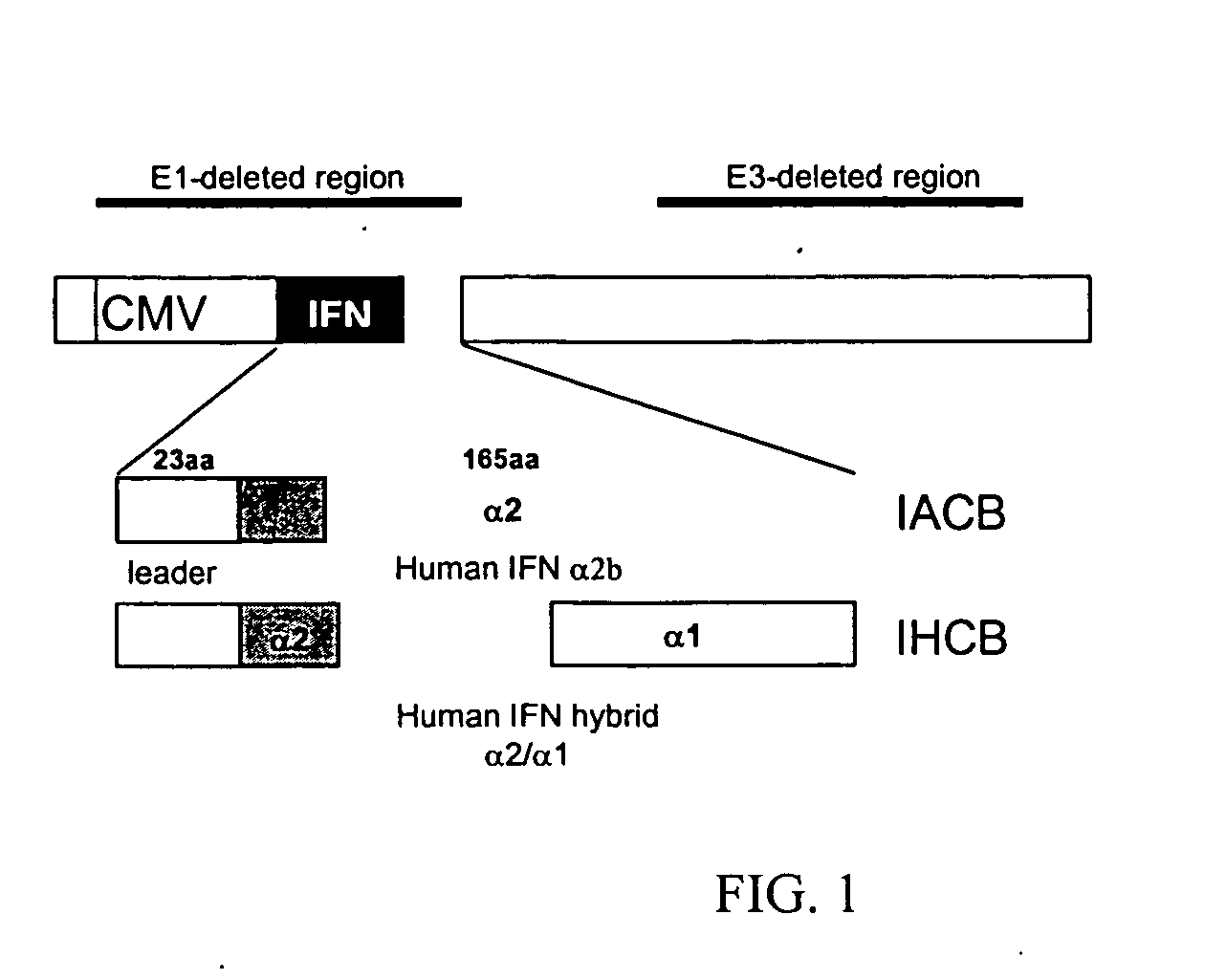
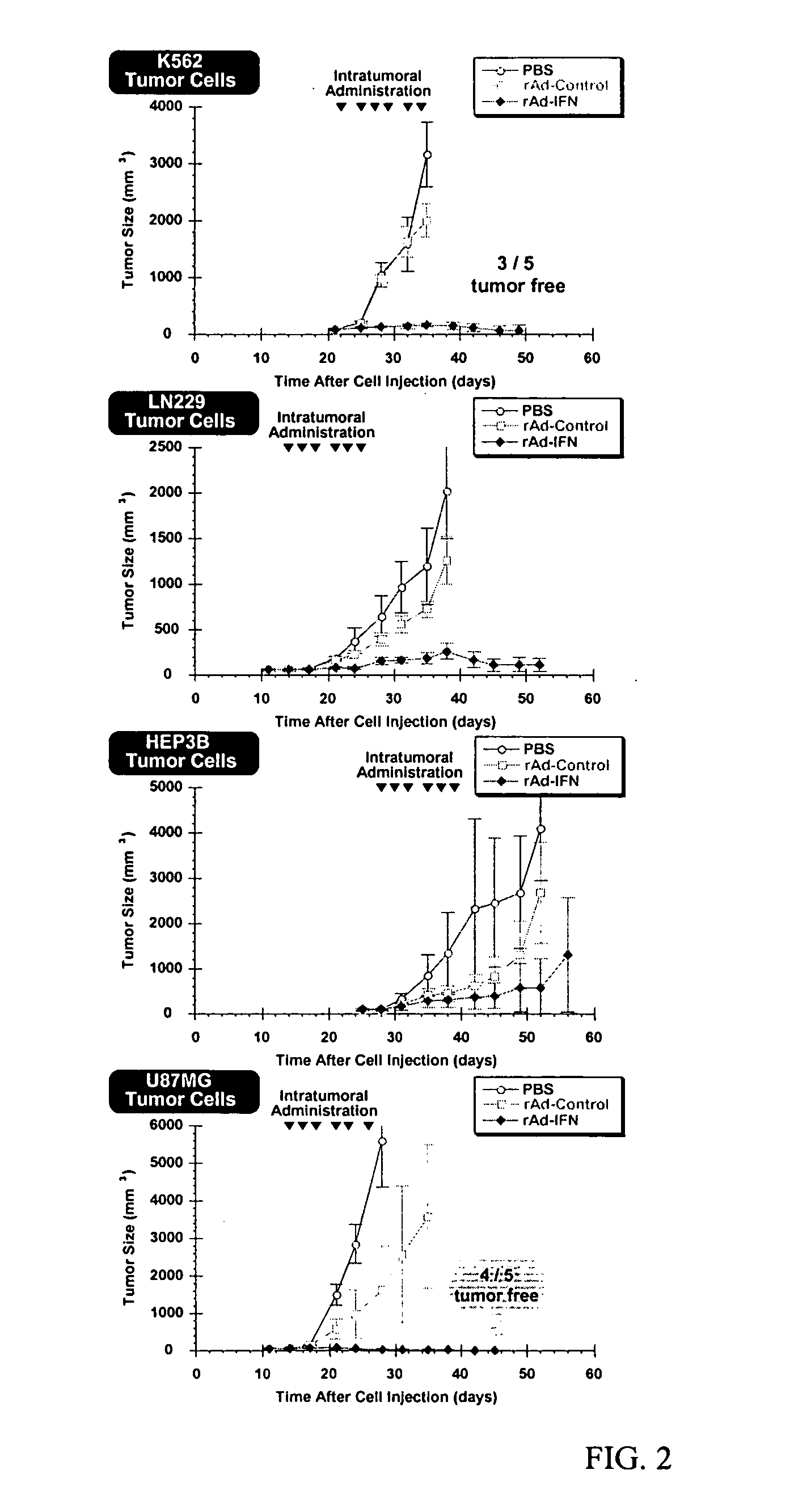
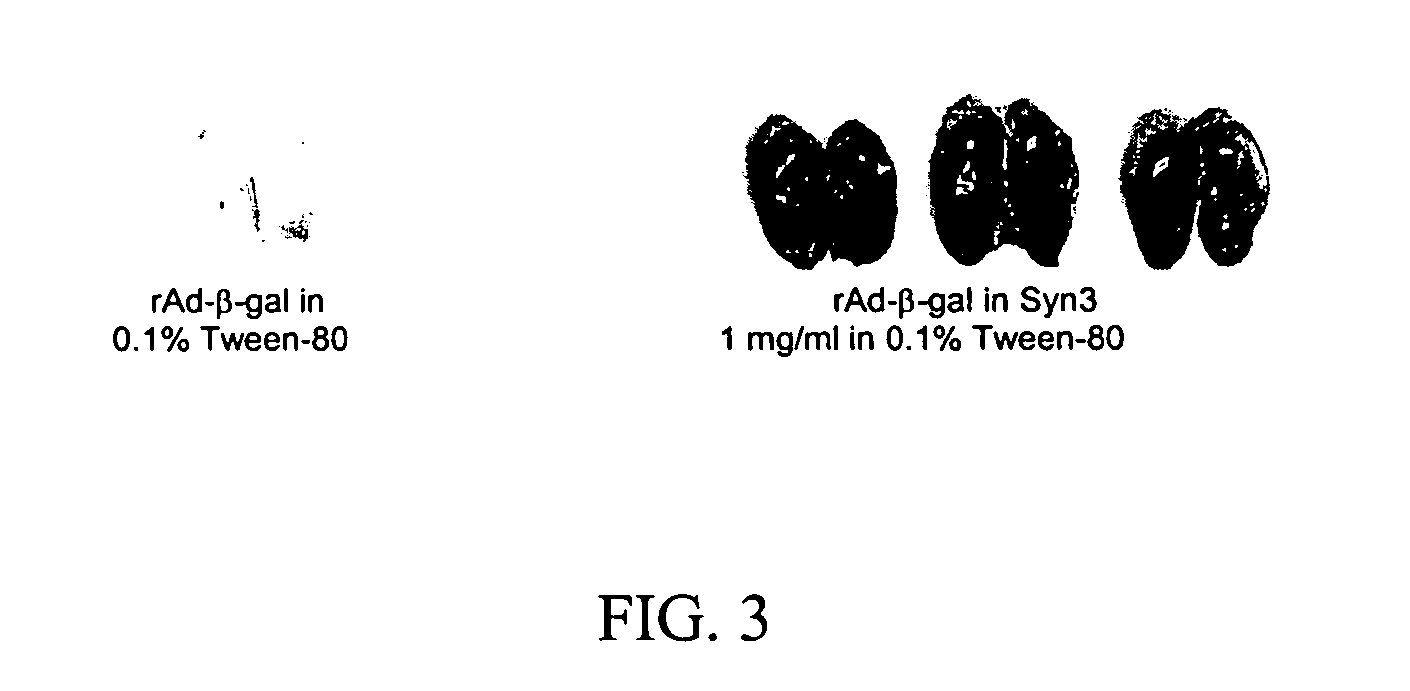
Methods and compositions for interferon therapy
a technology of interferon and composition, applied in the field of methods and compositions for interferon therapy, can solve the problems of high tumor recurrence, high tumor recurrence, and inability to perform surgical intervention in situ treatment of carcinoma, and achieve the effect of prolonging the contact time of the epithelial membran
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
U.S. Pat. No. 6,312,681 discloses a method for delivering an adenoviral vector which comprises a transgene to a cancer cell in the epithelial membrane of a bladder, the method comprising administering to the epithelial membrane the adenoviral vector and between 1% and 50% (v / v) ethanol or another delivery enhancing agent, wherein the adenoviral vector infects the cell and the transgene is expressed in infected cells. U.S. Pat. No. 6,312,681 assigned to the same assignee as the present application is hereby incorporated by reference in its entirety.
