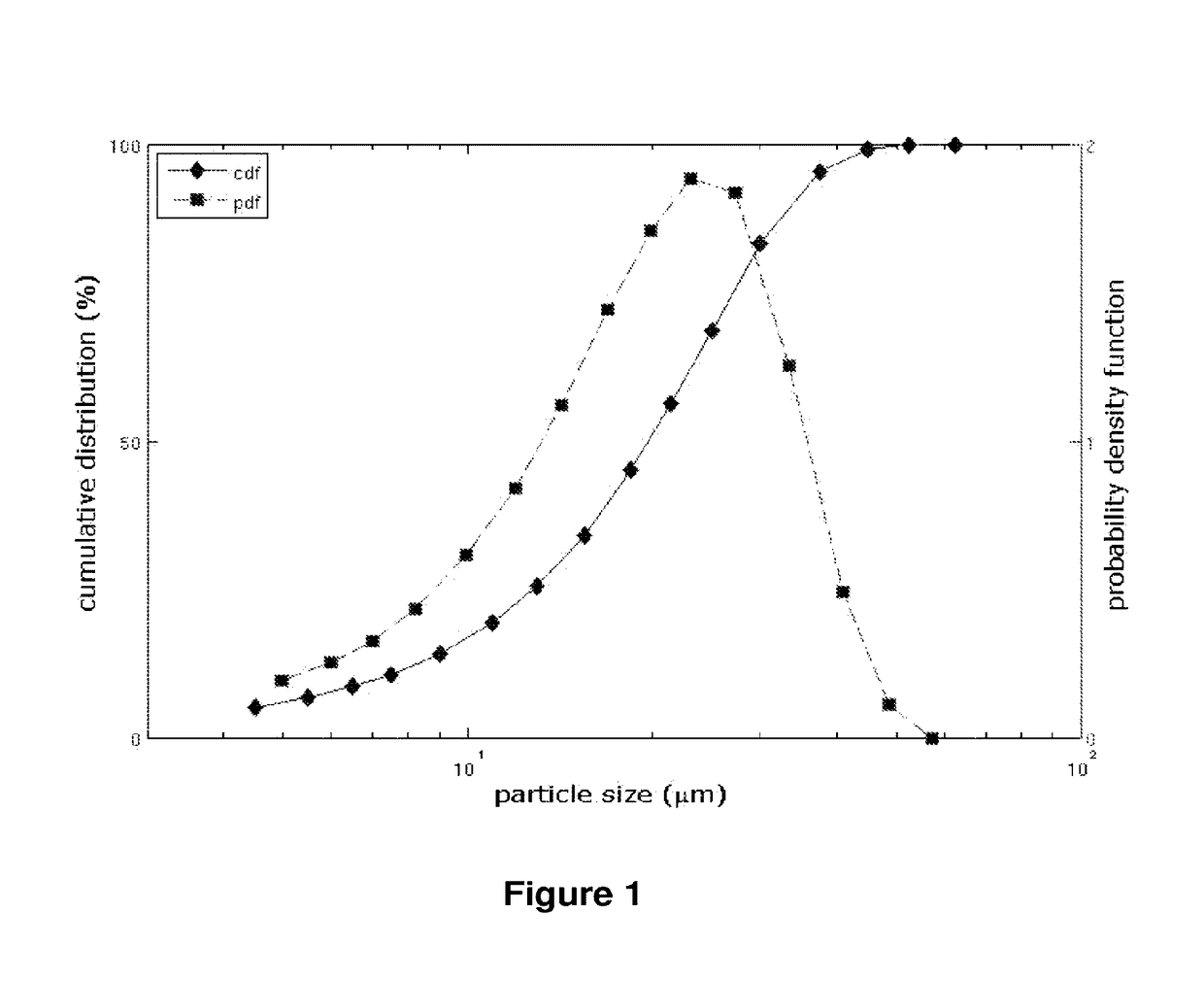
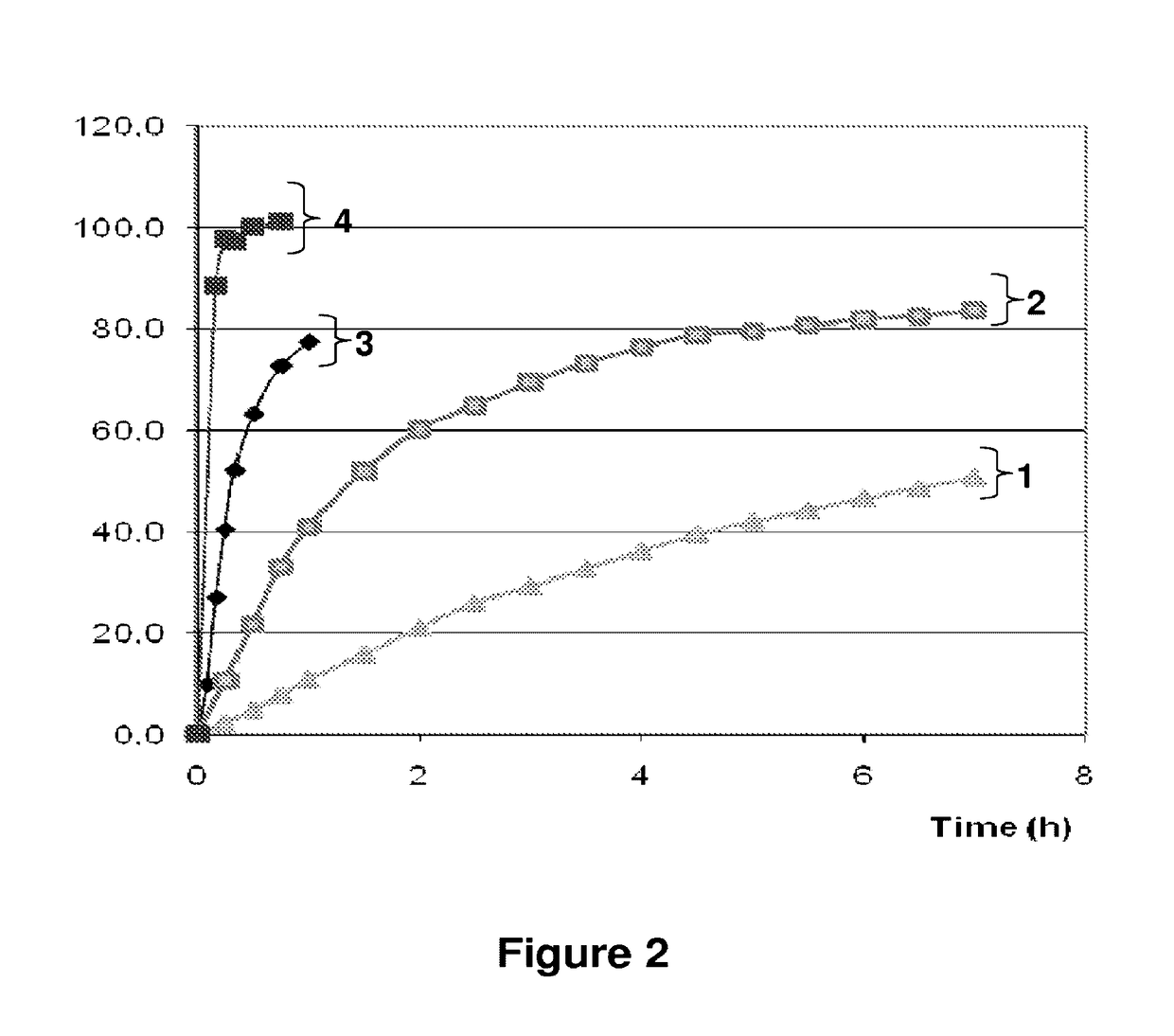
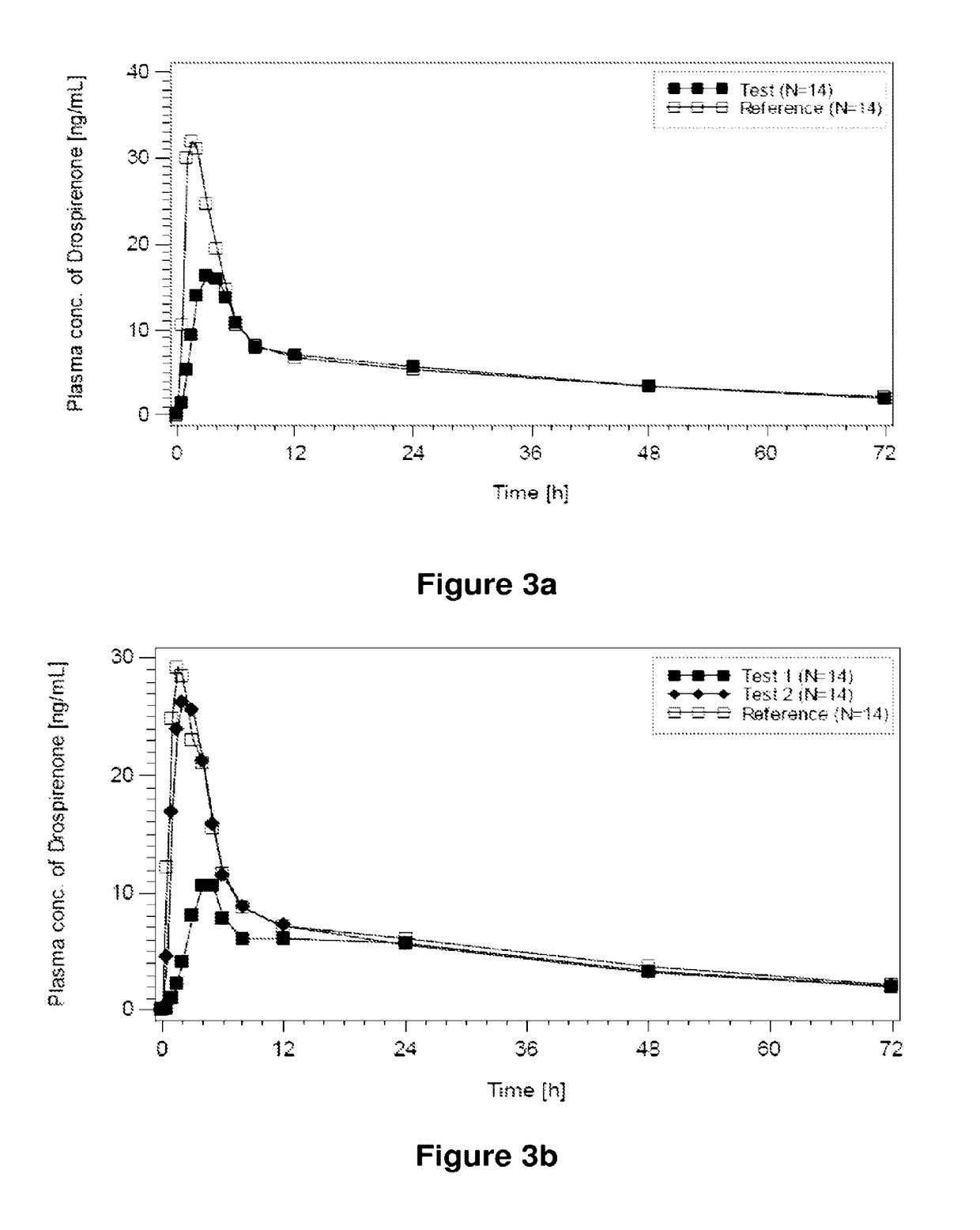
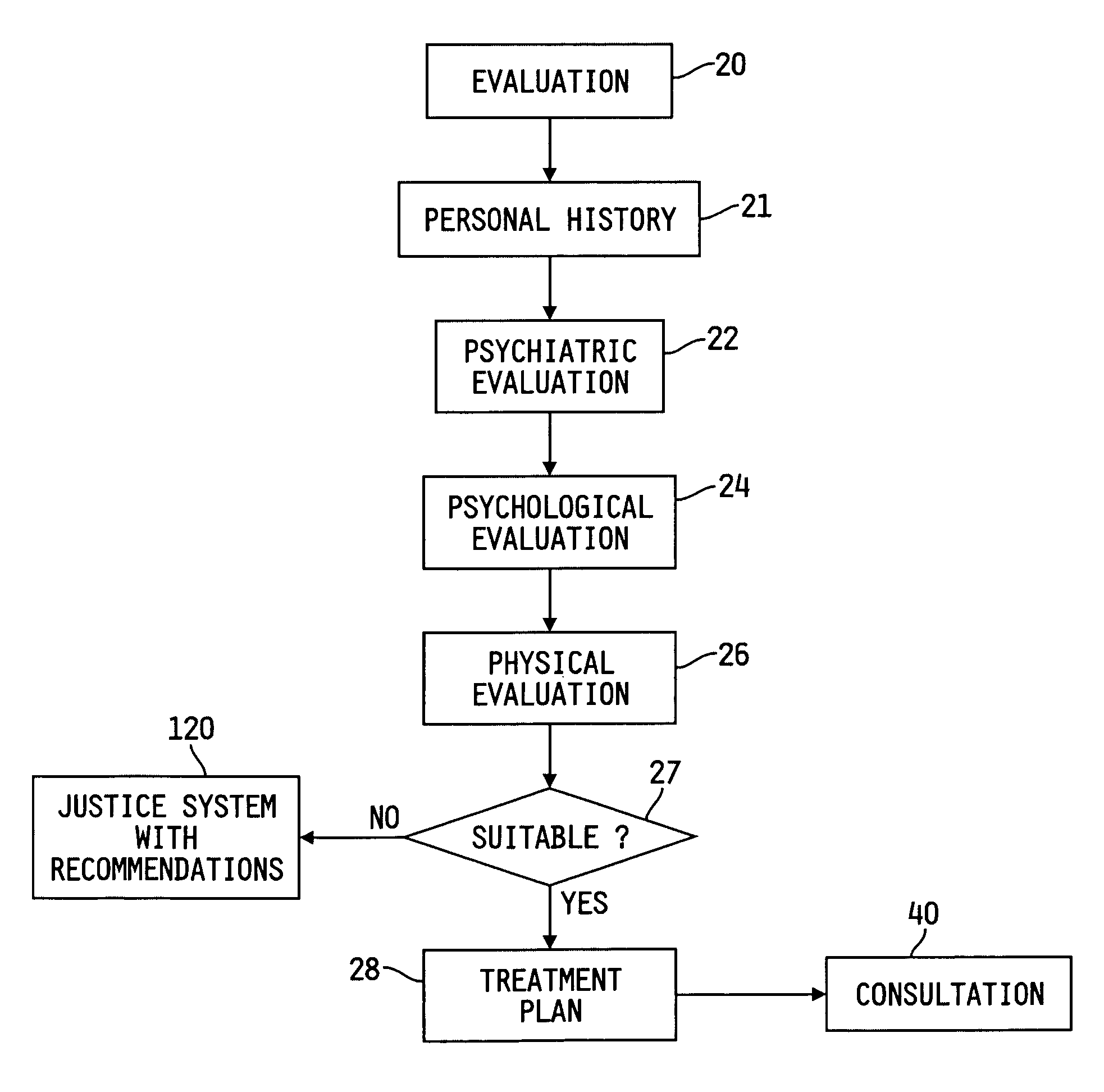
Patents
Literature
183 results about "Initial treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
First-line treatment or therapy simply refers to the initial, or first treatment recommended for a disease or illness. This may also be referred to as primary treatment, initial treatment, or induction therapy.
