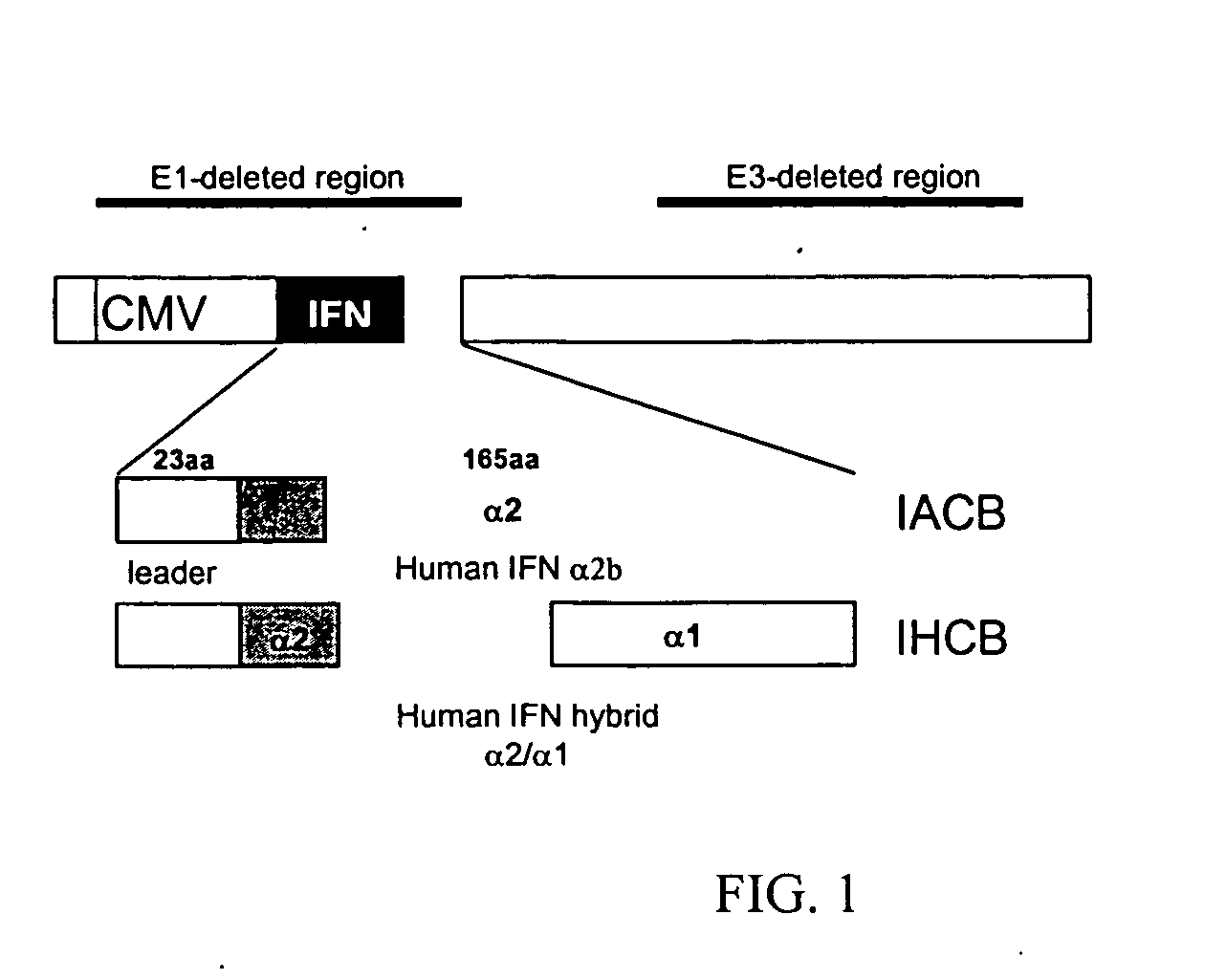
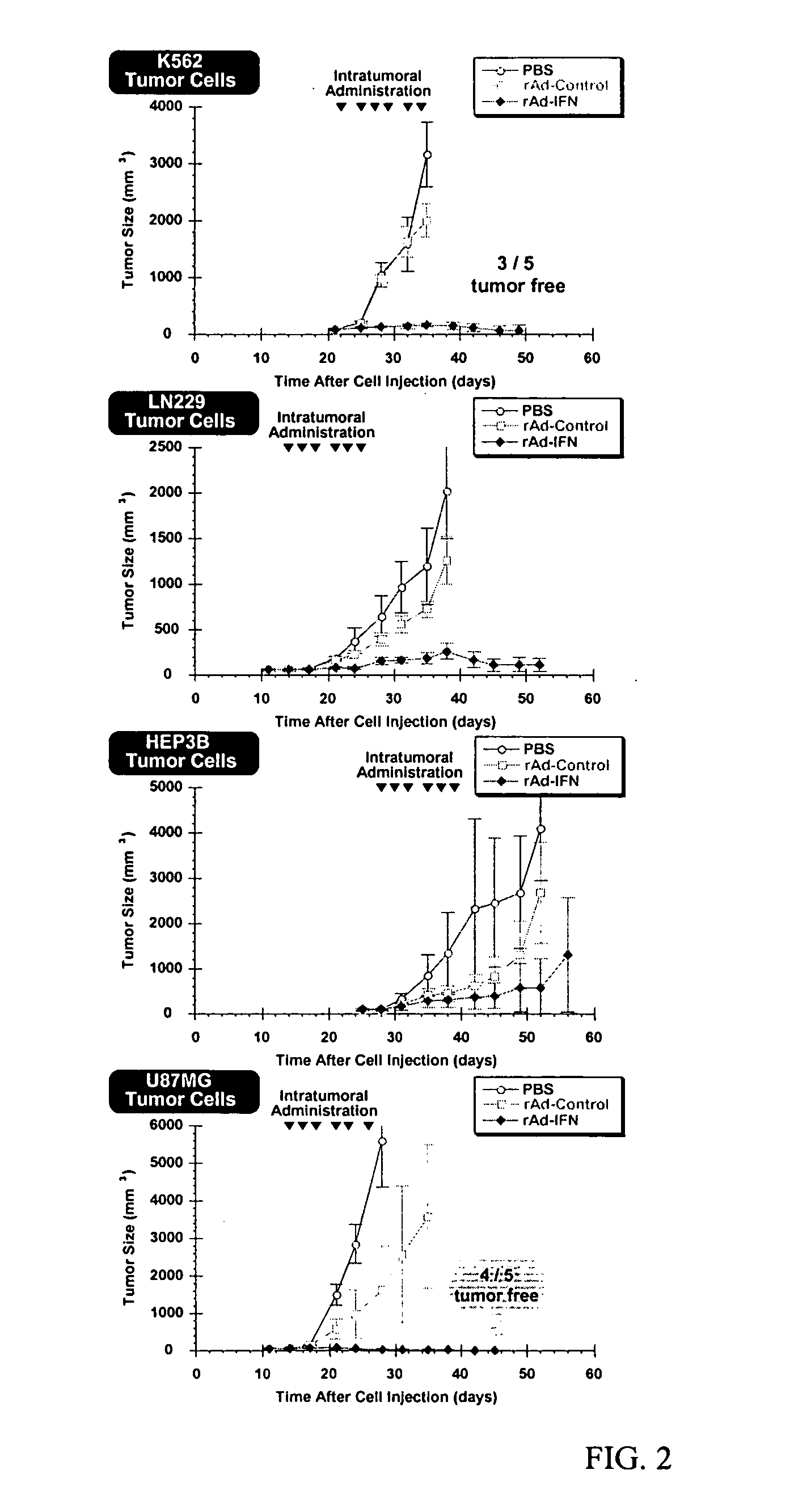
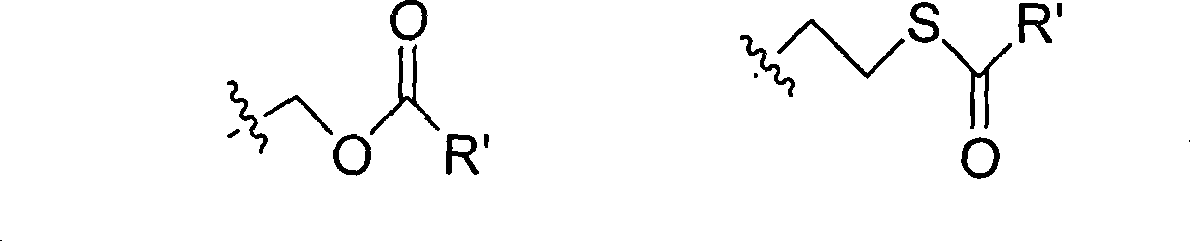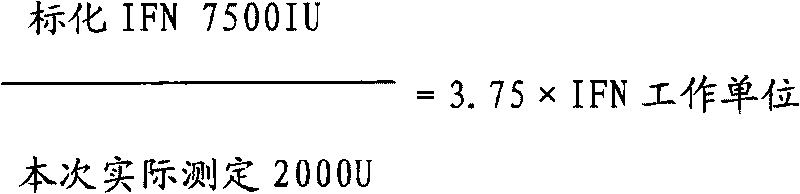Patents
Literature
171 results about "Alpha interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A protein produced by the body in response to an infection
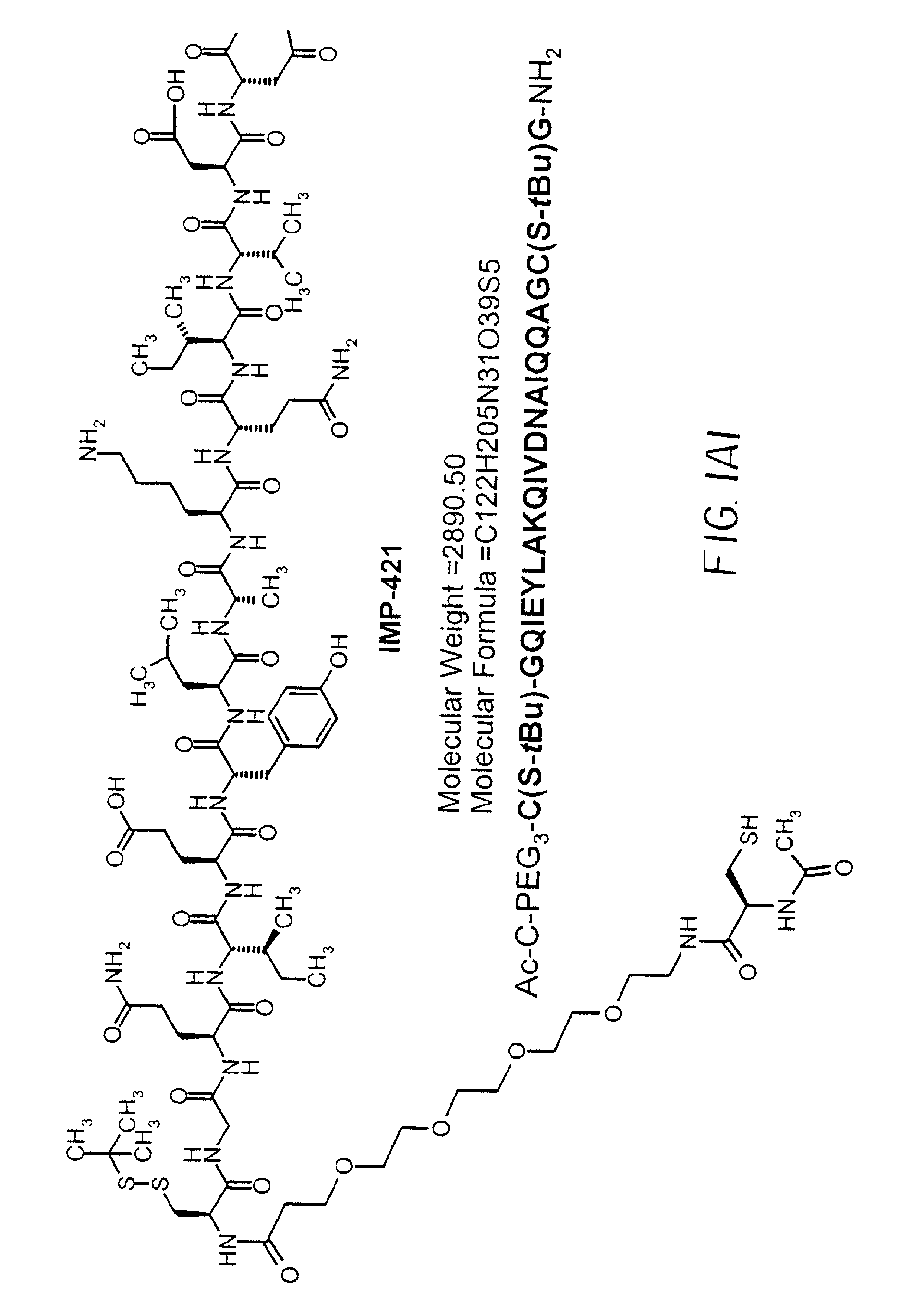
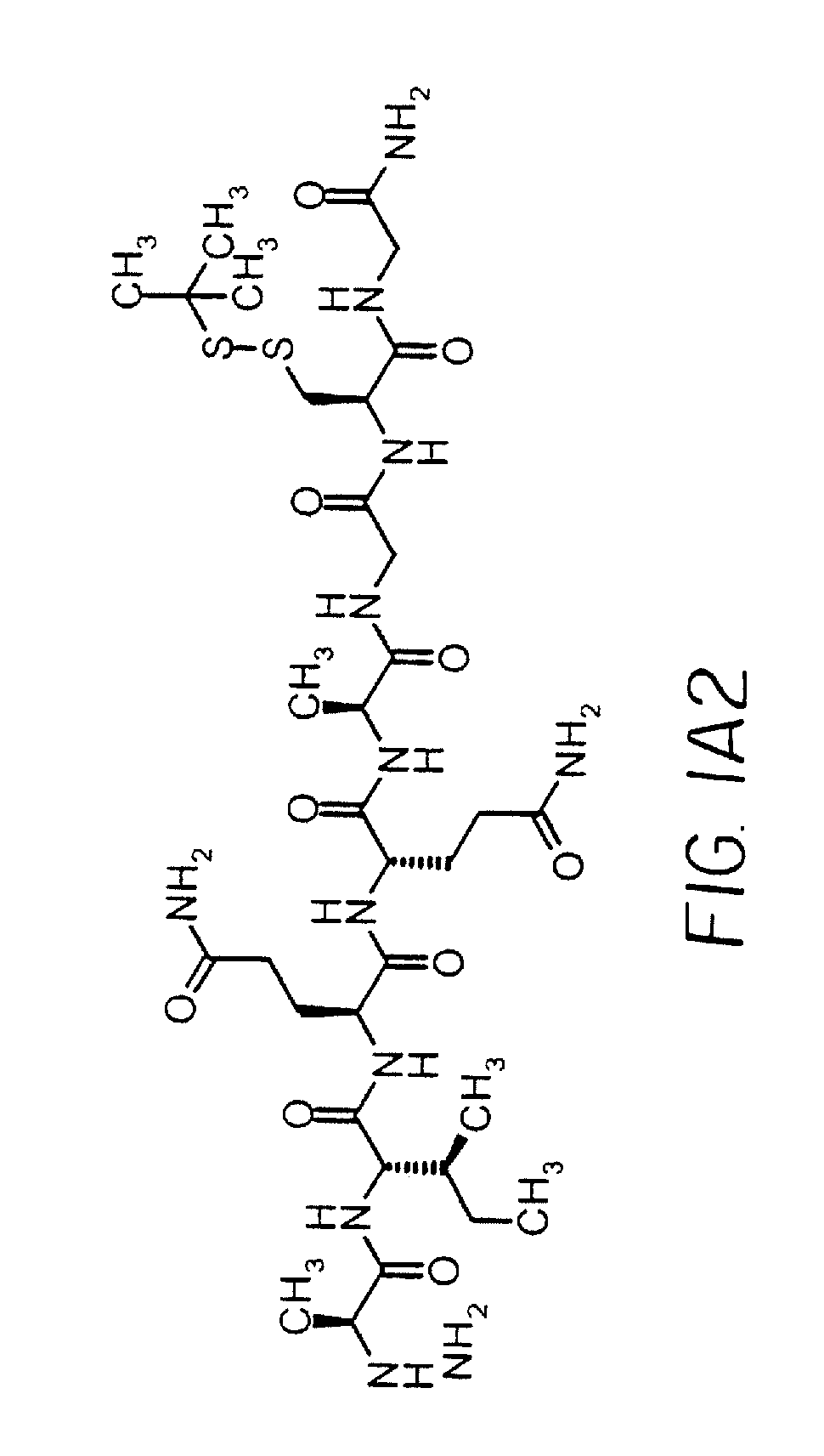
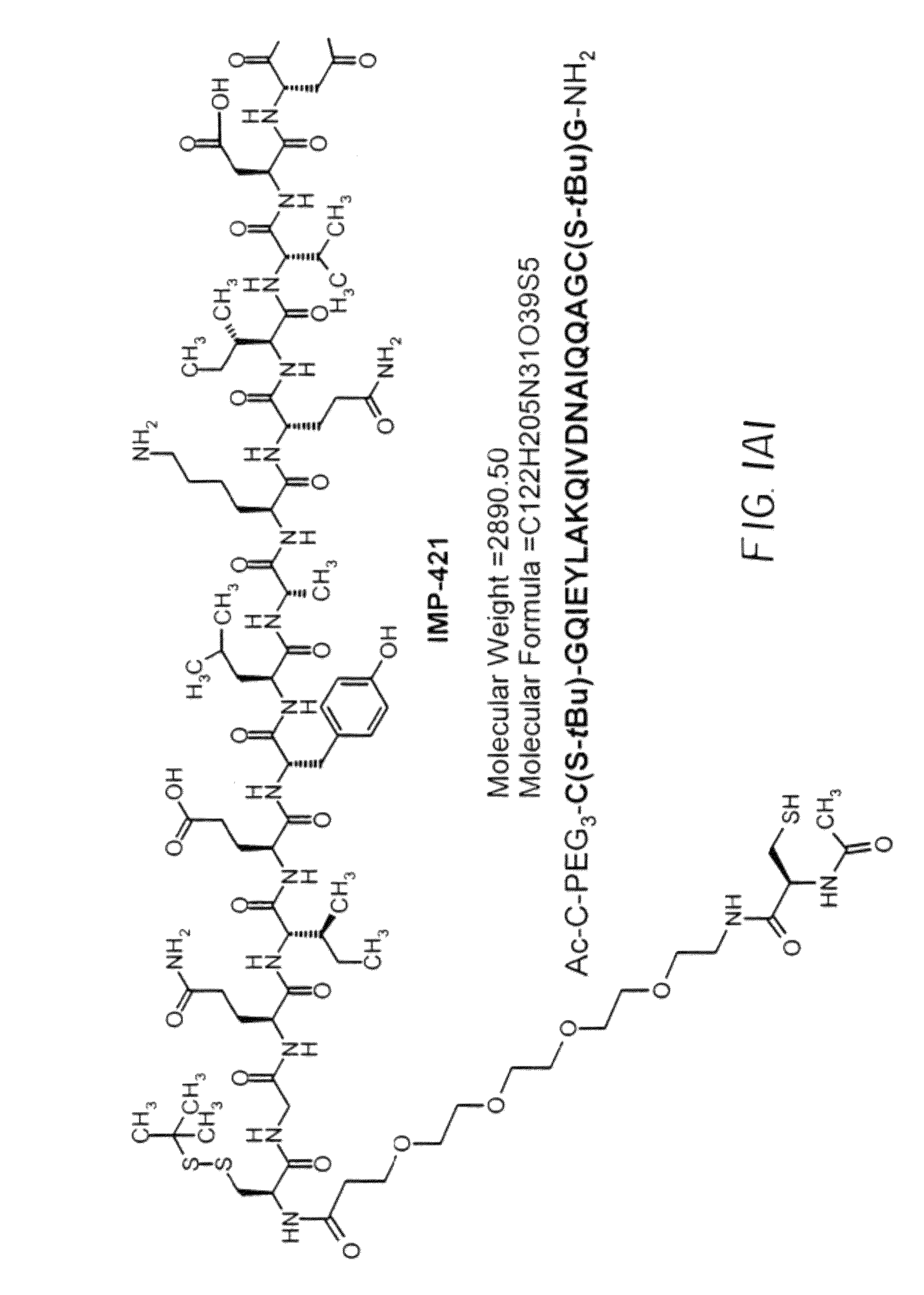
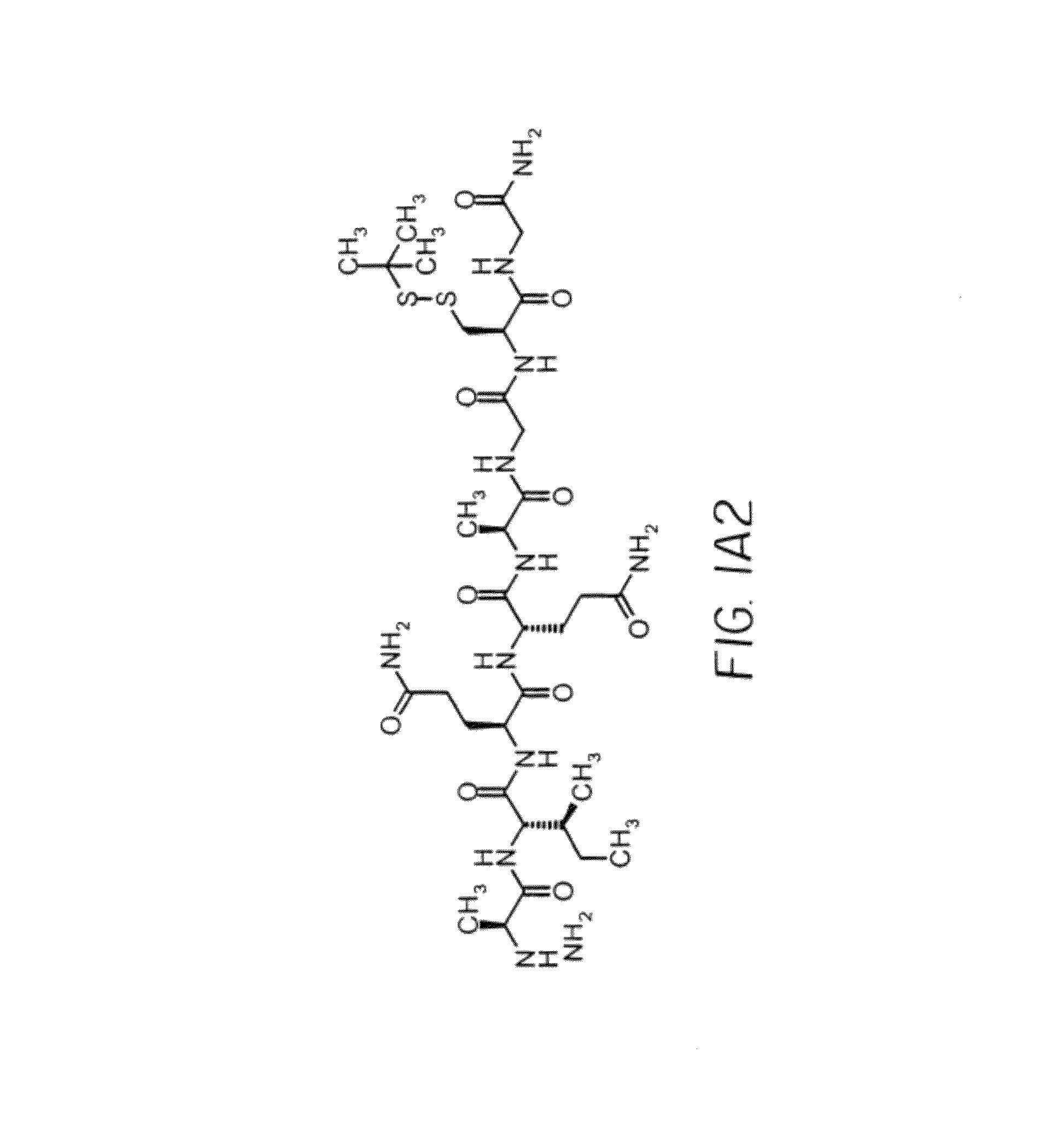
Dimeric alpha interferon pegylated site-specifically shows enhanced and prolonged efficacy in vivo
The present invention concerns methods and compositions for forming PEGylated complexes of defined stoichiometry and structure. In preferred embodiments, the PEGylated complex is formed using dock-and-lock technology, by attaching a therapeutic agent to a DDD sequence and attaching a PEG moiety to an AD sequence and allowing the DDD sequence to bind to the AD sequence in a 2:1 stoichiometry, to form PEGylated complexes with two therapeutic agents and one PEG moiety. In alternative embodiments, the therapeutic agent may be attached to the AD sequence and the PEG to the DDD sequence to form PEGylated complexes