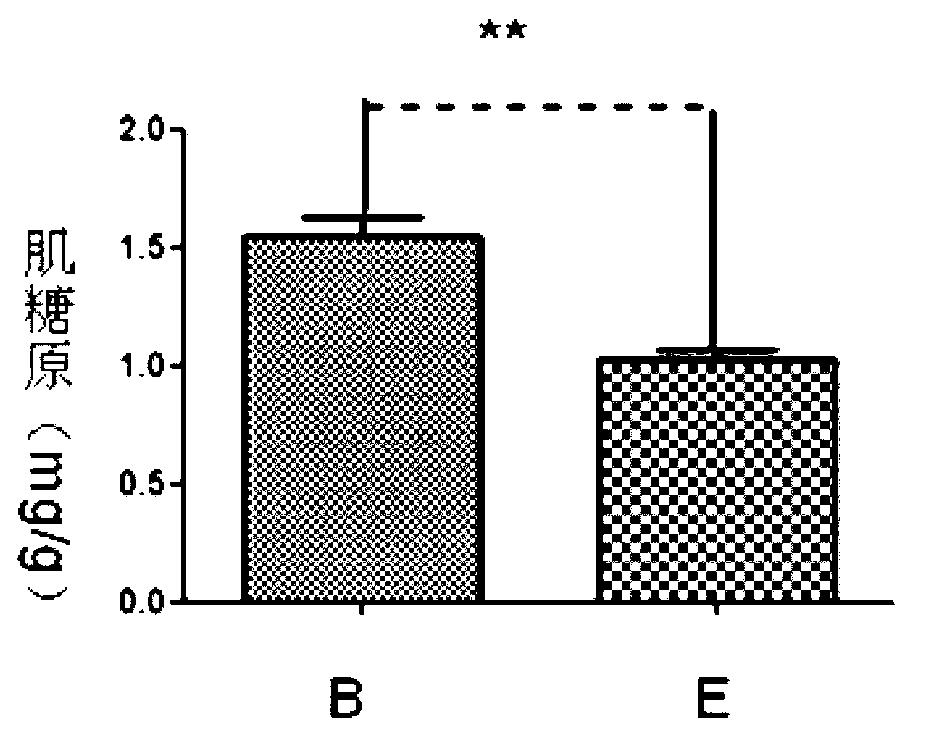
The invention discloses a new application of recombinant human thymosin alpha collagens, which relates to a recombinant human thymosin alpha collagen. The invention also provides the application of the recombinant human thymosin alpha collagen in preparing anti-fatigue drugs. The recombinant human thymosin alpha collagen (referred to as thymosin alpha collagen) refers to a recombinant human thymosin alpha collagen and a tissue separated and purified thymosin alpha collagen. The effect of the thymosin alpha collagen in various anti-fatigues is implemented through increasing the glycogen storage of the liver and muscles and reducing the level of serum urea nitrogen so as to improve the fatigue resistance.
Owner:XIAMEN UNIV
Thymosin alpha peptide for preventing, reducing the severity of, and treating infection
The present invention provides methods for preventing, treating, or reducing the severity of infection, including bacterial, viral, and fungal infections, and including infections of more complex etiology. The invention involves the administration of an alpha thymosin peptide regimen, so as to prime or enhance a patient's immune response for pathogen exposure. In certain embodiments, the alpha thymosin regimen is scheduled or timed with respect to potential or expected pathogen exposures. The regimen of alpha thymosin peptide as described herein provides the patient with a more robust immune response to pathogen exposure, including higher antibody titers and / or a more rapid antibody response. In certain embodiments, the patient is immunodeficient or immunecompromised, and / or the patient is hospitalized or scheduled for hospitalization, such that the regimen of alpha thymosin peptide helps to protect the patient from, or reduce the severity of, nosocomial infection or illness.