Apparatus and method of providing continuous positive airway pressure
InactiveUS6401713B1RespiratorsOperating means/releasing devices for valvesInitial treatmentInitial therapy
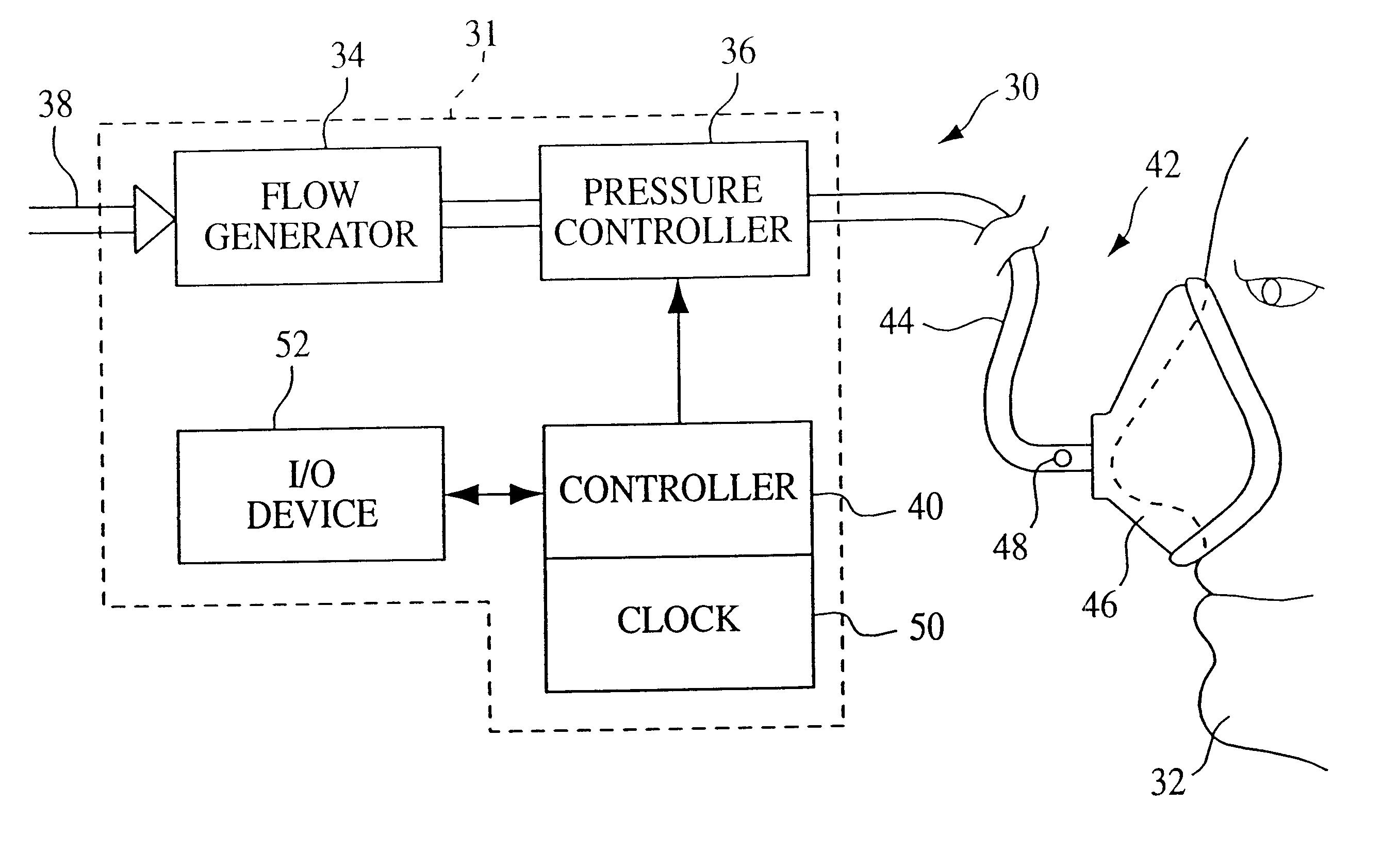
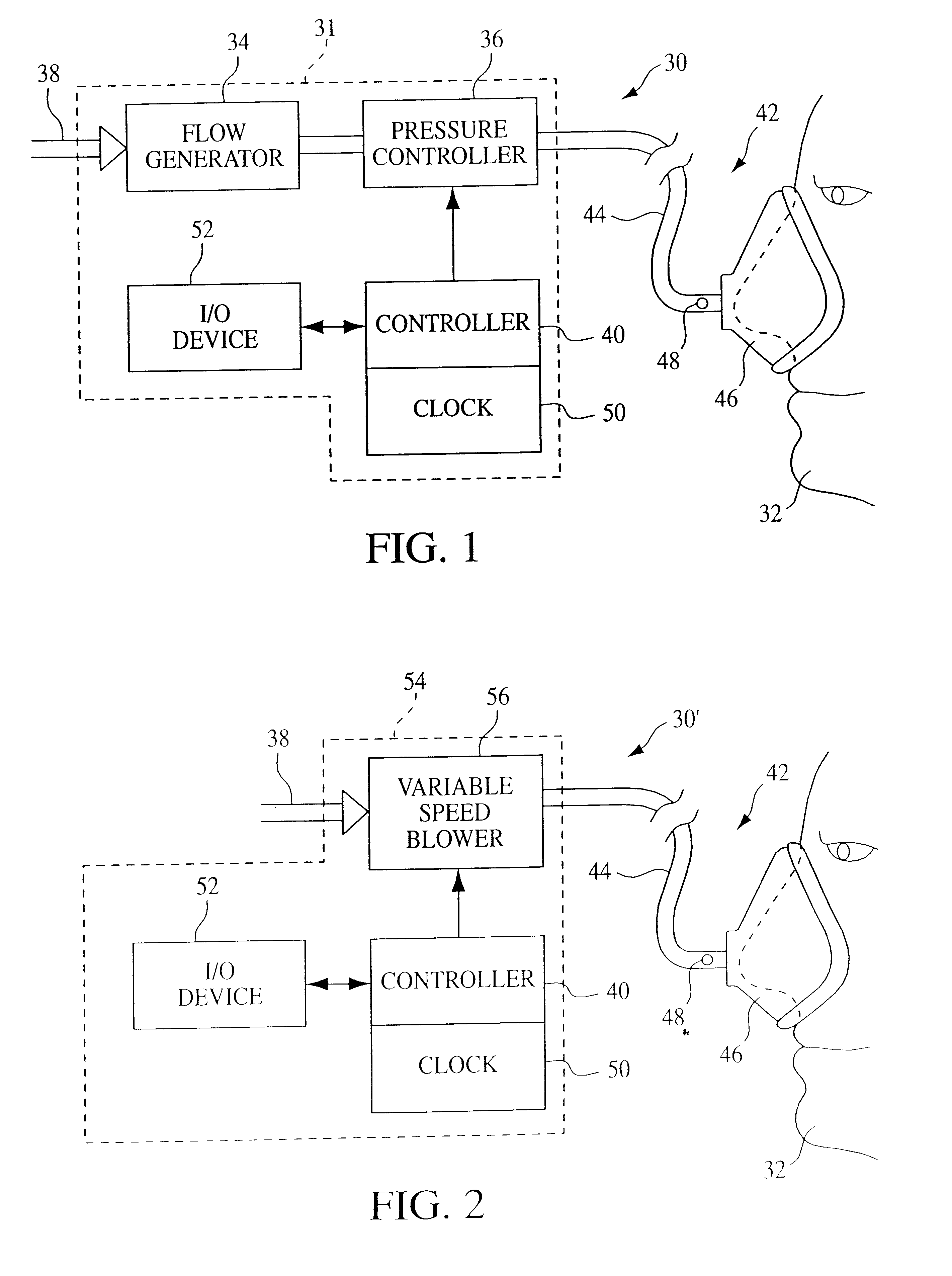
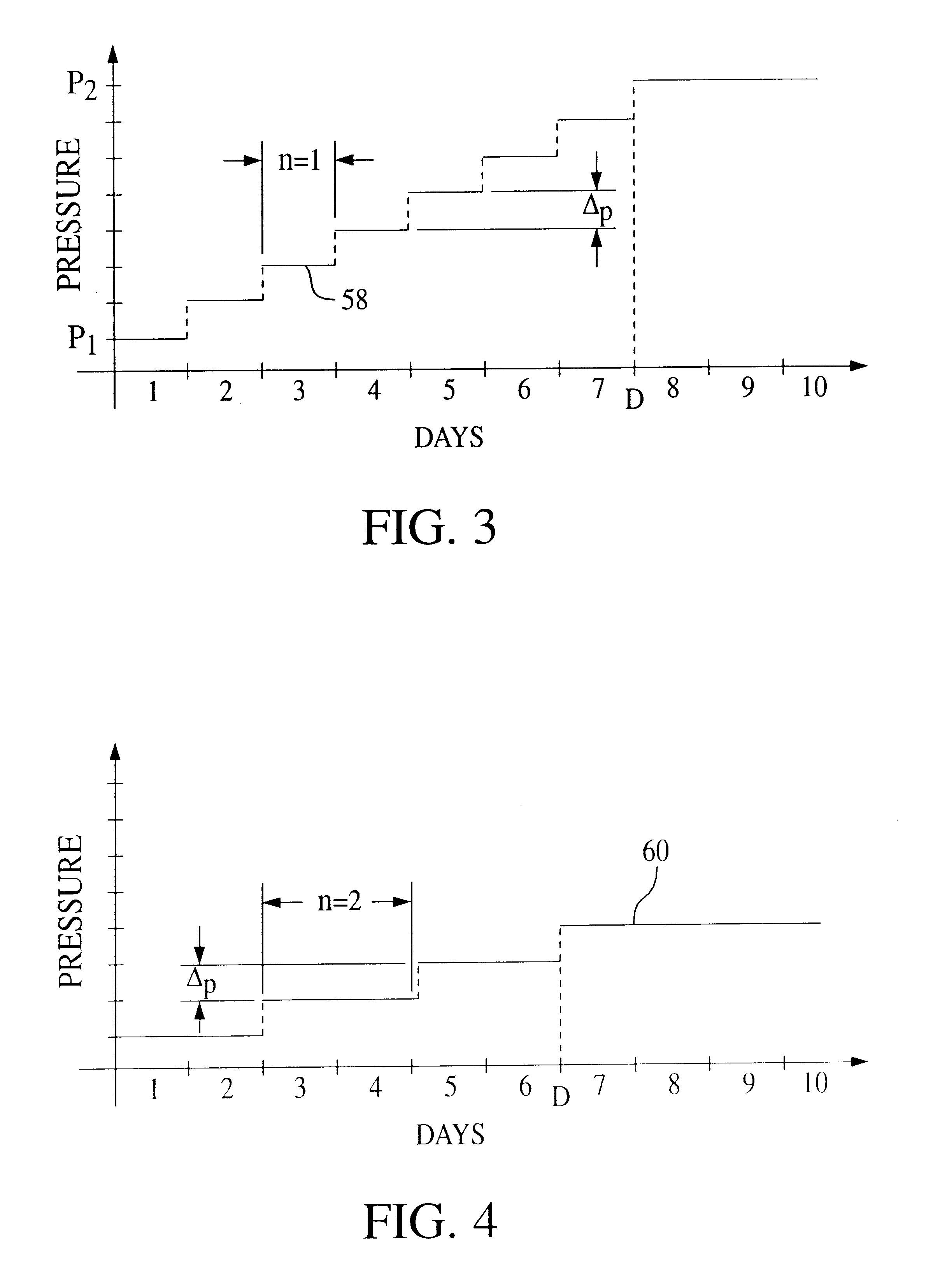
A pressure support system and method in which a gas flow generating system provides a continuous flow of breathing gas at selectable pressure levels. A patient circuit and interface communicate the continuous flow of breathing gas to the airway of a patient. A controller causes the gas flow generating system to provide the continuous flow of breathing gas to the patient at a first pressure level P1 in an initial therapy session. Thereafter, the pressure level of the continuous flow of breathing gas is increased from the first pressure level P1 to a final pressure level P2 over a first predetermined number D of days. More specifically, in one embodiment, the pressure level is incrementally increased by a predetermined incremental amount DELTAp after n therapy sessions taking place on separate days over this first predetermined number D of days. As a result, the current therapy pressure in each therapy session that takes place on separate days following the initial therapy session is incrementally and automatically increased.
Owner:RIC INVESTMENTS LLC
Combination therapy for depression, prevention of suicide, and various medical and psychiatric conditions
InactiveUS7973043B2Preventing disease progression/modifyingDelaying/preventing relapseBiocideNervous disorderInitial treatmentTherapy resistant
The present invention relates to a new method of treatment for persons meeting diagnoses for major depressive disorder, or other unipolar (non-bipolar, non-psychotic and non-treatment resistant) depression. The method comprises administering a combination of two categories of drugs, antipsychotics or dopamine system stabilizers, in combination with a newer antidepressant such as a selective serotonin reuptake inhibitor, as initial treatment or as soon as possible. The method targets the prevention of suicide, and provides other benefits including preventing disease progression development of tolerance toward the antidepressants. Another aspect of the invention relates to using the method for alleviating cognitive distortion and related functional impairment or health risks, and / or using the method for smoking cessation or nicotine withdrawal.