with two PEG moieties and one therapeutic agent. In more preferred embodiments, the therapeutic agent may comprise any peptide or protein of physiologic or therapeutic activity, preferably a cytokine, more preferably interferon-α2b. The PEGylated complexes exhibit a significantly slower rate of clearance when injected into a subject and are of use for treatment of a wide variety of diseases.
Owner:IBC PHARMACEUTICALS INC
Process for preparation of nucleoside phosphoric acid ester compound and application thereof
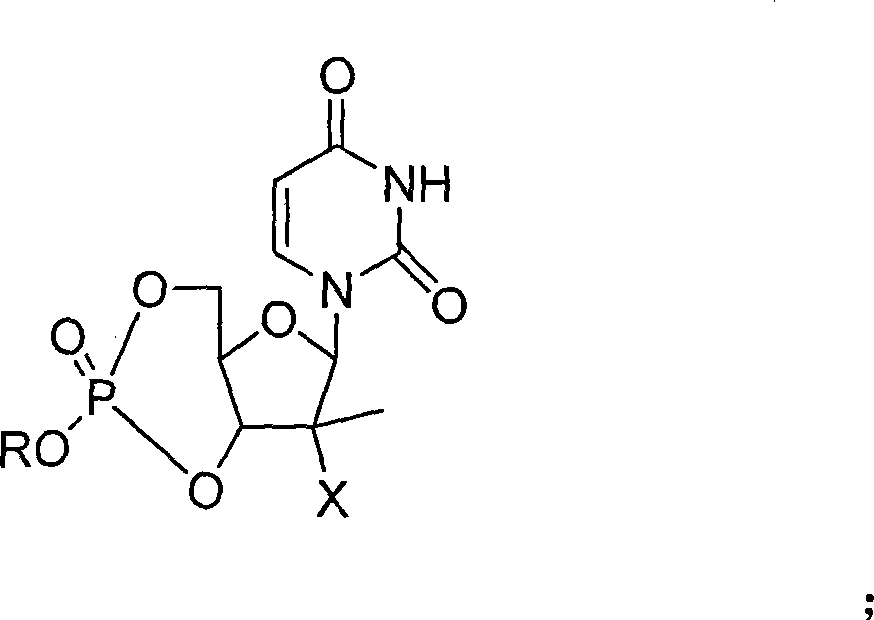
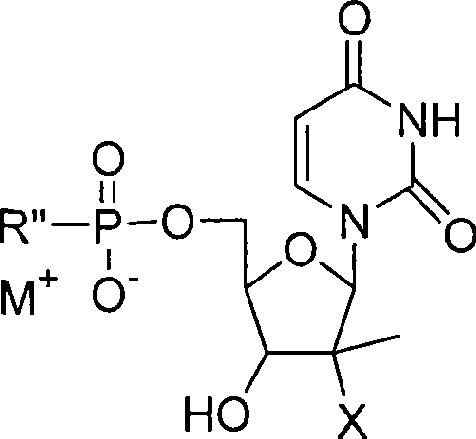
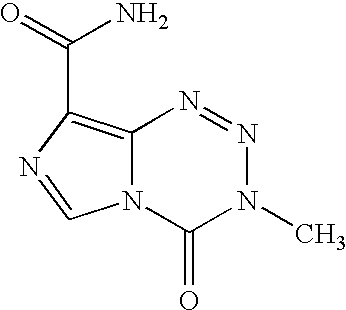
The invention provides the nucleoside phosphate compound and the preparation and application, which comprises the nucleoside 3', 5'-cyclic phosphate and the 5'-phosphodiester compound. Wherein, the 3', 5'-cyclic phosphate and the derivatives work as the novel HCV inhibitor and the structural formula is shown in the drawing (I), wherein, X equals to OH and F; R equals to H, NH4, R' 4N, R' 3NH and metals such as Na, K, Ca and Li or the structural formula in the drawing (II), R' equals to linear chain or substituted alkane or cyclane, aromatic