Fusion protein of human thymosin alpha1 and human composite interferon and preparation thereof
A technology of fusion protein and function, which is applied in the direction of peptide/protein components, medical preparations containing active ingredients, hybrid peptides, etc., and can solve problems such as reduced affinity, decreased antiviral specific activity, and difficulties in clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
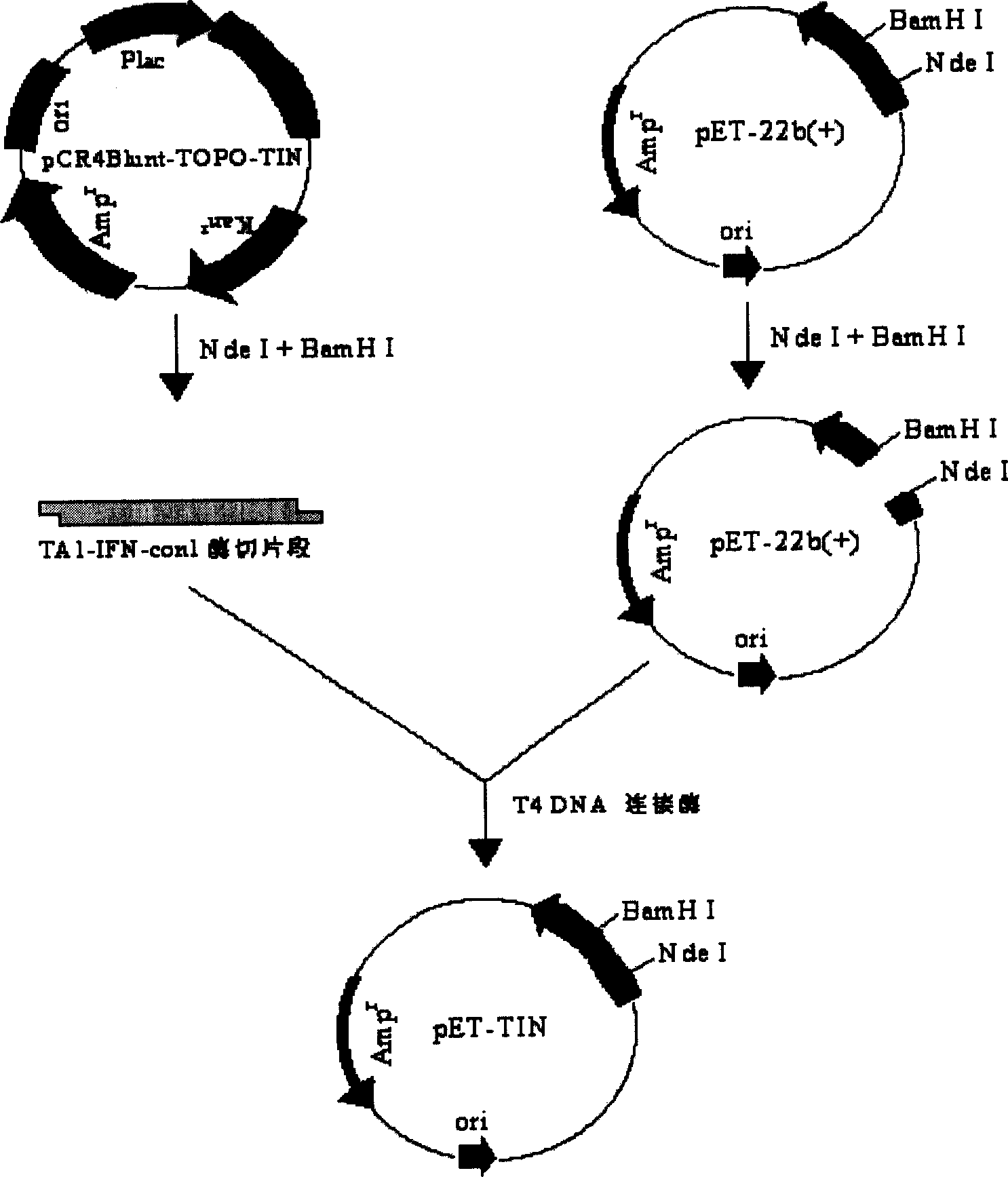
[0068] Synthesis of fusion genes
[0069] Design and Synthesis of Synthetic Fragments
[0070] According to the customary code of Escherichia coli, the IFN-con-Linker-TM-alpha1 fusion protein gene sequence (see figure) was artificially designed, and the whole gene sequence was entrusted to Shanghai Sangon Bioengineering Co., Ltd. to synthesize.
[0071] Construction of Engineering Bacteria for Fusion Gene Expression
[0072] Construction of Fusion Gene Expression Plasmid
[0073] Take the TM-alpha-Linker-IFN-con1 fusion gene plasmid and recover the small fragment after double digestion with endonucleases Nde I and BamH I. The prokaryotic expression plasmid pET-22b(+) with the T7 promoter was passed through Nde I and BamH I. Large fragments were recovered after BamHI double digestion. Ligated by T4 ligase, transformed into Escherichia coli DH5α, and screened recombinants. The clone confirmed by enzyme digestion and sequence analysis was named pET-TIN.
[0074] Construction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com