Owner:MIGALY PETER
Mobile station and methods for diagnosing and modeling site specific full-scale effluent treatment facility requirements
InactiveUS20110257788A1Reduce financial riskHigh continuity of operationWater/sewage treatment by neutralisationSustainable biological treatmentInitial treatmentIon exchange
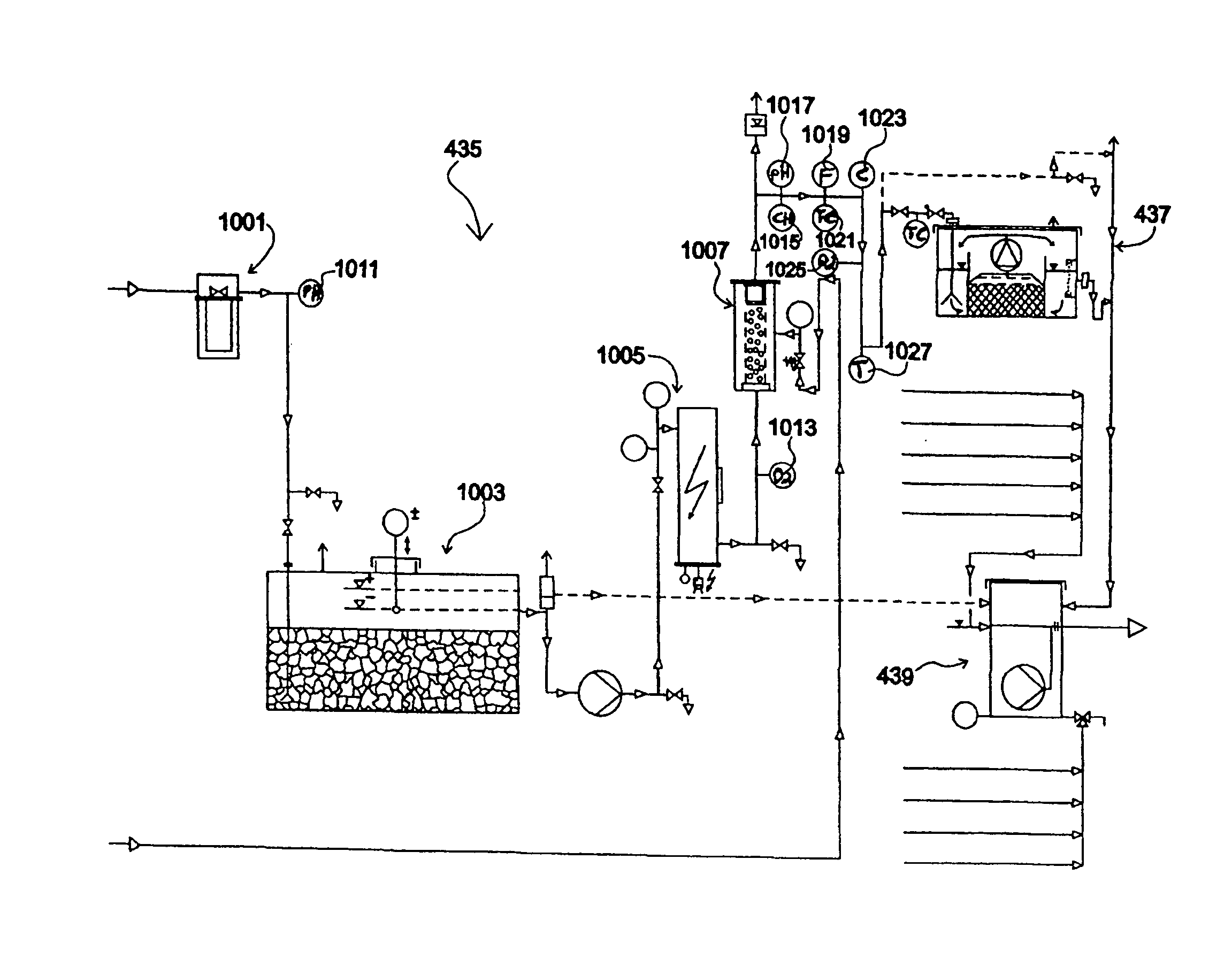
A mobile station and methods are disclosed for diagnosing and modeling site specific effluent treatment facility requirements to arrive at a treatment regimen and / or proposed commercial plant model idealized for the particular water / site requirements. The station includes a mobile platform having power intake, effluent intake and fluid outflow facilities and first and second suites of selectably actuatable effluent pre-treatment apparatus. An effluent polishing treatment array is housed at the station and includes at least one of nanofiltration, reverse osmosis and ion-exchange stages. A suite of selectively actuatable post-treatment apparatus is housed at the station. Controls are connected at the station for process control, monitoring and data accumulation. A plurality of improved water treatment technologies is also disclosed. The modeling methods include steps for analyzing raw effluent to be treated, providing a field of raw effluent condition entry values and a field of treated effluent condition goals entry values, and utilizing said fields to determine an initial treatment model including a selection of, and use parameters for, treatment technologies from the plurality of down-scaled treatment technologies at the facility, the model dynamically and continuously modifiable during treatment modeling.
Owner:ROCKWATER RESOURCE
Rehabilitation methods