hydrocarbon, or substituted aromatic hydrocarbon or heterocyclic aromatic hydrocarbon; the four R' and three R' in the amine are the same or different. The invention has the advantages that the nucleoside phosphate compound is used for treating the HCV or is combined with the Alpha-interferon, ribavirin or other anti-HCV drugs to treat the infection of the hepatic c virus or HCV.
Owner:冷一欣
Compositions comprising IMPDH inhibitors and uses thereof for treating HCV infection
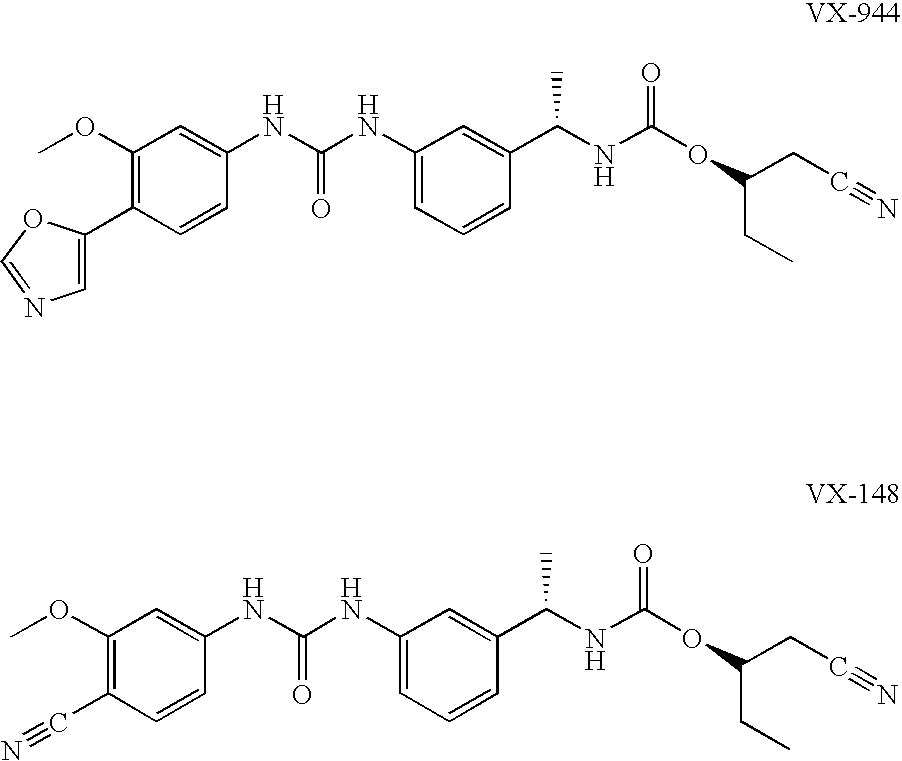
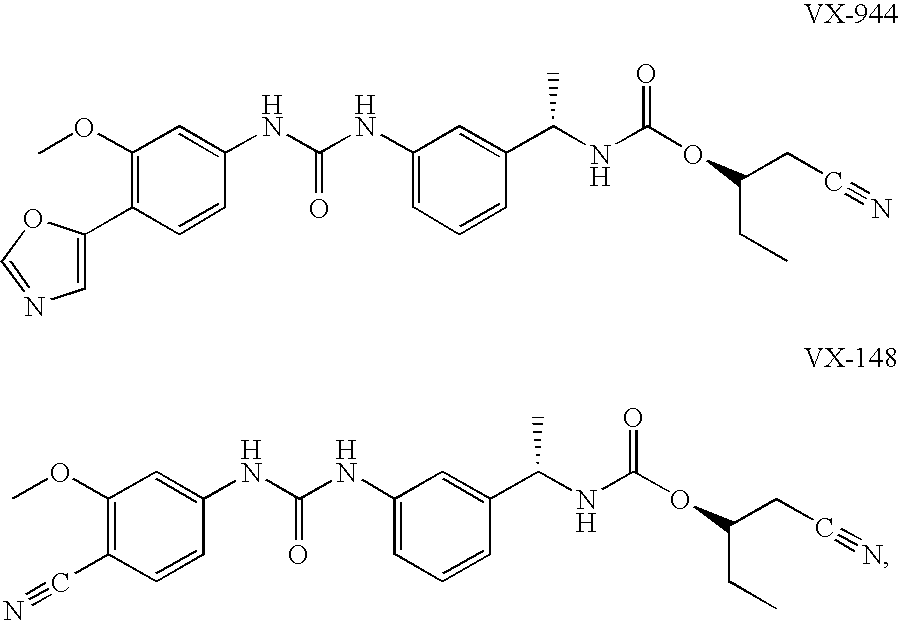
The present invention relates to optimal compositions useful in treating HCV infections in humans. These compositions comprise alpha-interferon or pegylated alpha-interferon and an IMPDH inhibitor selected from VX-148 or VX-944, wherein the IMPDH inhibitor is present in an amount such that a ratio of Cavg / Cmin is between 1 to 10, wherein:Cavg is average plasma concentration produced by said IMPDH inhibitor in said human; andCmin is estimated trough concentration produced by said IMPDH inhibitor in said human.The present invention also relates to methods of producing and using the optimal compositions to treat HCV infections in humans.