Owner:SCICLONE PHARMACEUTICAL INC
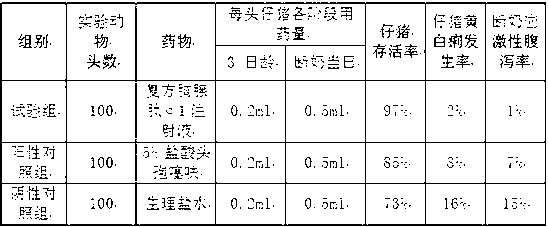
Compound thymosin Alpha 1 injection for improving livability of piglet and preparation method
ActiveCN103071148AImprove immunityReduce weaning stressAntibacterial agentsPeptide/protein ingredientsWeaningVitamin C
The invention provides a compound thymosin Alpha 1 injection for improving the livability of piglet and the preparation method thereof, wherein the compound thymosin Alpha 1 injection is prepared by the followings: 1 to 2 portions of thymosin Alpha 1, 3 to 5 portions of taurine, 5 to 10 portions of glutamine, 10 to 15 portions of vitamin C, 20 to 35 portions of radix sophorae flavescentis, 10 to 15 portions of Chinese pulsatilla root, 5 to 10 portions of atractylis ovata, and 3 to 5 portions of medicine terminalia fruit. The compound provided by the invention has high curing effect, is safe, reliable and nontoxic, and has no residuals, can obviously improve the immunity of piglet, and can effectively prevent immune suppressed diseases caused by swine fever, porcine circovirus and the like; toxin inside intestinal canal can be neutralized and damaged intestinal mucosa can be repaired, the colony structure inside the intestinal tract can be optimized, stress reaction caused by piglet weaning can be alleviated, and yellow and white scour of piglets, weaning stress diarrhea and diarrhea can be effectively prevented and cured. The medicine produced can be used for effectively increasing the survival rate of piglet, practical application shows that the survival rate of piglet can reach more than 98 percent after injection. In the invention, chitosan is used as flocculant for researching, developing and purifying the traditional Chinese medicine, the cost is low, safety and innocuity can be ensured, and the operation is simple.
Owner:HUBEI WUDANG ANIMAL PHARMA
Escherichia coli overexpressing riml and its application in the preparation of n-acetylated thymosin α
ActiveCN102277327AHigh yieldEasy to separateBacteriaMicroorganism based processesEscherichia coliGene
The invention discloses a colon bacillus for over-expressing RimL and an application on preparing N-extrasin alpha acetylate. The invention provides a recombinant strain and the recombinant strain is obtained by introducing a coding gene of the RimL and a coding gene of the extrasin alpha into host bacteria. The experiment provided by the invention shows that the invention constructs the recombinant strain which is obtained by co-expressing the coding genes of N-acetylase RimL and the extrasin alpha, the recombinant strain is fermented to obtain the extrasin alpha, and a large part of the extrasin alpha is the N-extrasin alpha acetylate; and the colon bacillus has a obvious application prospect.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Preparation method of fusion protein of porcine interferon-alpha 1 and thymosin-alpha 1
InactiveCN103937828AThe source is safe and sufficientReduce manufacturing costHybrid peptidesVector-based foreign material introductionInterferon alphaGlutathione S-transferase
The invention belongs to the field of veterinary biological pharmacy, and discloses a preparation method of a fusion protein of porcine interferon-alpha 1 and thymosin-alpha 1. The preparation method comprises following steps: an amplification primer is synthesized based on porcine interferon-alpha 1 (sequence number DQ249000) gene sequence and thymosin-alpha 1(sequence number 770552A) sequence which are disclosed by GenBank; fusion amplification of complete genome sequences of porcine interferon-alpha 1 and thymosin-alpha 1 is carried out; cloning and inducible expression of glutathione-s-transferase-interferon alpha 1-thymosin alpha 1 fusion protein are carried out; detection and identification are carried out; and the fusion protein is separated and purified. The fusion protein is capable of maintaining respective biological functions of porcine interferon-alpha 1 and thymosin-alpha 1 preferably, possesses certain activity in protecting cells from virus attack and promoting cell surface receptor expression, and is capable of providing industrialized production of porcine interferon-alpha 1 and thymosin-alpha 1 fusion protein preparations with technological support.
Owner:南京洲邦生物科技有限公司
Solid phase synthetic technique for thymosin alpha1
ActiveCN101104638BEasy to purifyHigh purityThymopoietinsPeptide preparation methodsAcetic anhydrideFluoroacetic acid
Owner:苏州天马医药集团天吉生物制药有限公司
4 connecting body thymosin alpha 1 gene order and preparation method of transgene tomato
InactiveCN101451137AImprove immunityReduce manufacturing costFermentationHorticulture methodsDiseaseNucleotide
The invention discloses a 4*thymosin alpha1 gene sequence and a method for preparing transgenic tomato, which belongs to the field of gene engineering. The method comprises the following steps: a nucleotide sequence with bioactive thymosin alpha1 polypeptide is encoded according to a plant preferential codon, and has at least 75 percent of homology with a nucleotide sequence containing 16th to 408th nucleotides in SEQ ID NO.2; or the nucleotide sequence can be hybridized with the nucleotide sequence containing 16th to 408th nucleotides in the SEQ ID NO.2 under moderate stringent conditions. The invention also provides a method for expressing thymosin alpha1 functional protein by utilizing a plant system. A 4*T alpha1 gene expresses the important effect and application value of a product in improving the immunity of human bodies and preventing and treating human major diseases such as hepatitis, HIV, cancer and diabetes in eukaryotic cells.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com