InactiveUS20060277074A1Improve efficiencyWaste less timePhysical therapies and activitiesData processing applicationsInitial therapyTime schedule
A computerized clinic management system. The system includes a database of patient records and a database of clinic resources reflecting a temporal availability of each individual resource in the database of clinic resources. The system optionally also includes and a scheduling module designed and configured to determine an initial subset of clinic resources required for an initial treatment plan for a patient based upon analysis of the database of patient records and to determine an initial schedule of therapy for said patient based upon further analysis of temporal availability of the subset of said clinic resources.
Owner:MOTORIKA
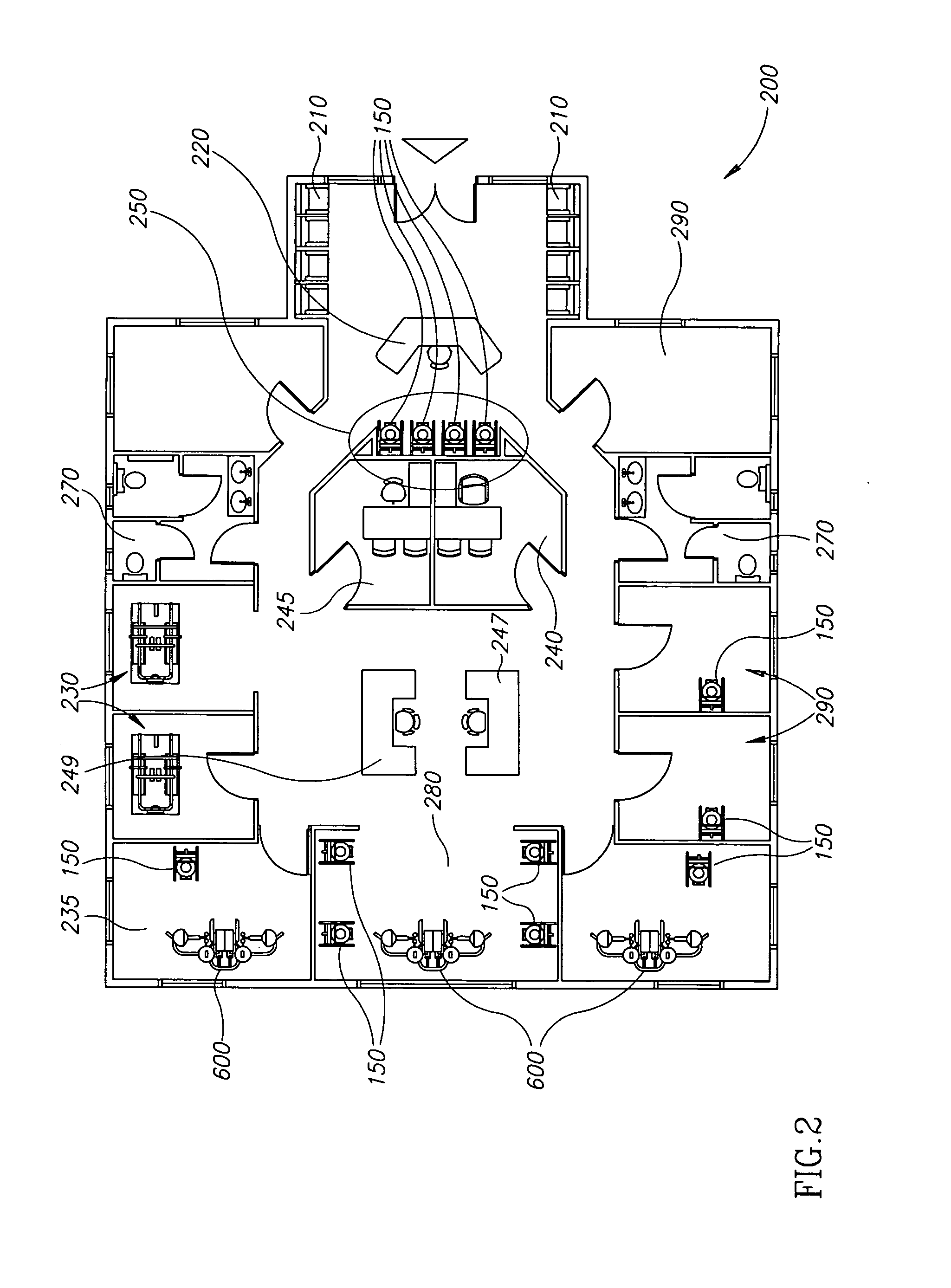
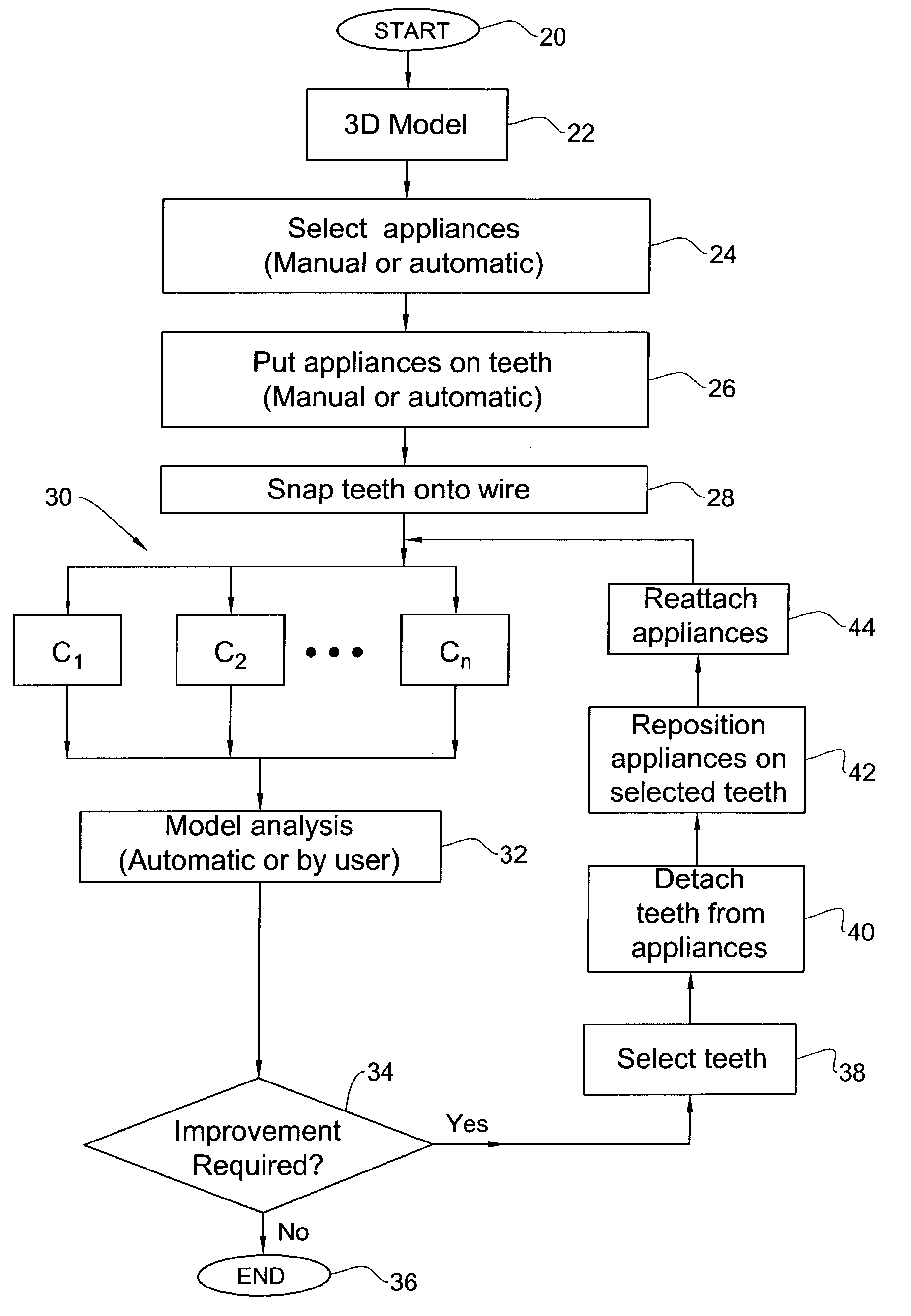
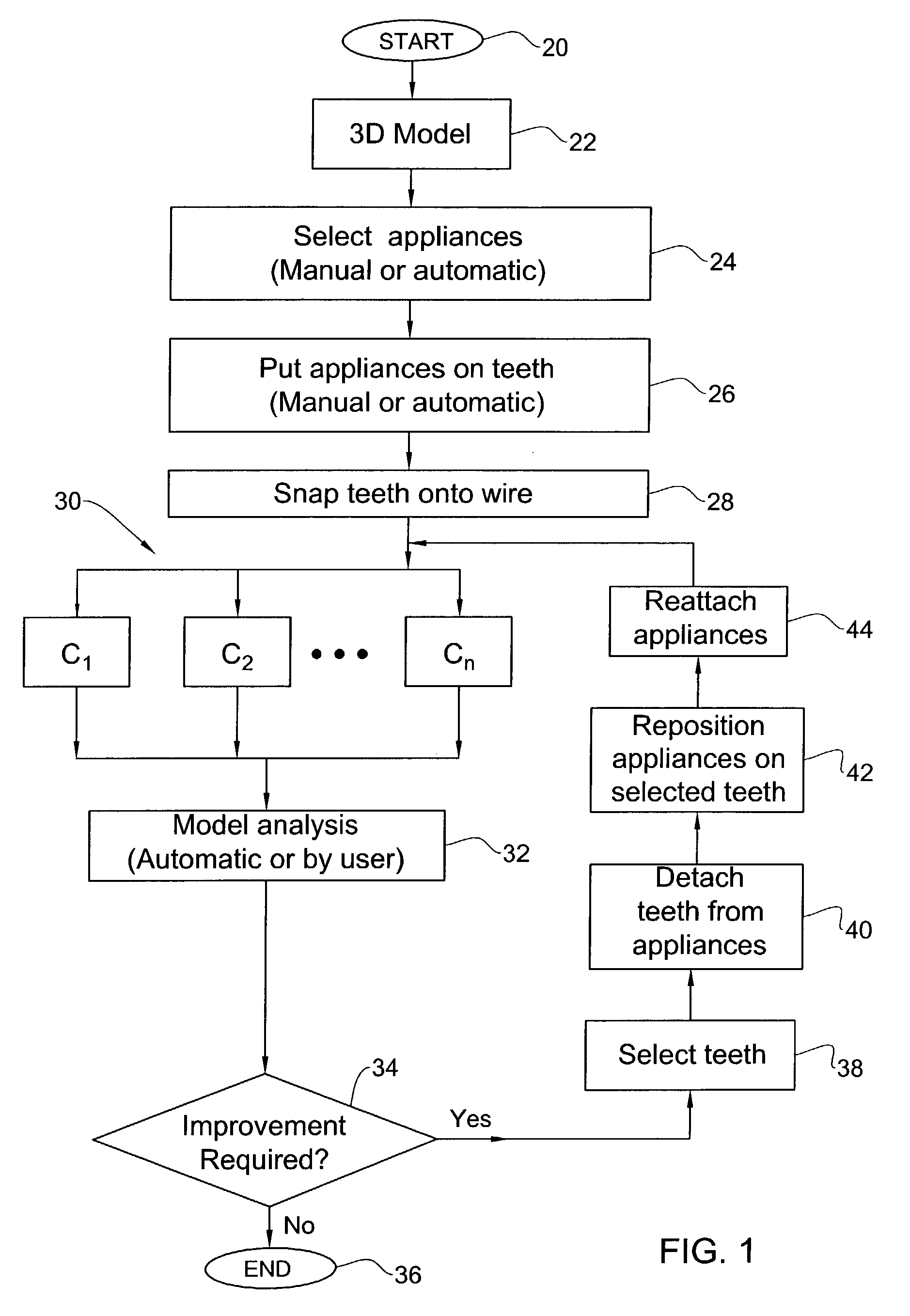
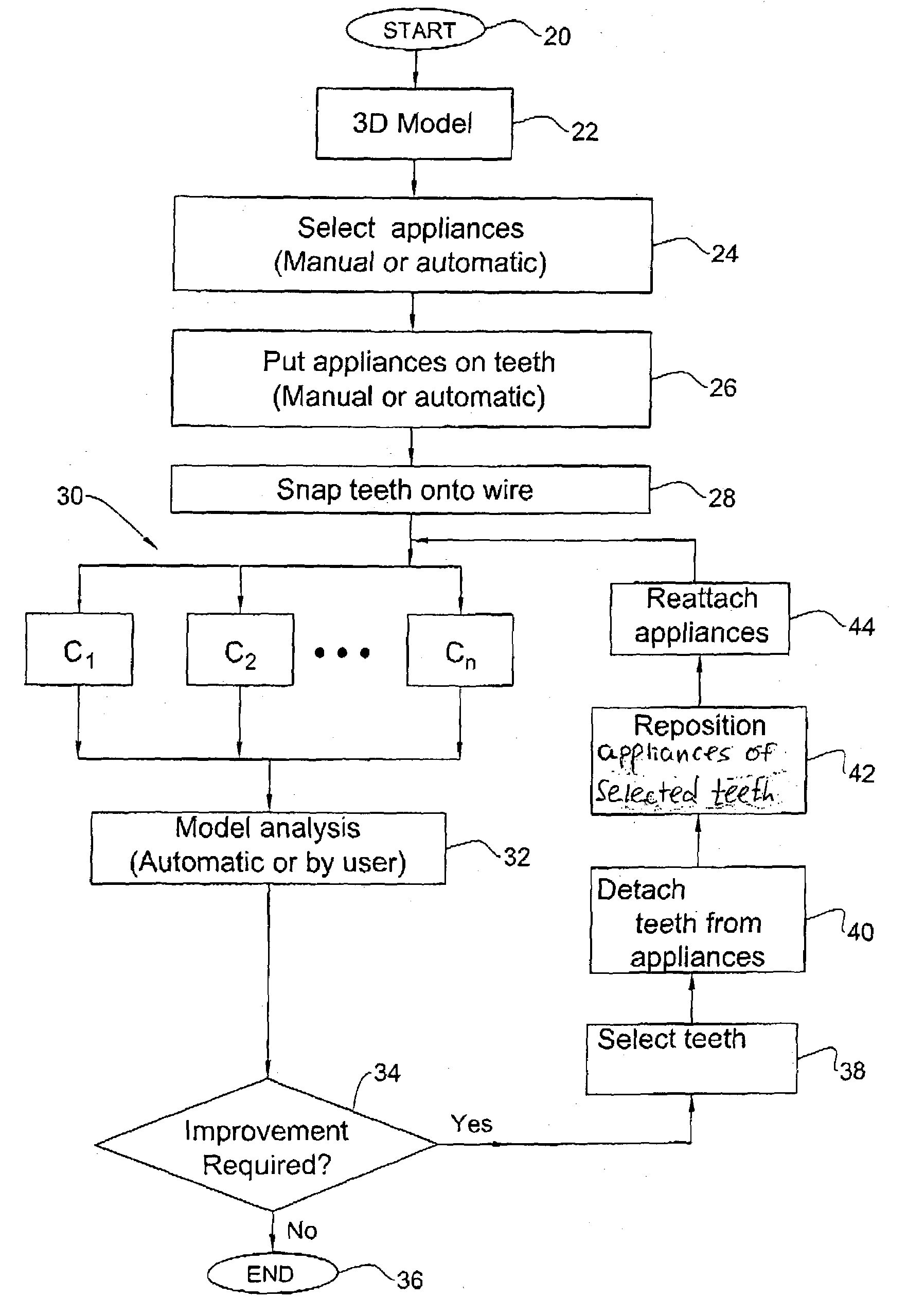
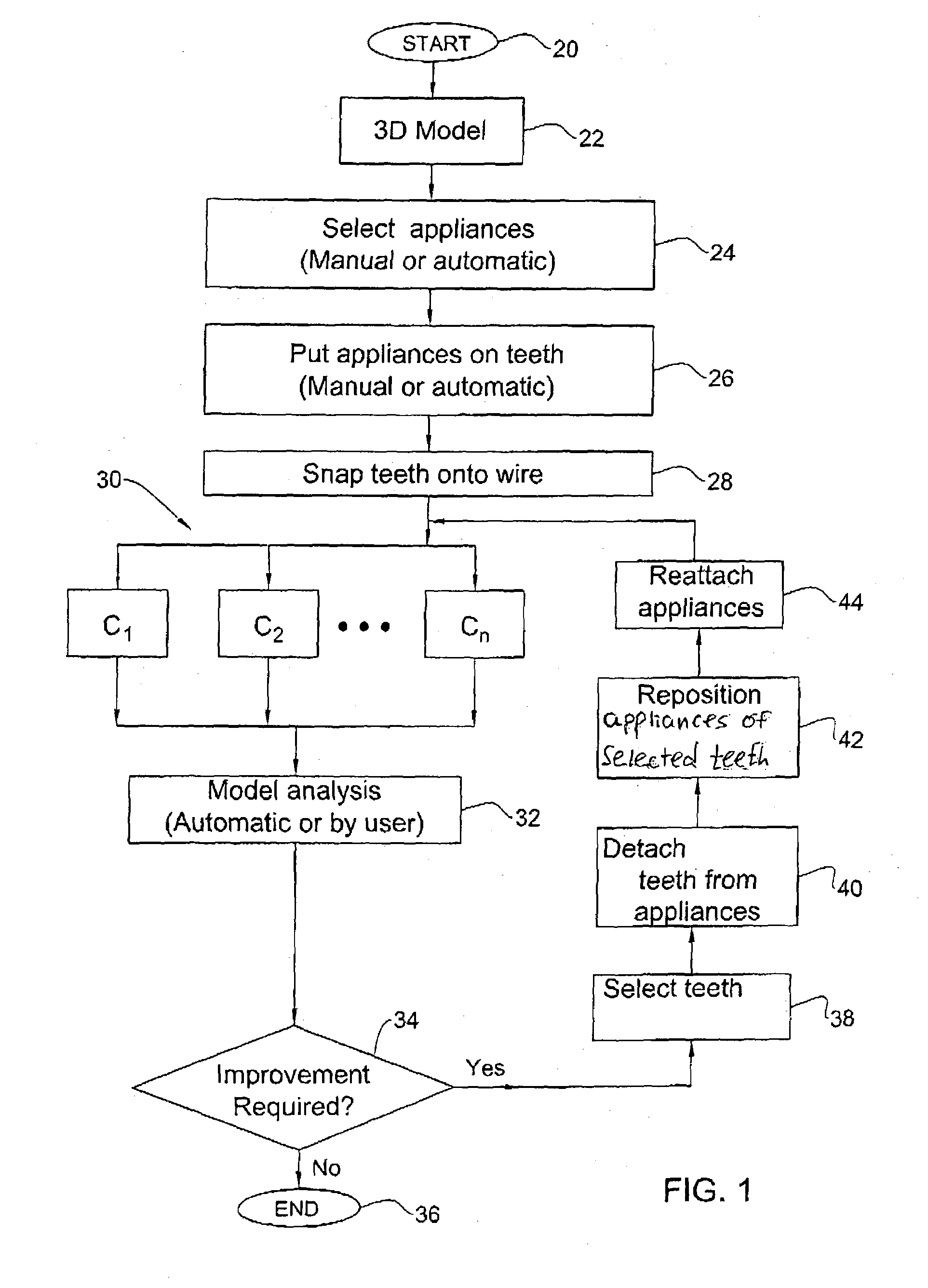
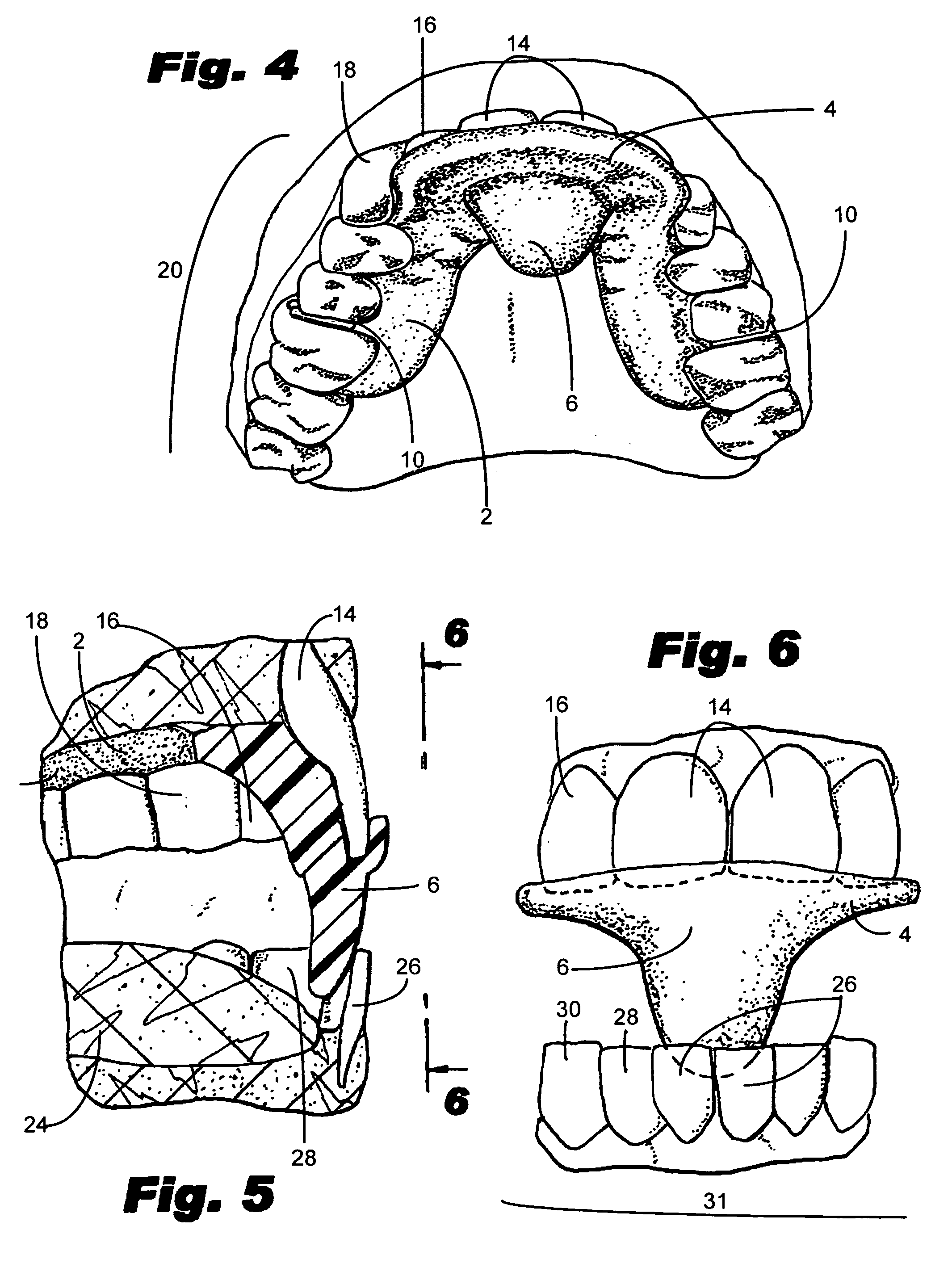
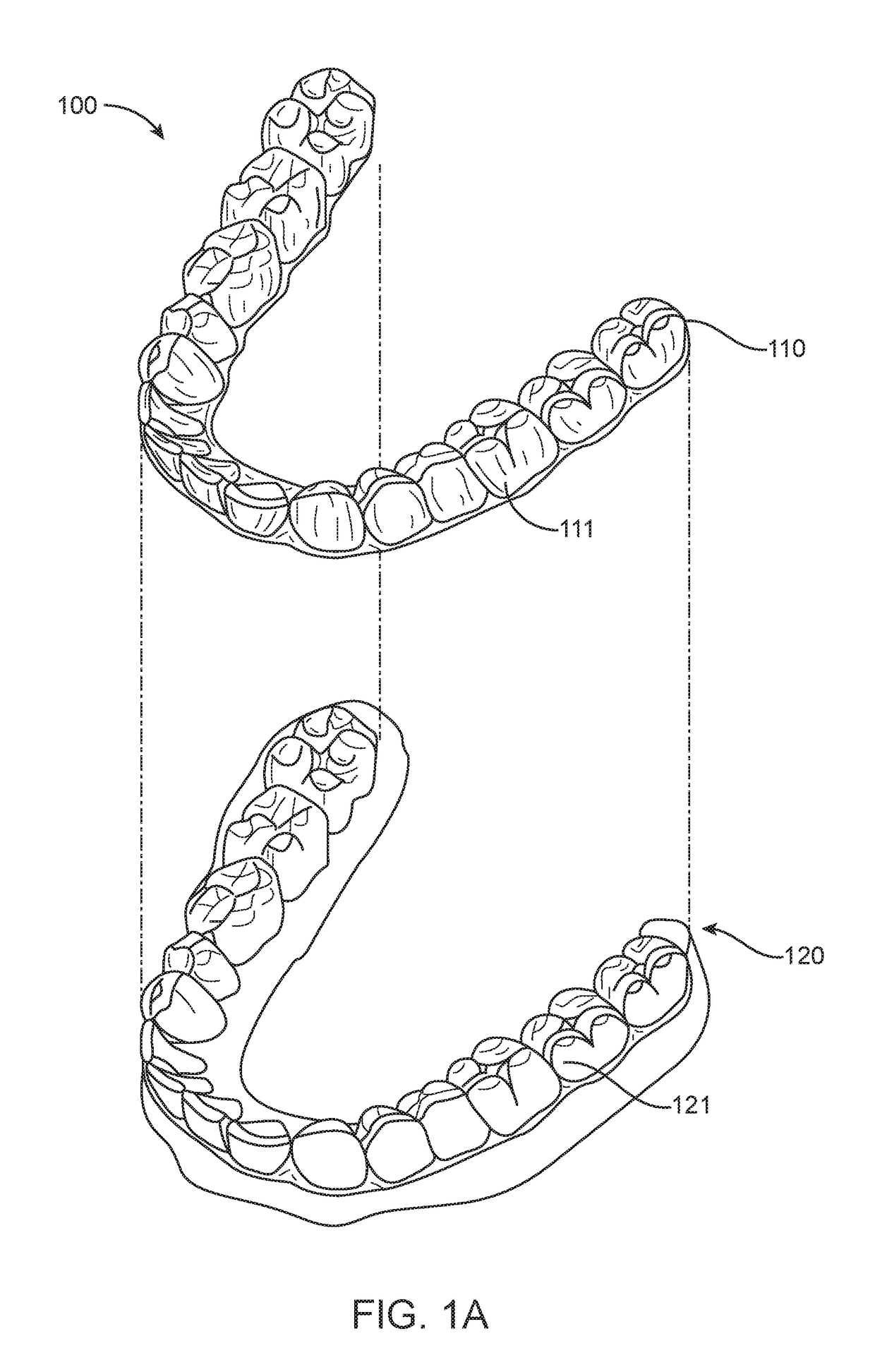
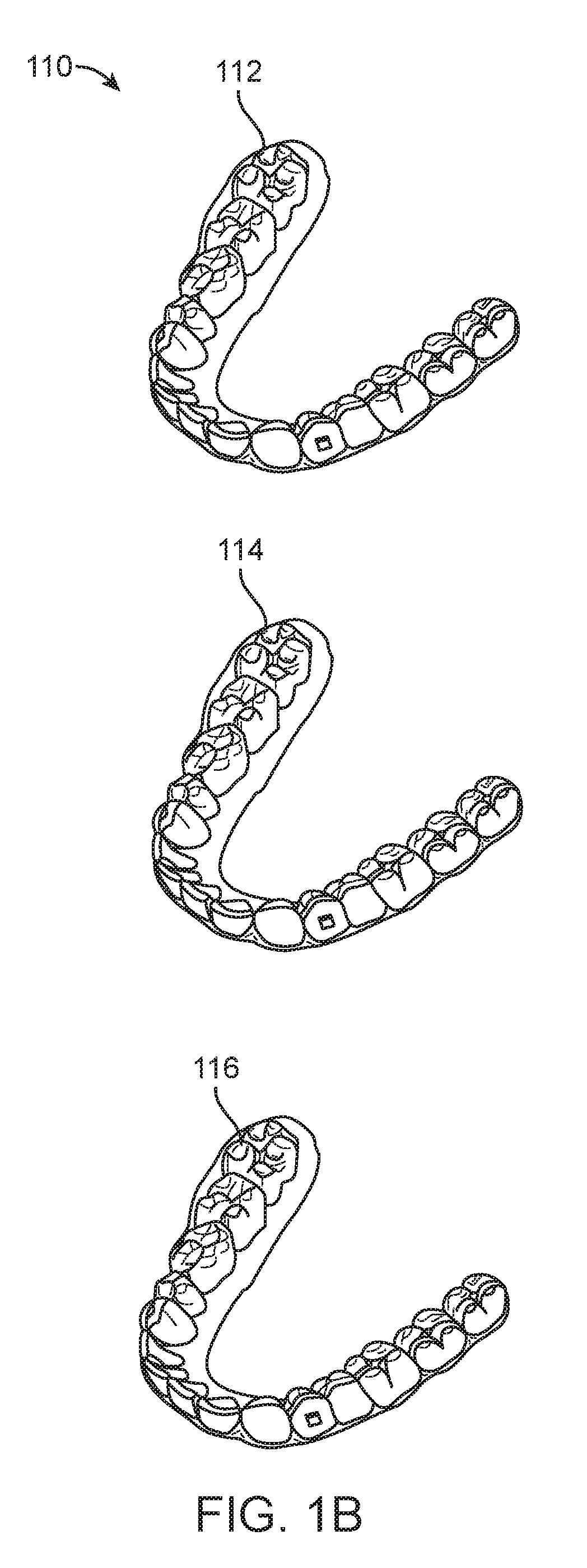
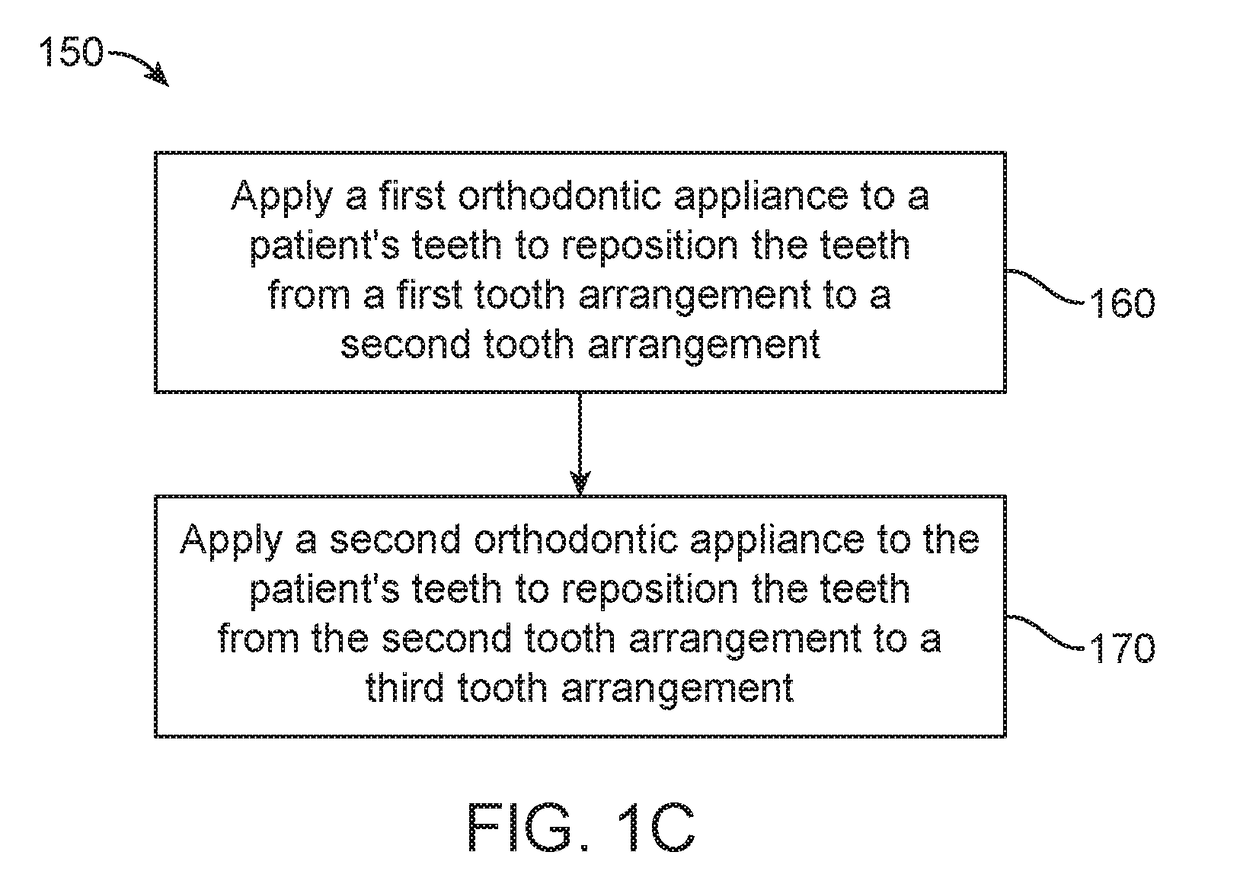
Method and system for assessing the outcome of an orthodontic treatment
The present invention provides a virtual orthodontic treatment method, comprising, providing a virtual diagnostic setup model of teeth of at least one jaw of an individual, associating virtual orthodontic appliances with all teeth in the model to obtain a first composite model and repositioning teeth into an initial treatment state according to pre-defined appliances-dependent rules; in the initial treatment state, detaching one or more teeth from their corresponding one or more orthodontic appliances, repositioning one or more appliances, reassociating one or more appliances with the teeth, permitting the teeth to reposition according to the appliances-dependent rules to obtain an altered treatment state, yielding a better grade, according to one or more systems for grading an orthodontic model, as compared to the grade of initial treatment state.