Owner:VERTEX PHARMA INC
Dimeric alpha interferon PEGylated site-specifically shows enhanced and prolonged efficacy in vivo
The present invention concerns methods and compositions for forming PEGylated complexes of defined stoichiometry and structure. In preferred embodiments, the PEGylated complex is formed using dock-and-lock technology, by attaching a therapeutic agent to a DDD sequence and attaching a PEG moiety to an AD sequence and allowing the DDD sequence to bind to the AD sequence in a 2:1 stoichiometry, to form PEGylated complexes with two therapeutic agents and one PEG moiety. In alternative embodiments, the therapeutic agent may be attached to the AD sequence and the PEG to the DDD sequence to form PEGylated complexes with two PEG moieties and one therapeutic agent. In more preferred embodiments, the therapeutic agent may comprise any peptide or protein of physiologic or therapeutic activity, preferably a cytokine, more preferably interferon-α2b. The PEGylated complexes exhibit a significantly slower rate of clearance when injected into a subject and are of use for treatment of a wide variety of diseases.
Owner:IBC PHARMACEUTICALS INC
Cysteine variants of alpha interferon-2
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby. Also disclosed are therapeutic methods for using the cysteine variants of the invention.
Owner:BOLDER BIOTECH
Methods and compositions for interferon therapy
InactiveUS20050025742A1Long contact timeKeep for a long timePeptide/protein ingredientsGenetic material ingredientsGene deliveryInterferon therapy
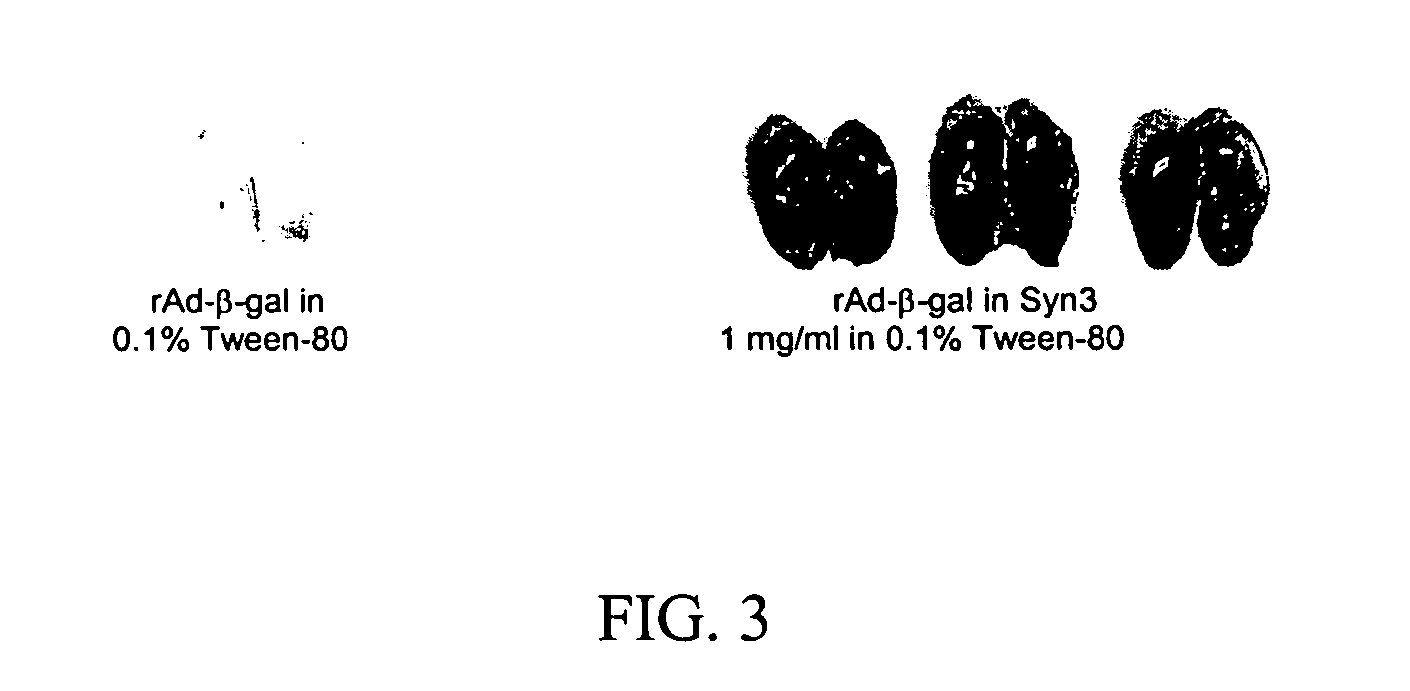
Methods and pharmaceutical compositions for administering protein or gene therapy to tissues or organs having an epithelial cell layer are provided. A protein or nucleic acid encoding the protein is administered to the target tissue or organ in combination with treatment with a delivery enhancing agent which increases the delivery of the interferon or nucleic acid to the cells of the target tissues or organs. The methods and