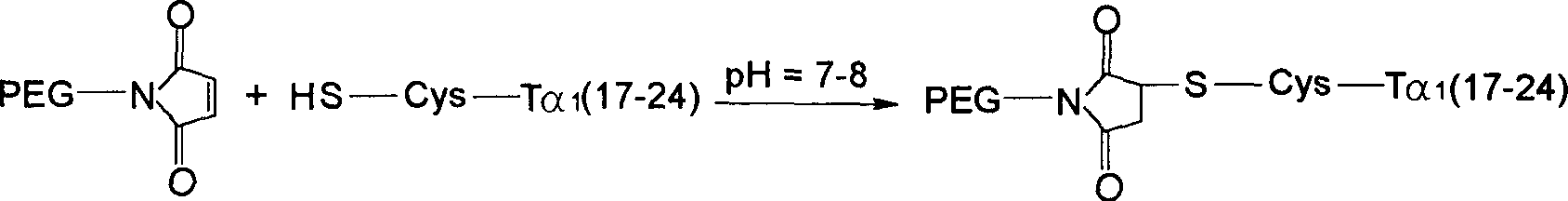Active fragment of thymosin alphal and its polyethylene glycol derivatives
A technology of derivatives and peptide derivatives, applied in the field of law, can solve the problems of large dosage, long cycle and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1 Phe(4-F) 21 Tα 1 Synthesis of (17-24)
[0127] Dissolve 0.285g of H-Phe(4-F)-OH.HCl (1.3mmol) in 10mL of methanol, 10mL of acetone and 10mL of water, add 0.218g of NaHCO 3 (2.6mmol), stirred to dissolve the solid and then added 0.438g Fmoc-OSu (1.3mmol), stirred at room temperature for reaction. After the completion of the reaction monitored by TLC, the organic solvent was removed by rotary evaporation, the pH was adjusted to 2-3 with 6N hydrochloric acid, the aqueous phase was extracted 3 times with ethyl acetate, the organic phase was combined, and anhydrous MgSO 4 dry. The desiccant was filtered off, and the filtrate was rotary evaporated to remove the solvent to obtain a white solid. Then recrystallized from ethyl acetate-petroleum ether to obtain 0.320 g of Fmoc-Phe(4-F)-OH, with a yield of 61.5%.
[0128] With 100mg Wang resin (0.05mmol) as solid phase carrier, Fmoc-Lys(Boc)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Val-OH as raw material, DCC-HOBT as condensatio...
Embodiment 2
[0130] Implementation of 2 mPEG 2000 -NHCOCH 2 CH 2 CO-Tα 1 Synthesis of (17-24)
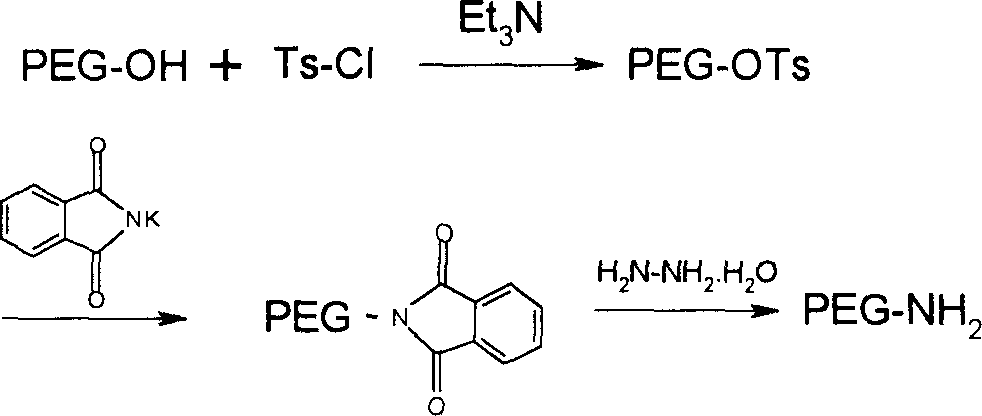
[0131] Weigh mPEG 2000 -OH 20g (10mmol) was placed in a 250ml reaction bottle, and 50ml CH was added 2 Cl 2 , the solid dissolved and then added 7.5ml Et 3 N (50mmmol) and 9.5g Ts-Cl (50mmol), stirred at room temperature. After TLC monitors that the reaction is complete, the solvent is removed by rotary evaporation, and 100 ml of anhydrous ether is added to precipitate a solid to obtain 18.9 g of mPEG 2000 -OTs, yield 94%
[0132] 12g mPEG 2000 -OTs (6mmol) was dissolved in 30ml DMF, 3.36g (18mmol) phthalimide potassium salt was added, and reacted at 120°C for 4 hours. The solvent was distilled off under reduced pressure, the residue was dissolved in 50ml of absolute ethanol, 4.0ml of hydrazine hydrate was added, and the mixture was refluxed for 4 hours. The solvent was removed by rotary evaporation and the residue was dissolved in CH 2 Cl 2 , filtered off the insoluble matter, and ...
Embodiment 3
[0136] Implementation of 3 Cys (mPEG 2000 -MAL)-Tα 1 Synthesis of (17-24)
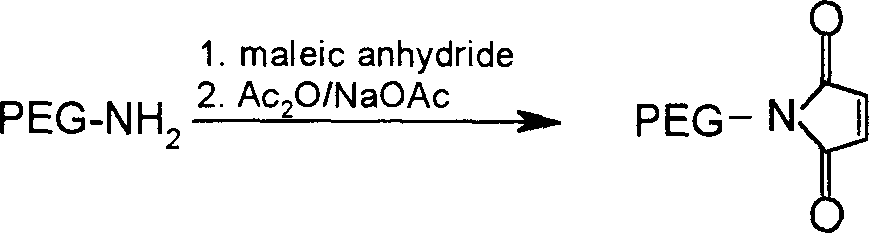
[0137] 1.0g mPEG 2000 -NH 2 Dissolve in 10ml of dioxane, add 0.4g of maleic anhydride, stir and react at 80°C for 30min. The solvent was evaporated under reduced pressure, 50ml of anhydrous diethyl ether was added, and a solid was precipitated by cooling. The solid was collected by filtration and dried to obtain 0.95g. The obtained solid was dissolved in 15ml of acetic anhydride, 1.0g of sodium acetate was added, and the reaction was stirred at 100°C for 45min. Evaporate the solvent under reduced pressure, dissolve the residue with dichloromethane, filter off the insoluble matter, add an appropriate amount of activated carbon to the filtrate, let it stand for 30 minutes, filter out the activated carbon, concentrate the filtrate to dryness, add anhydrous ether, precipitate a solid, filter After collection and drying, light yellow solid 0.55g mPEG was obtained 2000 -MAL, yield 55%.
[0138] With 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com