Owner:ALIGN TECH
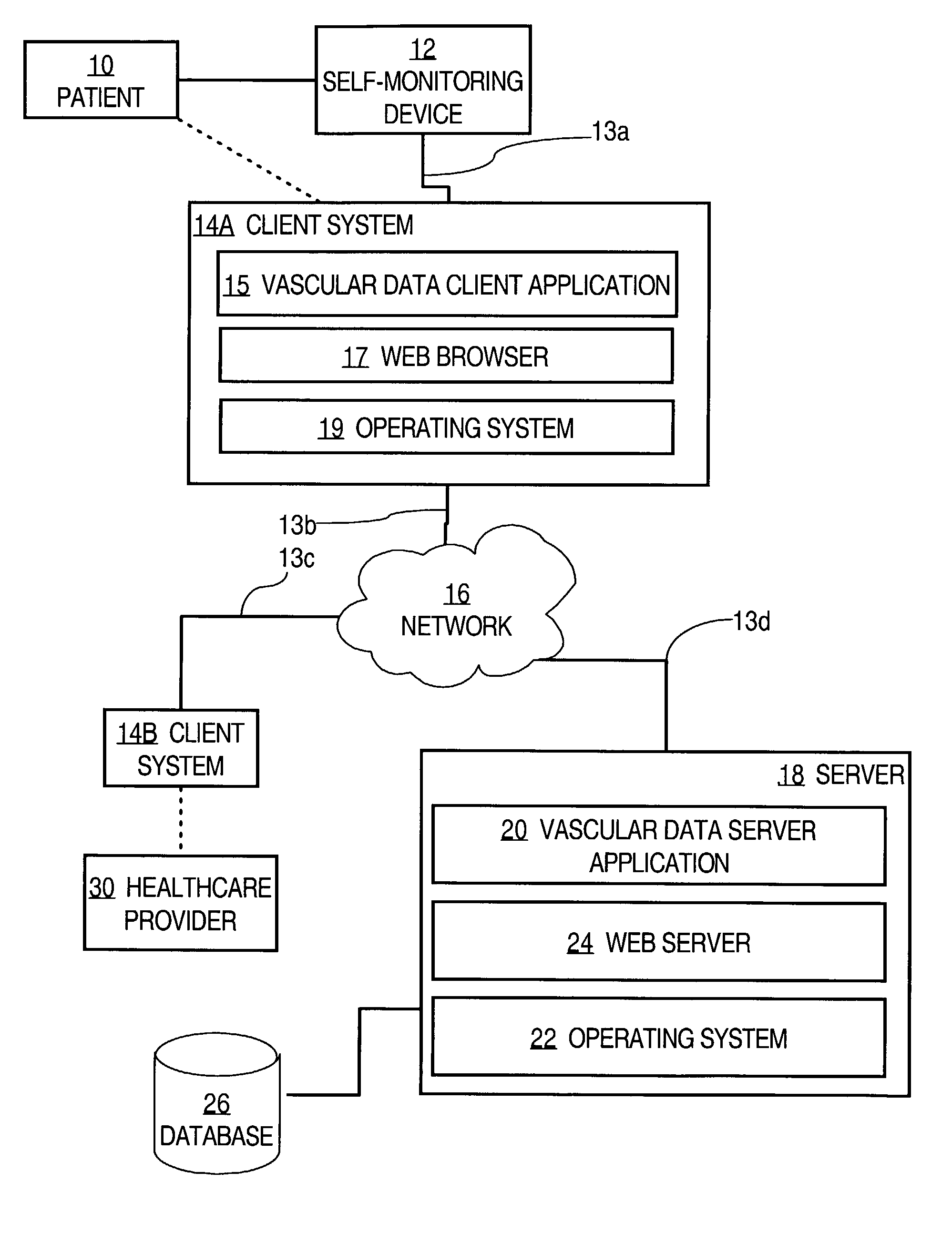
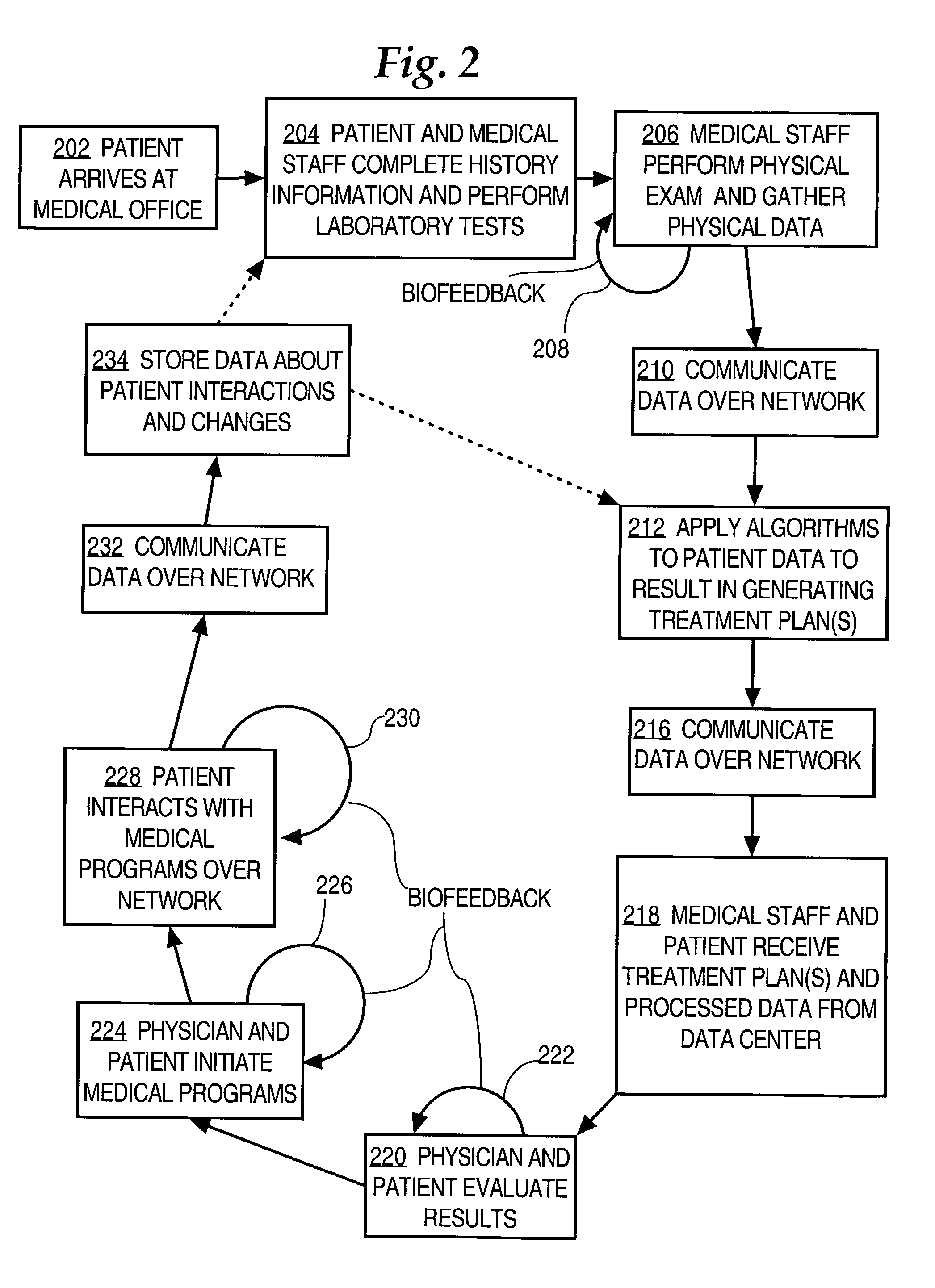
Method and system for improving vascular systems in humans using biofeedback and network data communication
InactiveUS7074183B2Convenience habitImproving lifestyle choicePhysical therapies and activitiesCatheterHuman useInitial treatment
Methods for treating vascular disease in humans and generating data representing treatment plans are disclosed. A first set of clinical vascular health data from a healthcare provider and representing a vascular health condition of a patient is received at a data center server that is communicatively coupled to a public data network. One or more vascular disease analysis algorithms are applied to the first set of vascular health data, to result in creating and storing an initial treatment plan for the patient. A second set of vascular health data is received from a monitoring device that is associated with the patient and that is communicatively coupled to the data network; the second set of data include Doppler monitor data obtained from the peripheral vascular system of the patient. One or more vascular analysis algorithms are applied to result in creating one or more supplementary treatment plans for the patient. At least one of the treatment plans includes a biofeedback interaction. The treatment plans are provided to the patient over the data network. The foregoing steps are iteratively repeated one or more times as determined by the physician and patient, resulting in improved vascular health.