combinations are particularly useful in the treatment of cancers and other conditions responsive to interferon therapy. An exemplary method comprises the transurethral intravesical administration to the bladder of a therapeutically effective amount of a pharmaceutical composition comprising an alpha-interferon or a gene delivery system encoding the interferon and SYN3 or a SYN3 homolog or analog. In the urinary bladder, as much as a 10 to 1000 fold increased in interferon levels and activity may be observed with the use of a SYN3 formulation as opposed to a PBS formulation of the same interferon protein or interferon gene delivery system.
Owner:CANJI
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of flavonoid quercetin dimmer as medicament for treating viral hepatitis B
InactiveCN101829103AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to the application of flavonoid quercetin dimmer as the medicament for treating viral hepatitis B, in particular to the application of flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof to the preparation of the medicament for eliminating HBsAg and HBeAg and inhibiting HBV DNA replication. The flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof has obvious HBsAg and HBeAg inhibiting activity, and at the concentration of 100mcg / ml, the flavonoid quercetin dimmer pharmaceutically acceptable salt thereof has the HBsAg eliminating strength of 65.7% and the HBeAg eliminating strength of 44.8% which are respectively 4.1 times and 2.7 times higher than the positive control medicament of Alpha-interferon and has the HBV DNA inhibiting ratio of 44.8% which is 117% of the HBV DNA inhibiting ratio of the Alpha-interferon at the highest test concentration. Therefore, the flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof can be expectedly used for preparing the non-nucleoside medicament for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating viral hepatitis B.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
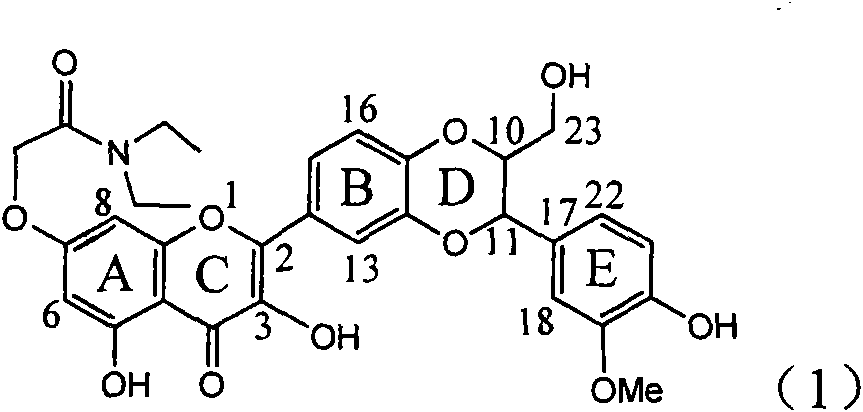