example 2
The following table represents exemplary ranges of the ingredients for non-aqueous liquid SYN3 formulations of the present invention. Prior to administration (e.g., for bladder cancer), the SYN3 solution is combined with the recombinant adenovirus preparation in a 1:50 v / v ratio to form an admixture that is administered to the patient.
Range of Ingredient ConcentrationsIngredientmg / mlmg / mlmg / mlmg / mlSYN30.001-1500.001-1500.001-1500.001-150Polysorbate 200.001-150Polysorbate 800.001-150Pluronic L640.001-100Pluronic L920.001-100N,N-Dimethylacetamide1 ml1 ml1 ml1 mlqs ad
To prepare, weigh approximately 75% of DMA into a glass beaker. To a separate beaker, charge the surfactant (Polysorbate 80, Polysorbate 20, Pluronic L64 or Pluronic L92) and dissolve in a small volume (approximately 10% of final volume) of DMA. Charge the DMA / surfactant solution into the DMA with constant stirring. Preweigh SYN3 in a separate container. Slowly charge the SYN3 into the solution while stirring. Once the...
example 3
The following table represents exemplary ranges of the ingredients for lyophilized SYN3 formulations of the present invention. The compounded solution is filled as indicated into a 20-ml capacity Type II glass vial and lyophilized. Preparation for administration requires addition of 20 ml of WFI to the vial containing the freeze-dried cake to redissolve the SYN3. The SYN3 solution is combined with p53, or any recombinant adenovirus preparation, in a v / v ratio of 1:5. The admixture is then administered to the patient for, for instance, bladder cancer.
Range of ConcentrationsINGREDIENTmg / mlmg / mlmg / mlSYN30.001-150 0.001-150 0.001-150 Citric Acid Monohydrate0.016-0.720.016-0.960.005-1.35Sodium Citrate Dihydrate 0.05-2.310.05-3 0.02-5.37Hydroxypropyl-β-cyclodextrin— 50-500—Big CHAP 20-360——Glycine 10-200——Mannitol—— 5-100Polysorbate 80— 1.0-36.0 10-200Ascorbic Acida0.001-0.6 0.001-0.6 —Water for Injection (WFI) 1 ml 1 ml 1 mlqs adPH Range 5-6 5-6 5-6ml Fill into 20-ml vialb5.35.35.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com