Owner:ALEXANDER FRANCIS CASTELLANOS 2002 TRUST
Compositions and methods for affecting movement of contaminants, bodily fluids or other entities, and/or affecting other physiological conditions
Compositions which self-assemble under physiological conditions are formulated for application to wounds. The formulations include a pharmaceutically acceptable carrier or are provided as part of a medical device or coating. The formulations may also include other therapeutic, prophylactic or diagnostic agents. The formulation can be administered as appropriate for treatment of one or more disorders or conditions. For example, the formulation may be applied to repair an injury or during surgery of the lung, eye or dura, or following an epidural or spinal tap, to stop leakage of blood, interstitial fluid, or cerebrospinal fluid. The formulation may be administered to a burn or ulcer. The formulation may be dispersed in a suture or adhesive for administration at the time of or as released following suturing or gluing of a wound, thereby limiting bleeding, loss of tissue fluids, or other fluids such as those produced by parenchymal tissues such as the liver, pancreas, and gastrointestinal tract. The formulation may be applied to any site of bleeding, in a bandage, gauze, sponge, or other material, for immediate control of bleeding, or released later to control bleeding if the initial treatment such as suturing or pressure is insufficient. In one embodiment, the formulation is provided as a dry or lyophilized powder. In another embodiment, the material is provided in water. In another embodiment, the material is provided in combination with an oil and forms a laminate. In another embodiment, the formulation is provided as a coating on a device, for example a stent or a catheter. The material is also useful to isolate tissue, for preservation of tissue for subsequent transplantation or reattachment, and as a bulking, stabilizing or hydrating agent.
Owner:VERSITECH LTD +1
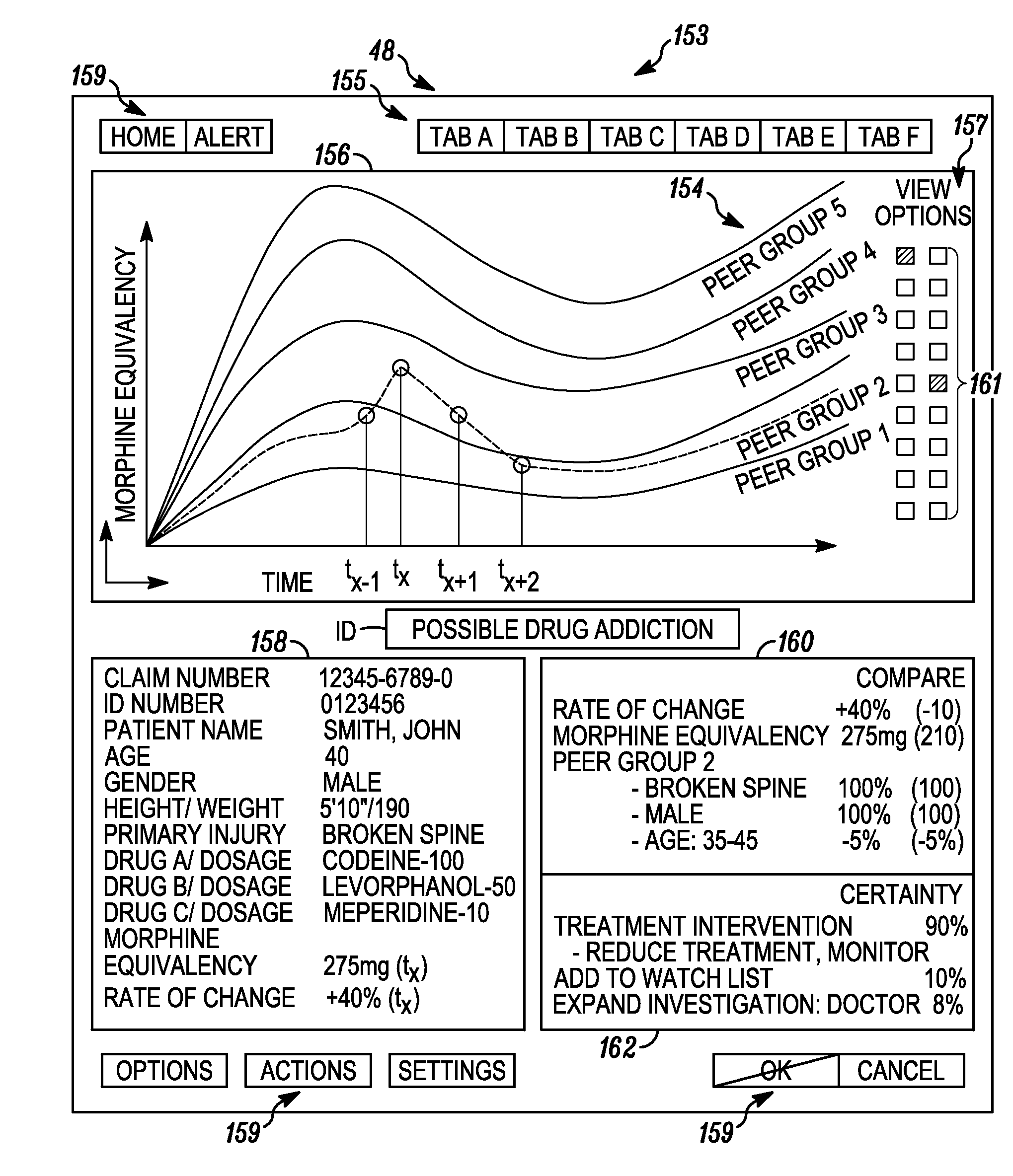
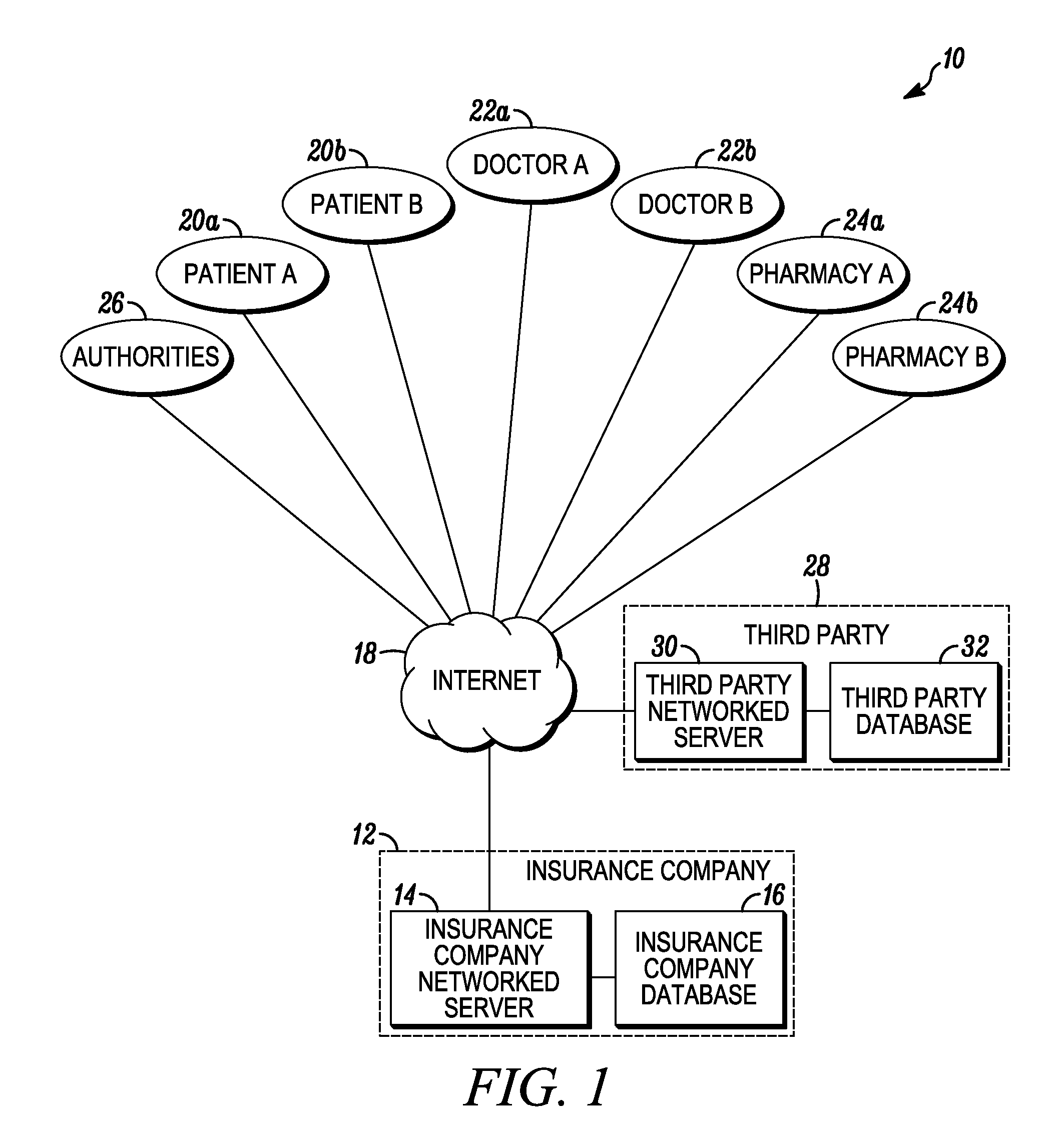
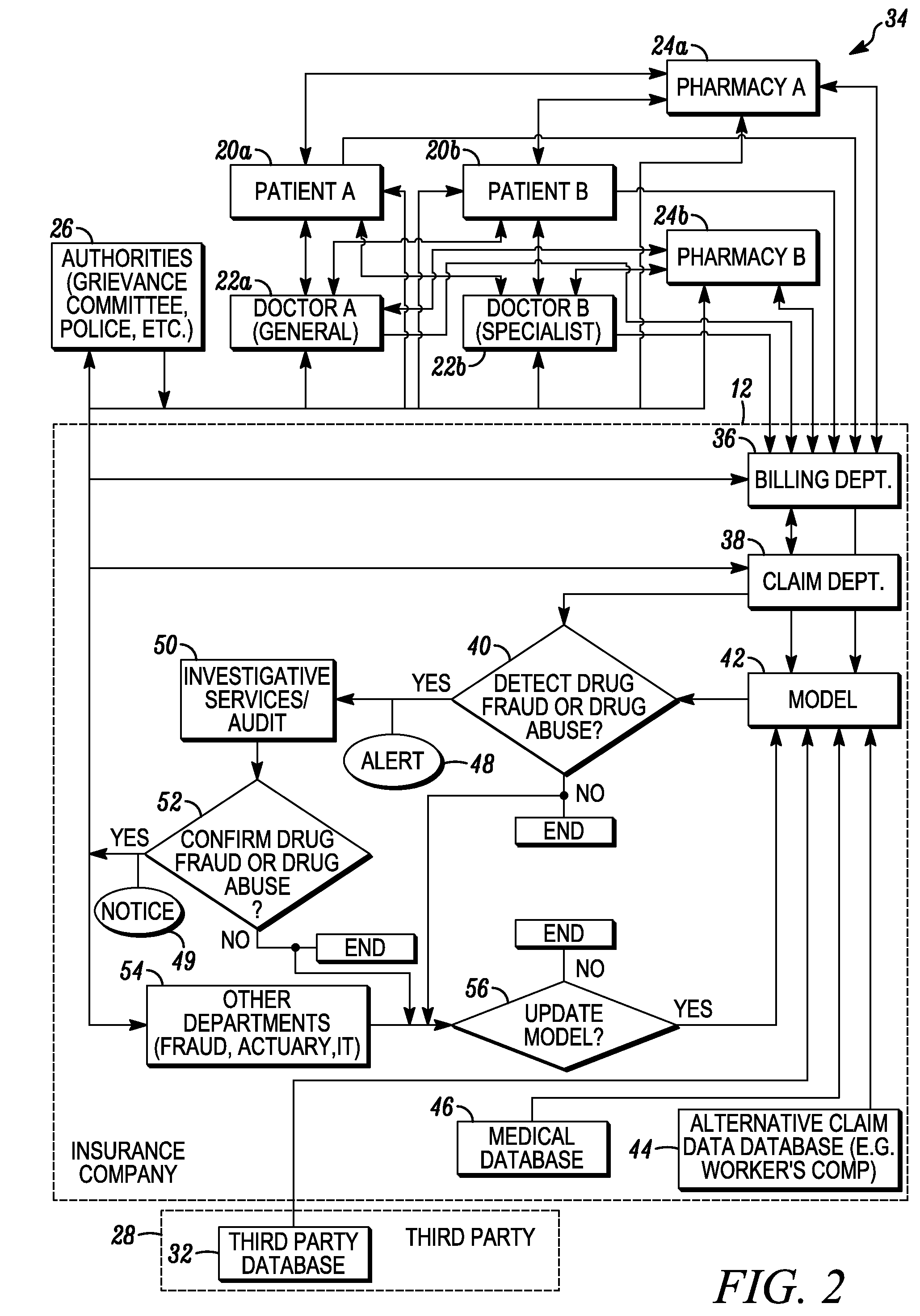
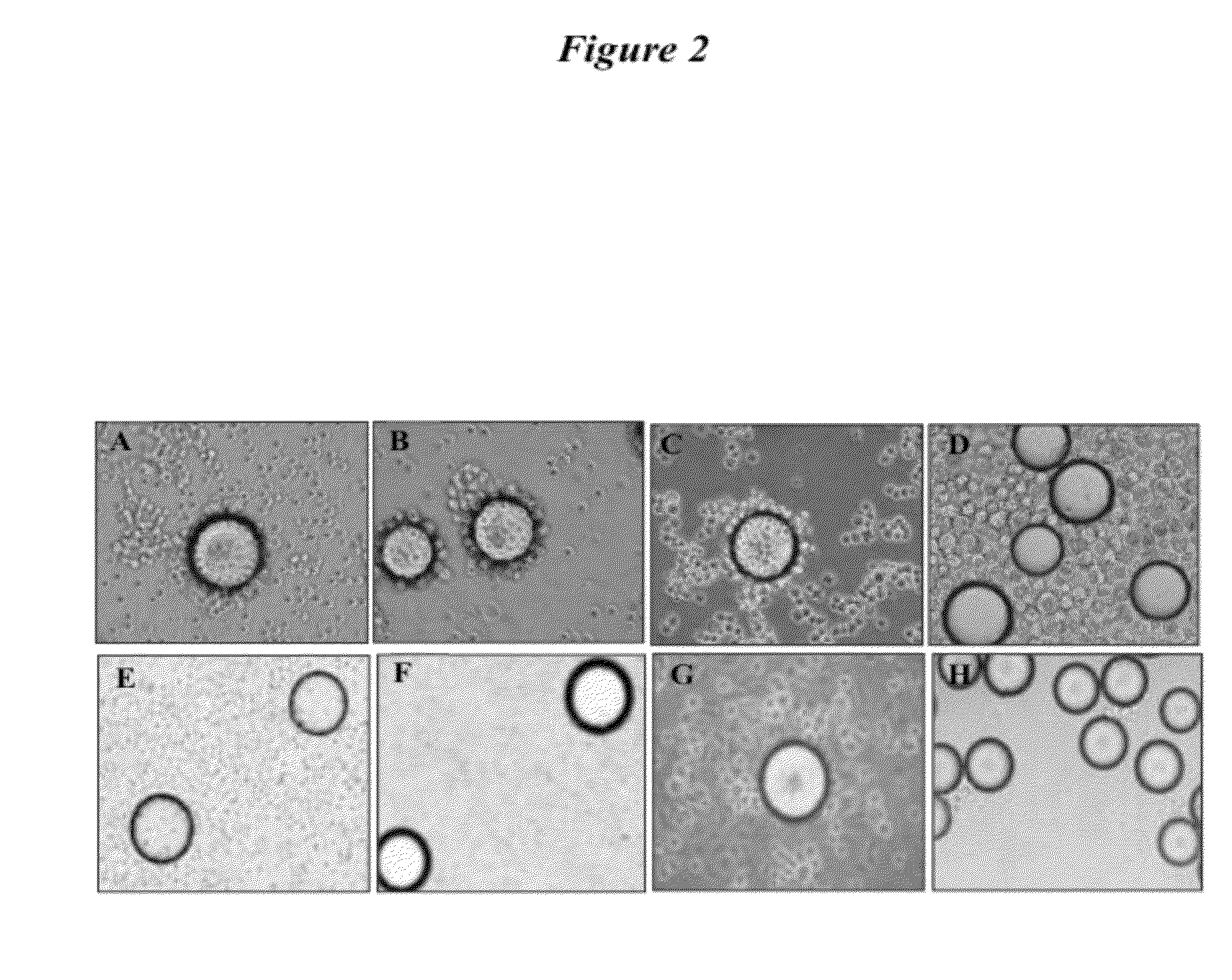
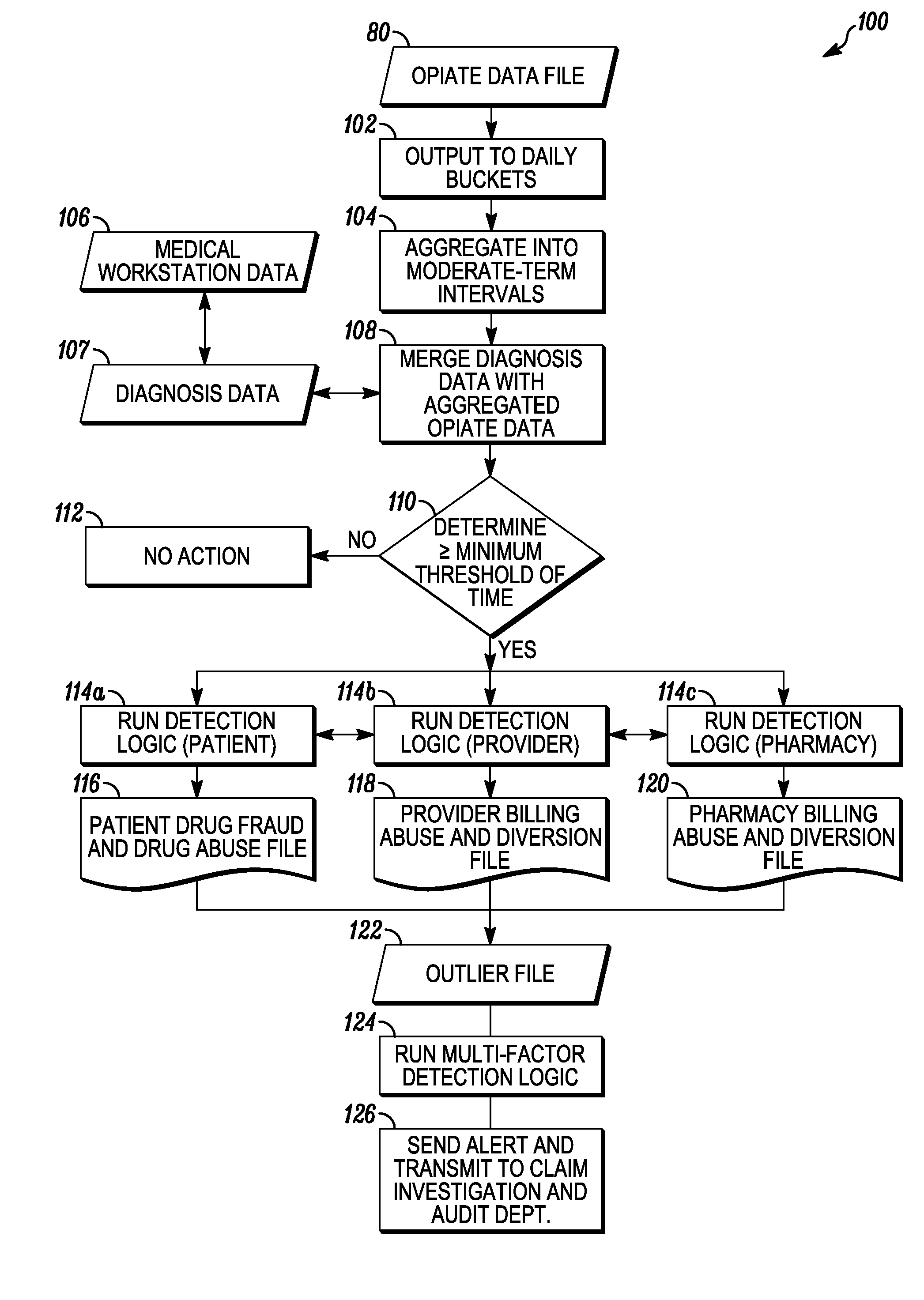
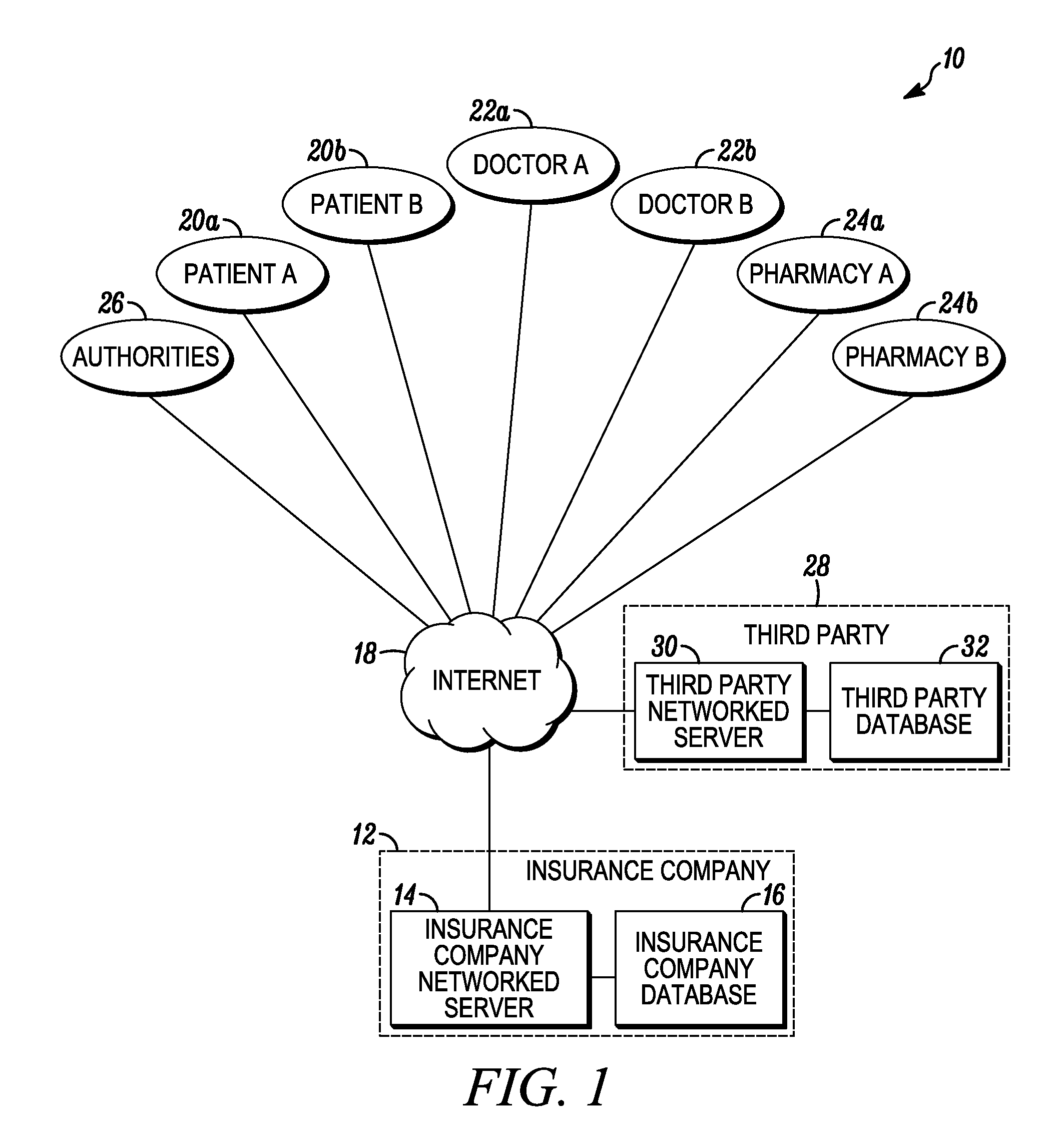
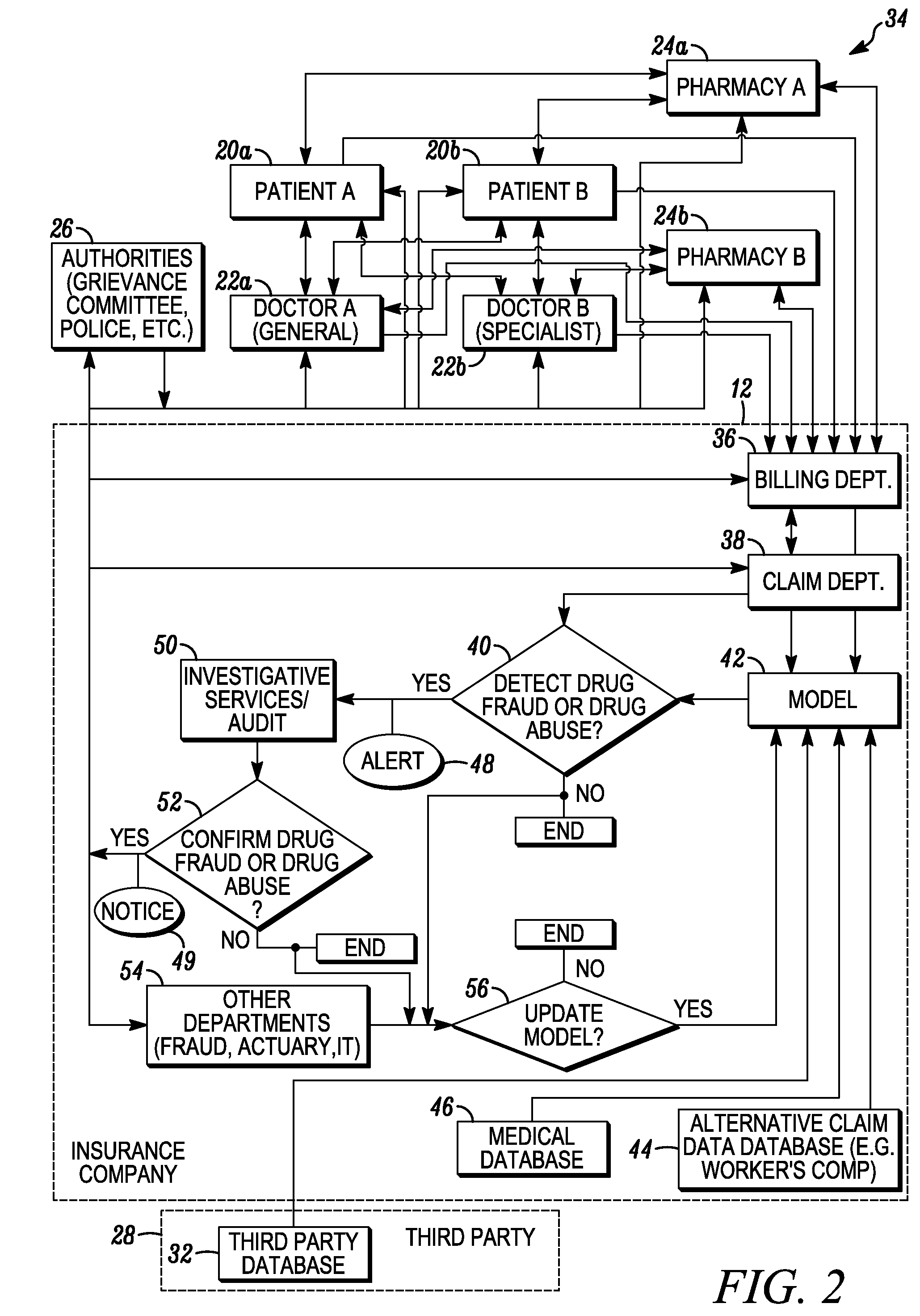
System and method for detecting drug fraud and abuse
A system and method for detecting improper drug use utilizes the morphine equivalency of a drug regimen of a patient having an injury to compare the morphine equivalency of the drug regimen of the patient to that of a peer group having the same diagnosis. The comparison may be conducted based on the aggregate morphine equivalency for respective periods of time following the injury or the initial treatment and based on the rate of change in the morphine equivalency for that or another period of time. Based on the results of the comparison, potential instances of drug fraud and / or drug abuse are identified and an alert is generated, and appropriate action is taken, where needed.