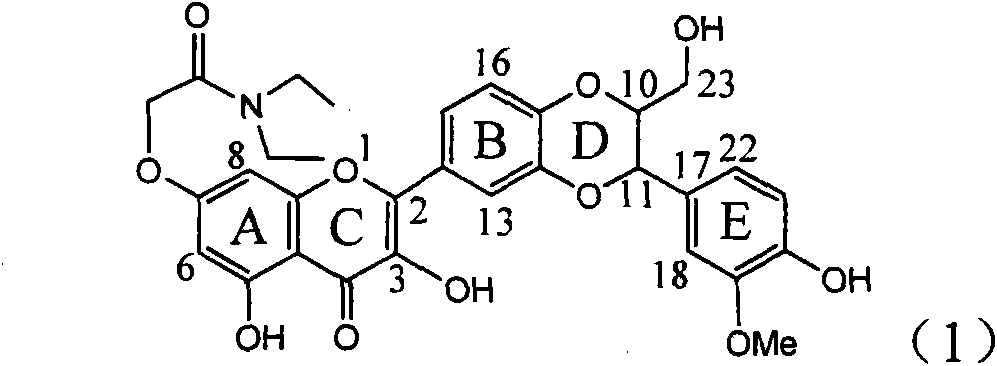
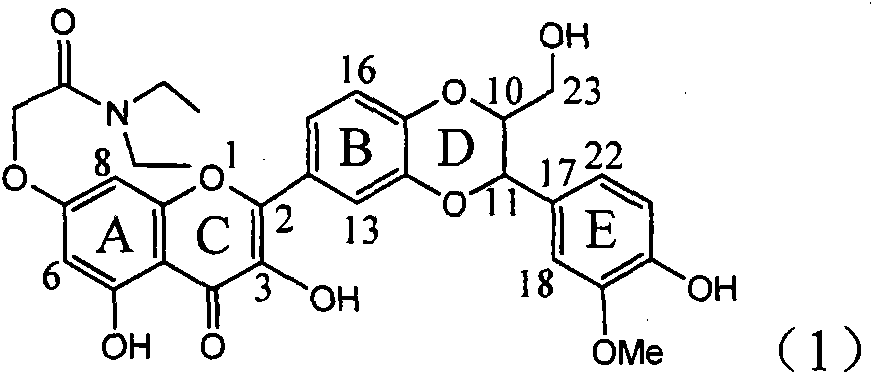
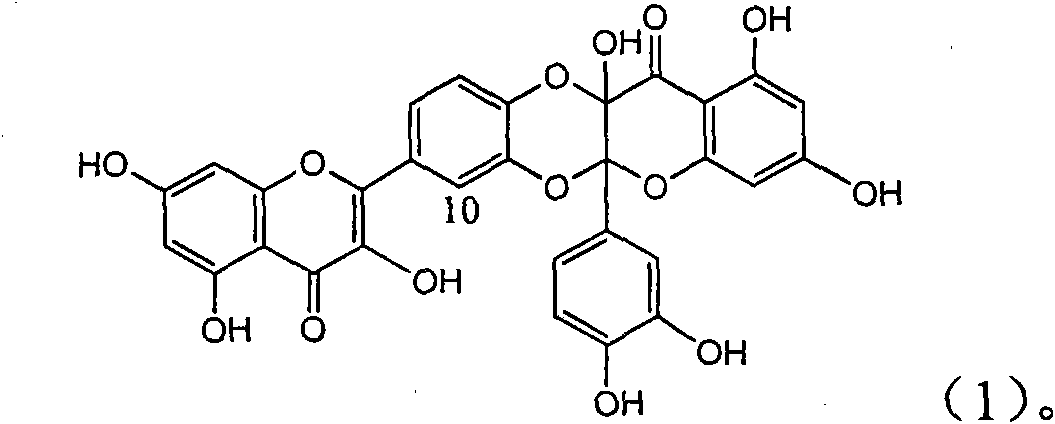
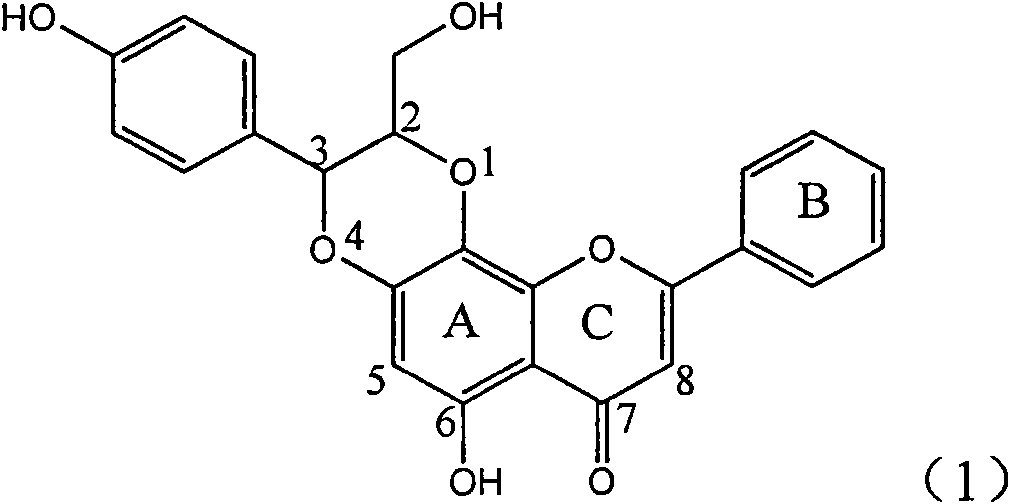
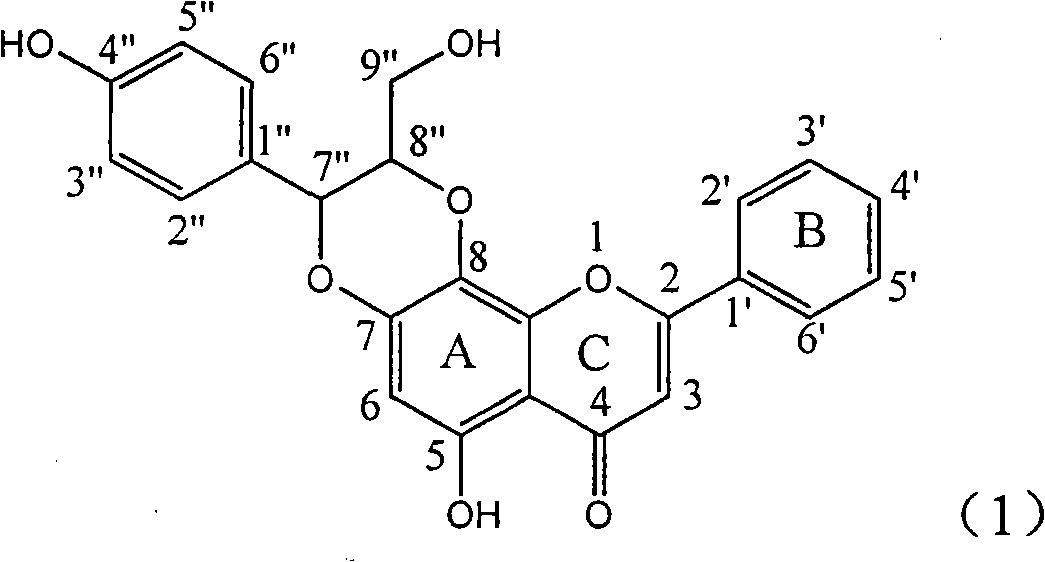
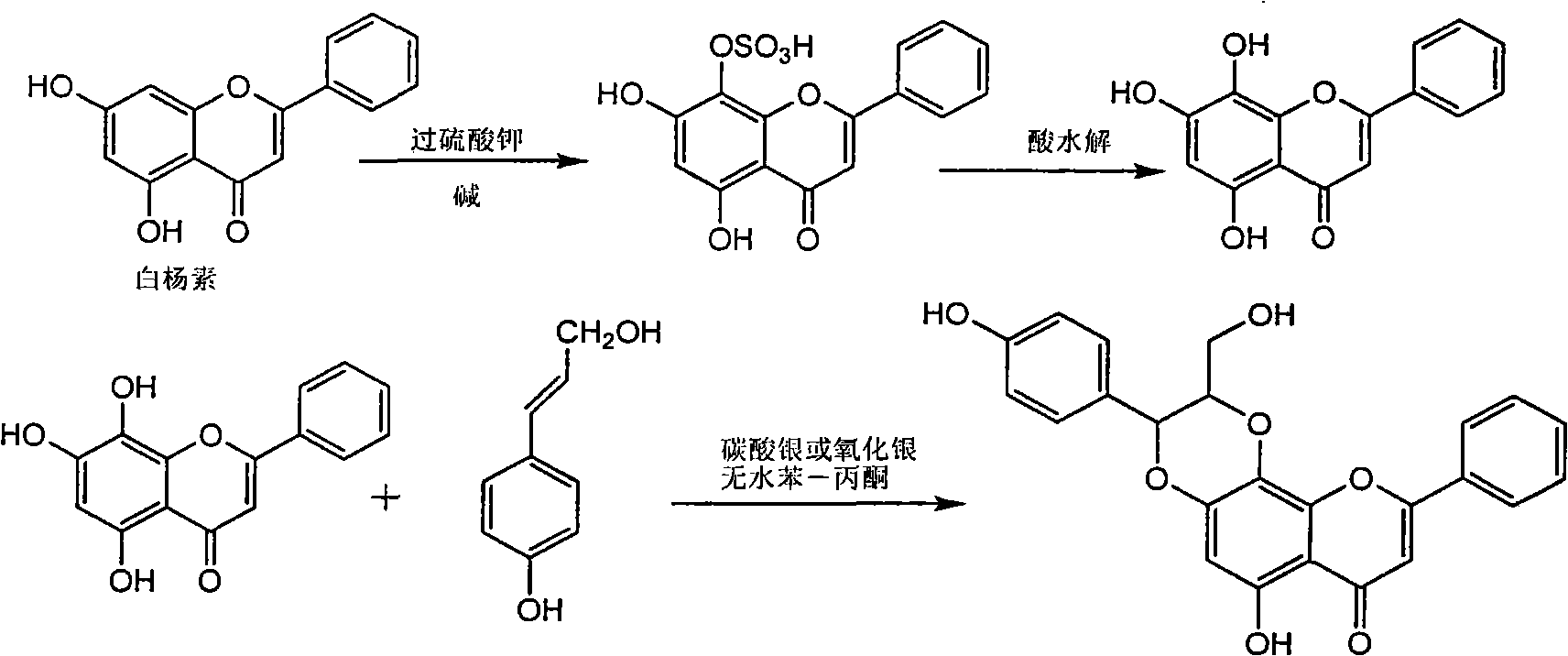
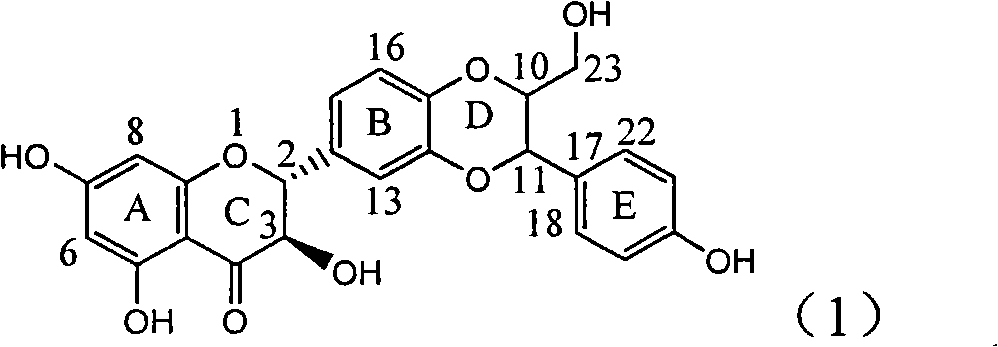
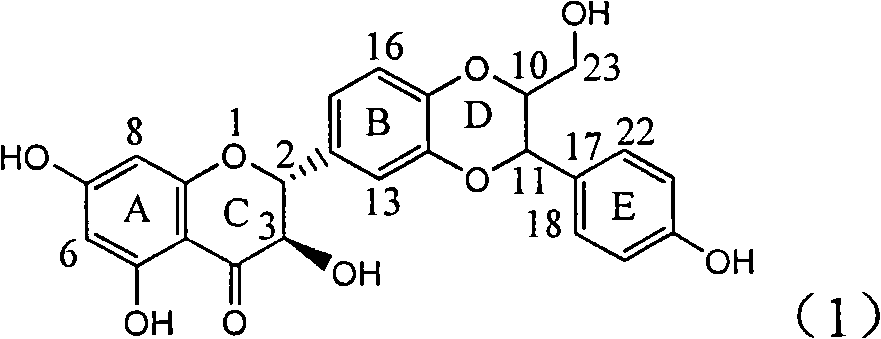
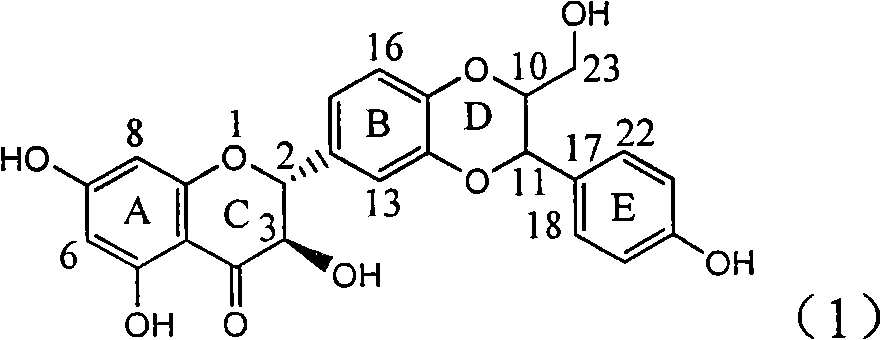
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
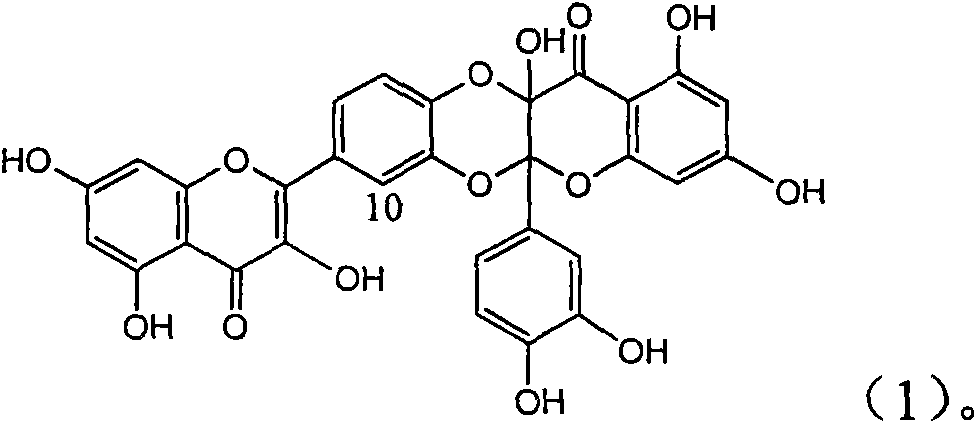
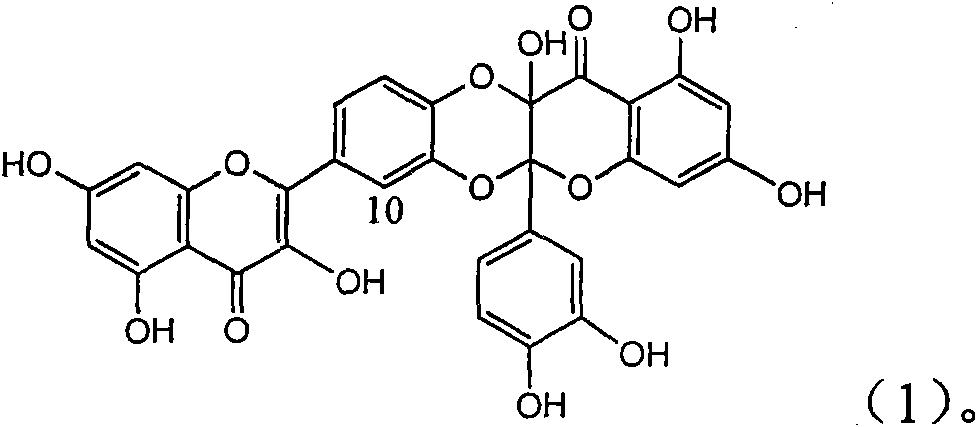
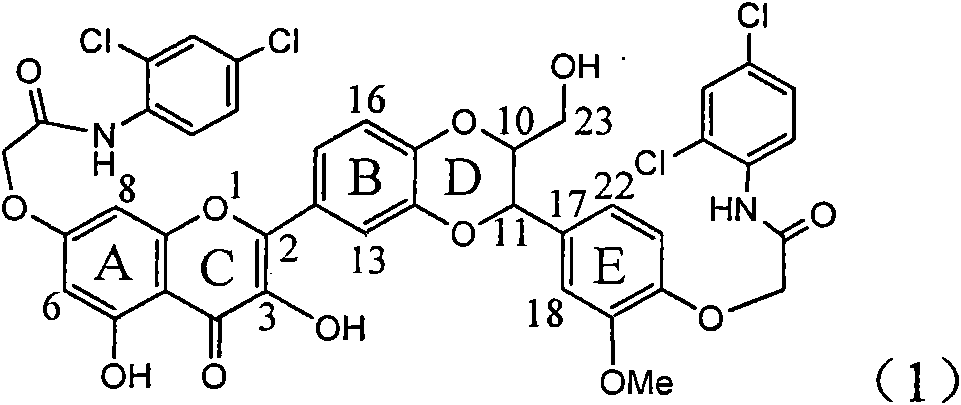
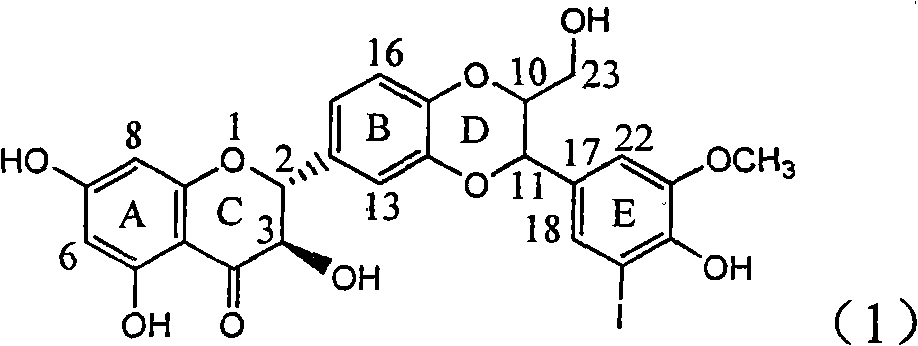
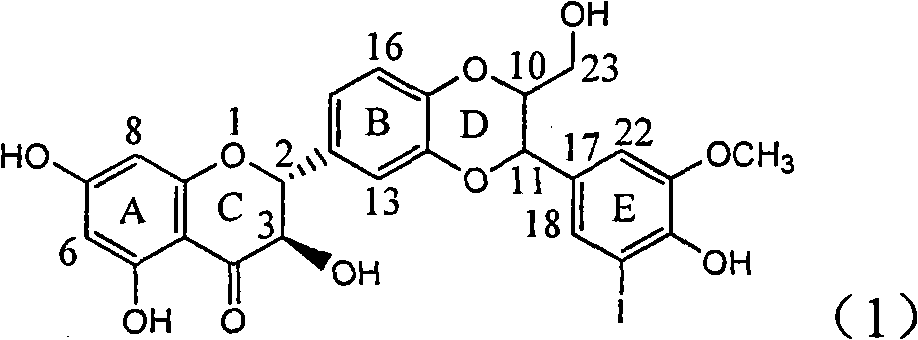
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
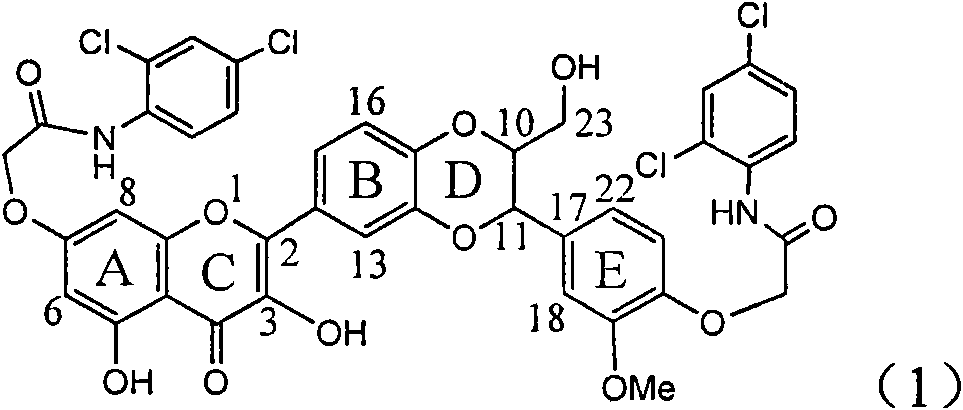
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
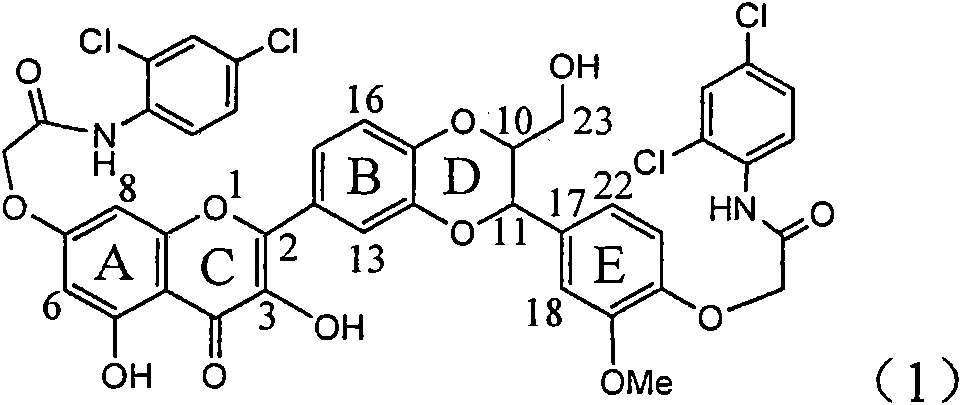
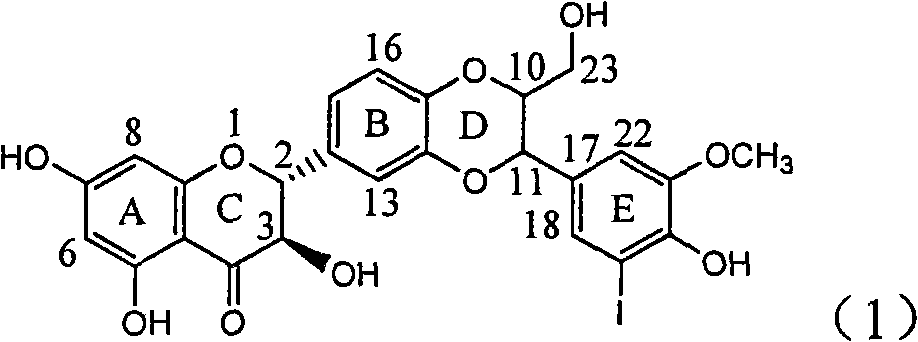
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Swine alpha-interferon gene synthesis, expression vector establishment and product preparing method
InactiveCN1611604AEfficient expressionBacteriaPeptide/protein ingredientsInfectious illnessAlpha interferon
One producing method of gene composition of pig a-interferon, expression vector structuring and its offspring includes synthesing pig a-interferon consisted of bacilluscoli preference codon and constructs highly active expression vector with this gene, and realizes the highly active expression in bacilluscoli of pig-a interferon, with 26% expression of the whole mycoprotein. At the same time, it provides a purely chemical engineering system of technique of induced expression, inclusion bodies separating and cracking, and complexity of albumen. Pig a-interferon has the functions of highly active antivirus and immunoregulation, and can be applied in preventing and treatment of pig contagion. This invention is able to produce efficient, safe, and cheap pig a-interferon which has no potential virus pollution. This lays a solid foundation for mass-production of pig a-interferon in our country.
Owner:BAOYITE BIOTECH INST QINGDAO
Production process of fusion expression recombinant chicken interferon alpha
InactiveCN106399321AStrong antiviral activityEfficient expressionFermentationInterferonsEscherichia coliInclusion bodies
The invention discloses a production process of fusion expression recombinant chicken interferon alpha. The process includes the steps of: S1, according to the preference of Escherichia coli codon, conducting codon optimization on a chicken interferon alpha gene sequence published in Genebank, and artificially synthesizing the chicken interferon alpha gene; S2, according to the codon optimized chicken interferon alpha gene, designing three specific primers; S3, constructing recombinant chicken interferon alpha plasmid containing ProS2 dissolution promoting label; S4, transforming and identifying the recombinant expression plasmid; S5, conducting inducible expression of recombinant chicken interferon alpha; S6, extracting an expression product and conducting protein renaturation purification: S61, inclusion body extraction and treatment; S62, inclusion body denaturation; S63, denaturation solution renaturation; and S64, nickel column affinity purification. By means of cell cytopathic inhibition, the invention detects that the interferon has the activity of inhibiting vesicular stomatitis virus proliferation, and the activity unit reaches 7.32*10<7>UI / mg.
Owner:SOUTH CHINA AGRI UNIV +1
Treatment of AIDS
InactiveUS20060194212A1Peptide/protein ingredientsMicrobiological testing/measurementTherapy HIVAntiretroviral therapy
The invention includes methods of treating HIV infection in a patient where the method includes administration of an antibody to TNF-alpha and an antibody to interferon-gamma to the patient and administering antiretroviral therapy to a patient. The invention further includes methods of treating HIV infection in a patient where the method comprises administration of an antibody to TNF-alpha and an antibody to alpha interferon to the patient and administering antiretroviral therapy to a patient. The invention further includes a method of treating HIV infection in a patient where the method includes administering an antibody to alpha interferon and antiretroviral therapy to a patient. The invention further includes a method of treating an HIV infection in a patient where the method comprises administering a chimeric TNF-alpha receptor and anti-retroviral therapy to a patient.
Owner:ADVANCED BIOTHERAPY
Recombined chicken alpha interferon gene and recombinant vector thereof
InactiveCN101338314AStrong antiviral activityEase of mass productionFungiMicroorganism based processesHigh concentrationAnti virus
The invention relates to a novel gene sequence of a recombinant chicken Alpha interferon, constructs a recombinant expression vector thereof and belongs to a gene engineering biological product obtained by a molecular biology method. The gene sequence of a newly designed chicken Alpha interferon is recombined into a pPICZ Alpha-A vector and then is confirmed on the special position of a microzyme by an electric conversion mode. Besides, a pichia expression system is adopted to express a foreign gene, thus being beneficial to the commercial production of the chicken interferon. The protein expressed by a gene group after being diluted by 4365158.3 times can completely restrain the attraction of vesicular stomatitis virus of 100-1000TCID50. Compared with a natural chicken Alpha interferon, the novel gene sequence of a recombinant chicken Alpha interferon has a higher anti-virus effect; the anti-virus effect thereof is improved by 8 times. Test also detects that the protein expressed by the gene can restrain the proliferation of a newcastle disease virus and an avian influenza virus; besides, the effect of the interferon with high concentration is more remarkable.
Owner:NANJING AGRICULTURAL UNIVERSITY
Porcine alpha interferon and interleukin 2 chimeric gene, construction method and protein purification method thereof
InactiveCN101570757AGuaranteed dual activityGood antiviral activityMicroorganism based processesAntiviralsAdditive ingredientInterferon alpha
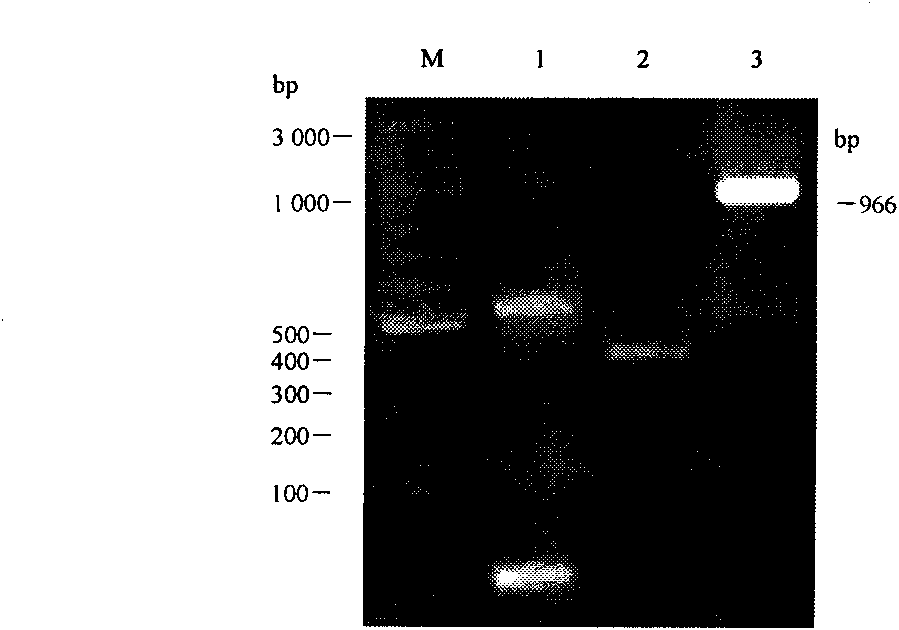
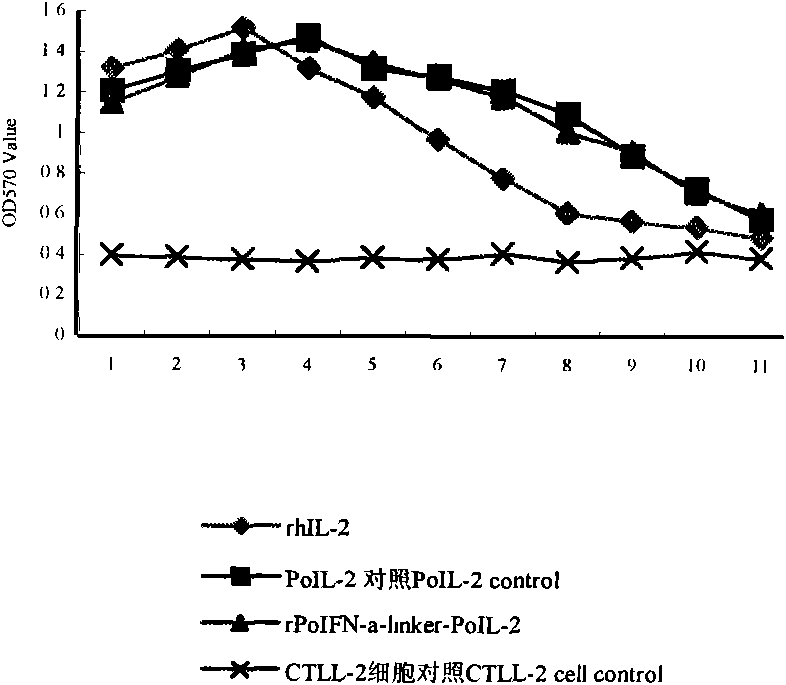
The invention belongs to the technical field of biological genetic engineering, and discloses technology for constructing and expressing porcine alpha interferon (PoIFN-alpha) and interleukin 2 (PoIL-2) chimeric gene and quickly renaturing and purifying expression protein. The technology constructs mature peptide genes of PoIFN-alpha and PoIL-2 into PoIFN-alpha-linker-PoIL-2 chimeric gene through a genetic flexible linker (linker) (G4S)3 and clones the chimeric gene into a pGEM-T Easy vector by adopting an overlap extension PCR method, and subclones the chimeric gene into a pQE-30 expression vector for prokaryotic expression. The recombinant fusion protein (rPoIFN-alpha-linker-PoIL-2) can be quickly renatured and purified through urea modification, renaturation by low concentration protein renaturing solution, PBS solution dialysis and other steps. The purified rPoIFN-alpha-linker-PoIL-2 fusion protein has proliferation activity for inhibiting vesicular stomatitis virus (VSV) on a cell, is used for prevention and treatment of porcine virosis as a main ingredient of an antivirus preparation, and has high efficiency, broad spectrum, safety and low price.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Method for producing recombinant canine interferon alpha from cultivated silkworm
InactiveCN101376885ASolve operational problemsFix stability issuesViruses/bacteriophagesFermentationEscherichia coliMammal
The invention discloses a method for expressing recombinant canine Alpha interferon with silkworm. Silkworm baculovirus serves as a carrier and recombinant virus containing a canine Alpha interferon gene is structured. Silkworm serves as a host of the recombinant baculovirus, and the recombinant canine Alpha interferon containing biological activity is expressed. The method solves the defects that complicated renaturation operations are needed in expressing the canine Alpha interferon in colon bacillus, the plasmid transformants in yeast expression are unstable and the production cost of mammal culture cells is too high, most of the recombinant canine Alpha interferon expressed by silkworm larvae is secreted into haemolymph, which is very good for the quick extraction of protein. Experimental results show that the method for using the recombinant baculovirus to produce recombinant canine Alpha interferon with silkworm is feasible. The largely expression of the recombinant canine Alpha interferon opens up broad prospects for the application of the Alpha interferon to medical and curing fields.
Owner:ZHEJIANG UNIV
Construction and modification method of recombinant porcine long-acting-alpha interferon and preparation method of lyophilized injection
ActiveCN104262480AIncrease productionRealize large-scale productionPowder deliveryPeptide/protein ingredientsImmunopotencyBacillus coli
The invention provides a construction and modification method of recombinant porcine long-acting-alpha interferon and a preparation method of a lyophilized injection. Recombinant porcine-alpha interferon provided by the invention is prepared by adopting colon bacillus soluble expression, and the recombinant porcine long-acting-alpha interferon is obtained by using polyethylene glycol to perform modification according to the characteristics of polyethylene glycol, so that the half life of the recombinant porcine long-acting-alpha interferon in a pig body is prolonged to 44.5h and the porcine immunity is enhanced. The recombinant porcine long-acting-alpha interferon provided by the invention has excellent expression under high density and high volume of bacteria, production and purification processes are simple and novel, the cost is low, and the product yield and purity are improved. The lyophilized injection of the recombinant porcine long-acting-alpha interferon, provided by the invention, has the advantages of low cost, significant curative effect and stable product quality.
Owner:CHONGQING UNIV OF TECH
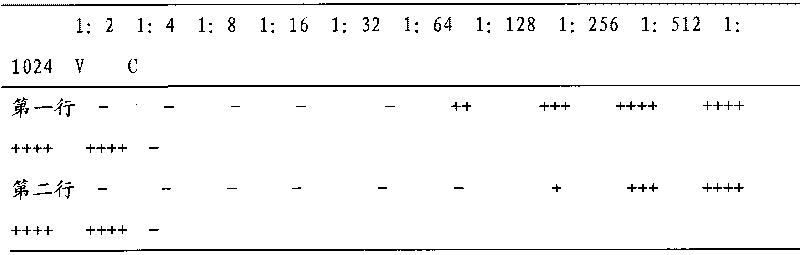
Method for preparing recombinant porcine alpha interferon standard substance
The invention discloses a method for preparing a recombinant porcine alpha interferon (rpoIFN-alpha) standard substance, which comprises the following steps of: performing fermentation, induction expression and total protein extraction on the recombinant strain of the recombinant porcine alpha interferon and obtaining the standard substance through two-step purification. The rpoIFN-alpha biologic activity reference standard substance prepared by the method can be applied to characteristic identification and potency assay of the rpoIFN-alpha so as to make the potency results of the rpoIFN-alpha measured in different batches or in the same batch but different times more credible by taking the standard substance as a standard.
Owner:ANHUI JIUCHUAN BIOTECH +1
Combination therapy (temozolomide and alpha-IFN) for advanced cancer
InactiveUS7294332B2High response rateEliminate side effectsBiocidePeptide/protein ingredientsInterferon alphaCombination therapy
There is disclosed a method for treating advanced cancer in patients in need of such treating. Temozolomide and alpha interferon are administered in combination in amounts sufficient to achieve a clinical response.
Owner:SCHERING CORP
Methods of inducing cytotoxic immune response and recombinant simian ademovirus compositions useful therein
InactiveCN1518457ASsRNA viruses negative-senseGenetic material ingredientsSimian AdenovirusesImmunodeficiency virus
The present invention provides a method of inducing a CD8+ T cell response against a selected molecule by delivering the molecule via a recombinant simian adenovirus. The present invention also provides a method for inducing alpha interferon and beta interferon by delivering a recombinant simian adenovirus to a subject. The methods and compositions of the invention are particularly suitable for use in the prevention and treatment of human immunodeficiency virus, human papillomavirus infections and cancer treatment.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY +1
Method of treatment of cancer patients
InactiveUS20120195911A1Improve efficiencyExpand spectrum of antitumor activityBiocidePeptide/protein ingredientsAntiviral drugOncology
This invention may be used in human and veterinary medicine in combination with traditional methods of treatment of oncological illnesses for the purpose of increasing their effectiveness.It is a method of treating patients with oncological diseases that is distinct in that an antiviral drug is used as an additional component of standard treatment before the beginning of and in parallel with standard treatment, in which Acyclovir, Valacyclovir, alpha interferon, a specific antiviral immunoglobulin or a combination of these substances are used as an antiviral drug, which may be used for inclusion in the standard surgical, radiological, or chemotherapeutic treatment plan for cancer patients.The proposed method allows us to significantly improve the results of the treatment of cancer patients, decrease the number of relapses, improve patients' quality of life, and significantly extend their lives. Because the proposed drugs are already approved for use, there are no technical difficulties facing their inclusion in cancer patients' treatment plans.
Owner:MARTYNOV ARTUR +2
Recombinant porcine alpha interferon and application thereof in preparing medicines for treating Porcine cytomegalovirus (PCMV)
InactiveCN102796758APeptide/protein ingredientsMicroorganism based processesInfected cellProtein target
The invention discloses a recombinant porcine alpha interferon, prepared by the following methods: A1, synthesizing an artificially modified porcine alpha interferon; A2, constructing a recombinant eukaryotic expression vector pGAPZ alpha-IFN alpha; and A3, highly expressing IFN alpha by an eukaryotic cell yeast expression system. The invention is characterized by taking a supernatant to purifying the porcine alpha interferon which is cultured by a lot of engineering bacteria and expressed with a chromatography column, then collecting a target protein eluate, filtering through a 0.22 mum microfiltration membrane, then determining the activity by cytopathic inhibition, and calculating the potency unit according to 50% pathology; wherein the cell used for determination is Madin-Darby bovine kidney (MDBK), and vesicular stomatitis virus (VSV) is used for attacking the virus. According to the invention, the recombinant porcine alpha interferon obtained by purification is applied in controlling PCMV, and experiments prove that the recombinant porcine alpha interferon has obvious protection effect on PCMV infected cells. The route of administration of the porcine alpha interferon is injection or mucous membrane administration, and the dosage form comprises injection or nasal drops.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Method for preparing recombinant canine alpha-type interferon
ActiveCN101100664ALow costReduce production capacityPowder deliveryPeptide/protein ingredientsEscherichia coliDisease
Production of recombinant alpha-canine interferon is carried out by augmenting alpha-interferon target gene of specific primer PCR, connecting with expression carrier PET-28a, converting colibacillus, expressing canine-alpha interferon efficiently, high-purifying, killing bacterium, split charging and freeze drying. It's simple, safe, reliable and non-toxic. It's convenient to transport and store. It can be used to treat canine parvoviral and virus diarrhea etc. diseases as broad-spectrum antiviral preparation.
Owner:ANHUI JIUCHUAN BIOTECH +1
Recombinant adenovirus for expression of goat alpha interferon and construction method and application thereof
ActiveCN103981192AHigh titerStrong antiviral activityGenetic material ingredientsViral/bacteriophage medical ingredientsRuminant animalMicroorganism
The invention discloses recombinant adenovirus for expression of goat alpha interferon and a construction method and application thereof. The invention first provides an optimized goat alpha interferon gene with a nucleotide sequence shown as SEQ ID NO:1; the invention also provides the recombinant adenovirus for stable expression of the goat alpha interferon, and the microbial preservation number is: CGMCC (China General Microbiological Culture Collection Center) No.8757. The invention further provides a construction method of the recombinant adenovirus. The recombinant adenovirus constructed by the construction method is capable of stable expression of the goat alpha interferon, high in virus titer, and high in antiviral activity; the recombinant adenovirus and the goat alpha interferon expressed by the recombinant adenovirus both have antiviral activity in swine cells IBRS2, bovine cells MDBK and primary sheep skin cells, and can be used in the prevention or treatment of ruminant animal virus infectious diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Gene synthesis of wild boar alpha-interferon, vector construction and method for producing outcome
InactiveCN101392256AOptimize free energySimple structurePeptide/protein ingredientsAntiviralsEscherichia coliInclusion bodies
The invention discloses a gene synthesis of Alpha-interferon of wild boars and vector construction thereof as well as a production method of a product. A number of problems of low expression capacity, products expressed in a fusion state, products with purification tags or no fermentation technology with high density exist in the domestic recombinant strains. The invention includes a method that a codon and codon pairs, preferred by Escherichia coli, are used for synthesizing an Alpha-interferon gene of the wild boar, establishing a high-efficiency expression vector and transforming a high-efficiency expression strain as well as methods of high-density fermentation of engineering bacteria, separation and purification of inclusion bodies, the modification, the renaturation and the purification of target protein and the determination of biological activity of the expressed product. The gene synthesis, the vector construction and the production method pertain to the technical field of the production of polypeptide drugs by genetic engineering in biopharmaceuticals.
Owner:黑龙江省农业科学院畜牧研究中心 +1
Artificial synthetic porcine interferon and method for preparing porcine interferon
The invention relates to a synthetic porcine interferon gene, a carrier-host cell containing same and a method for preparing porcine interferon. The synthetic porcine interferon gene has an SEQ ID NO.1 nucleotide sequence shown in a figure 1. The preparation method comprises the steps of synthesizing a synthetic porcine alpha-IFN gene and the carrier-host cell containing the gene, recombining pichia for fermentation to produce the porcine interferon and purifying the obtained porcine alpha interferon. An expression system has the characteristics of high expressivity, high stability and high secretion. The pichia as host bacteria is small in the secretion of own protein, large in the secretion of high-activity foreign protein and beneficial to downstream separation and purification, is a eukaryotic expression system suitable for expressing foreign gene, and is beneficial to large-scale batch production.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Preparation method of recombinant human alpha interferon
The invention discloses a method for preparation of interferon. The method includes extracting inclusion body after the fermentation of bacillus coli having coding interferon alpha ribonucleotide sequence, applying affinity tomographic column to perform reclamation and purification for the inclusion body, then performing ion-exchange chromatography, anti-phase high performance liquid chromatography and gel exclusion chromatography to obtain high-purity recombination alpha interferon.
Owner:SHENYANG SUNSHINE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com