Owner:THE TRAVELERS INDEMNITY +1
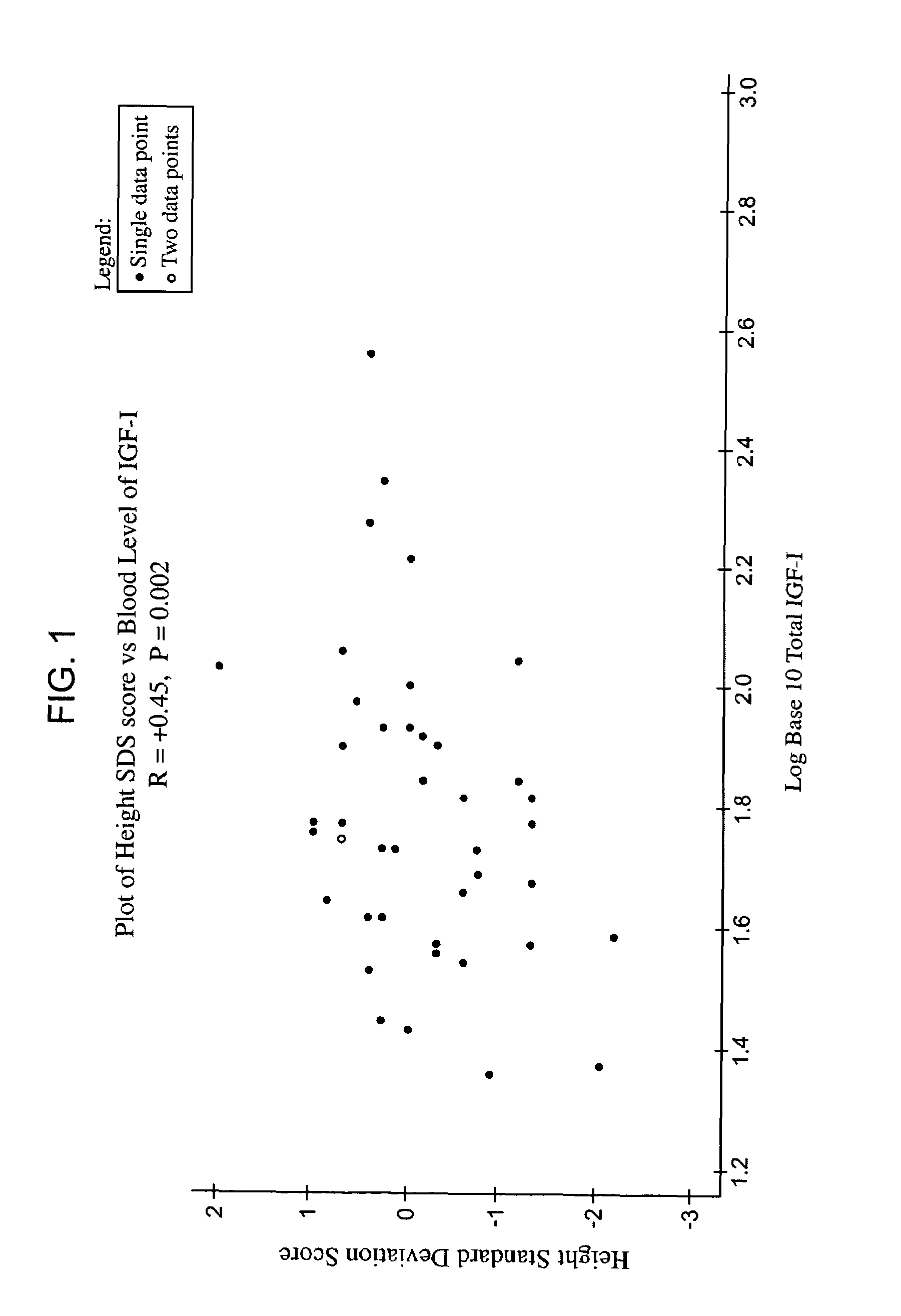
Methods for treatment of insulin-like growth factor-1 (IGF-1) deficiency
ActiveUS7258864B2To promote metabolismIncrease probabilityPeptide/protein ingredientsPeptide preparation methodsInitial treatmentBlood level
Owner:IPSEN BIOPHARMACEUTICALS INC
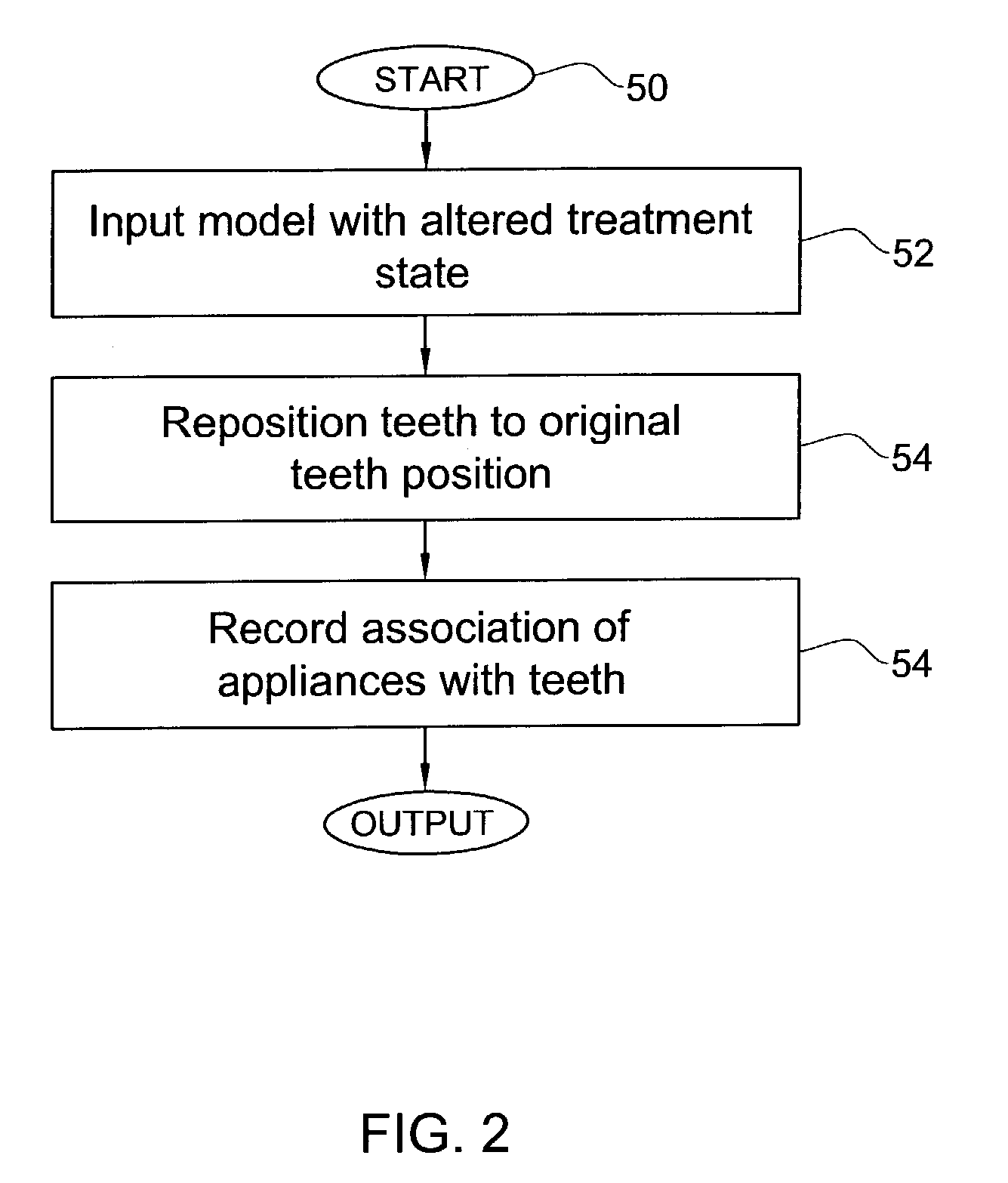
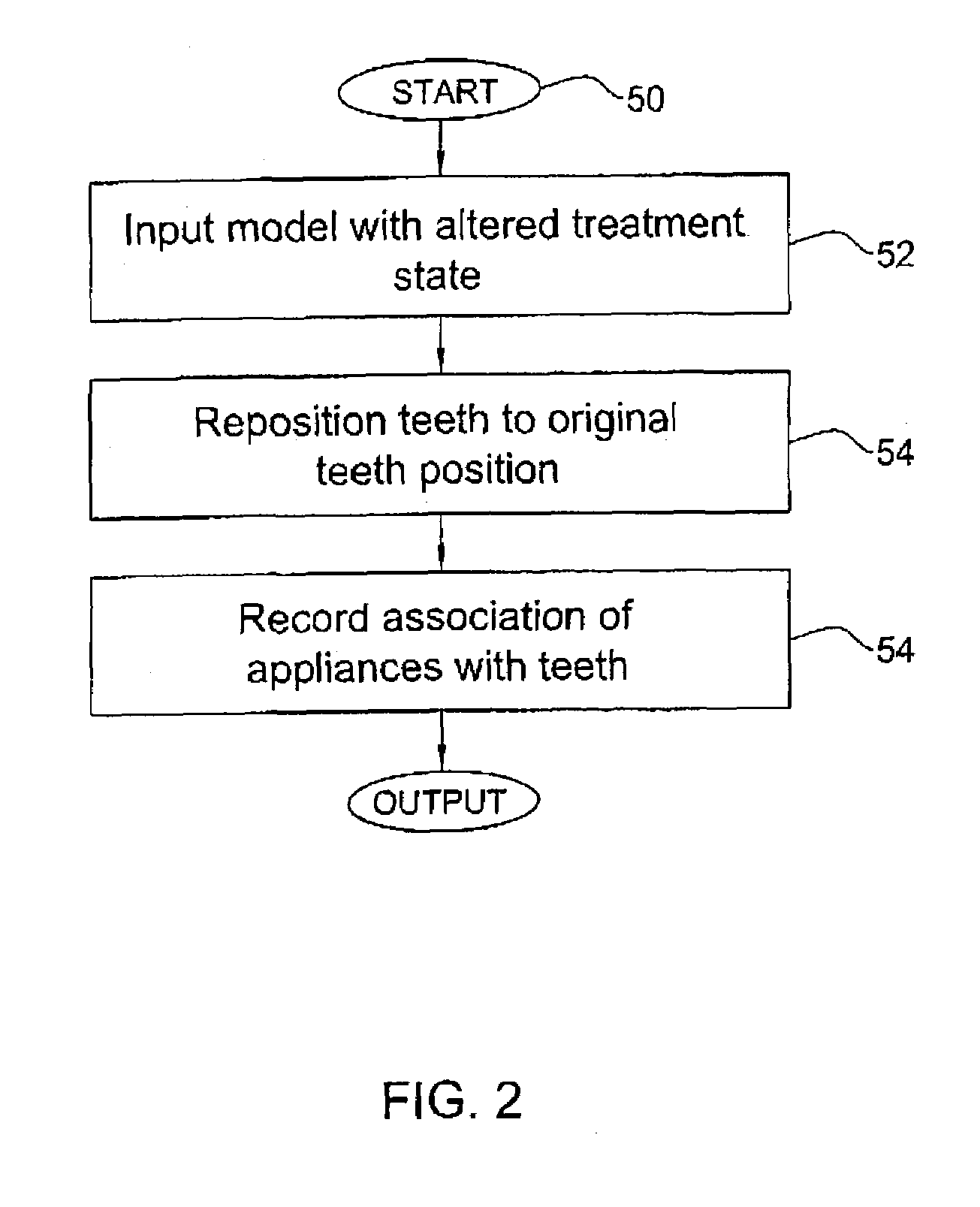
Method and system for assessing the outcome of an orthodontic treatment
A virtual orthodontic treatment method and a system thereof, the method comprising the following: providing a virtual diagnostic setup model of teeth of at least one jaw of an individual, associating each teeth in said model with its corresponding virtual orthodontic appliance to obtain a first composite model and repositioning teeth into an initial treatment state according to pre-defined appliances-dependent rules. In the initial treatment state, selecting one or more teeth and reassociating the teeth with their corresponding virtual orthodontic appliance by changing the position of the appliance corresponding to the selected tooth thereby causing repositioning of the teeth according to the appliance-dependence rules. An altered treatment state is obtained, yielding a better grade, according to one or more systems for grading an orthodontic model, as compared to the grade of the initial treatment state.
Owner:ALIGN TECH
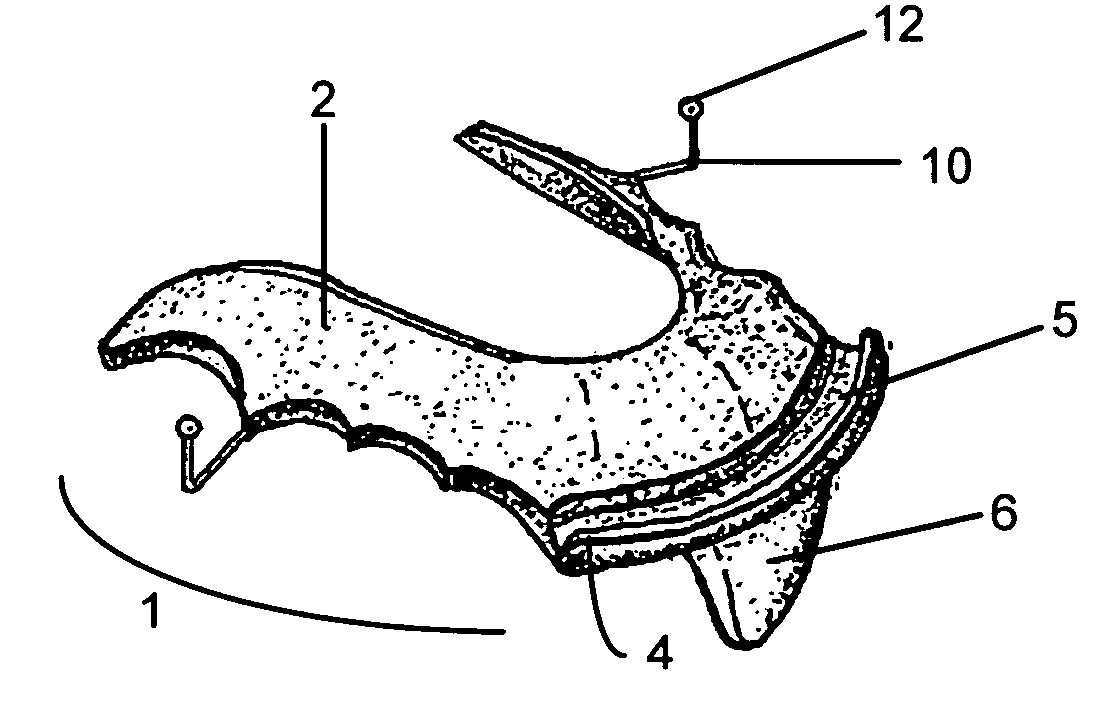
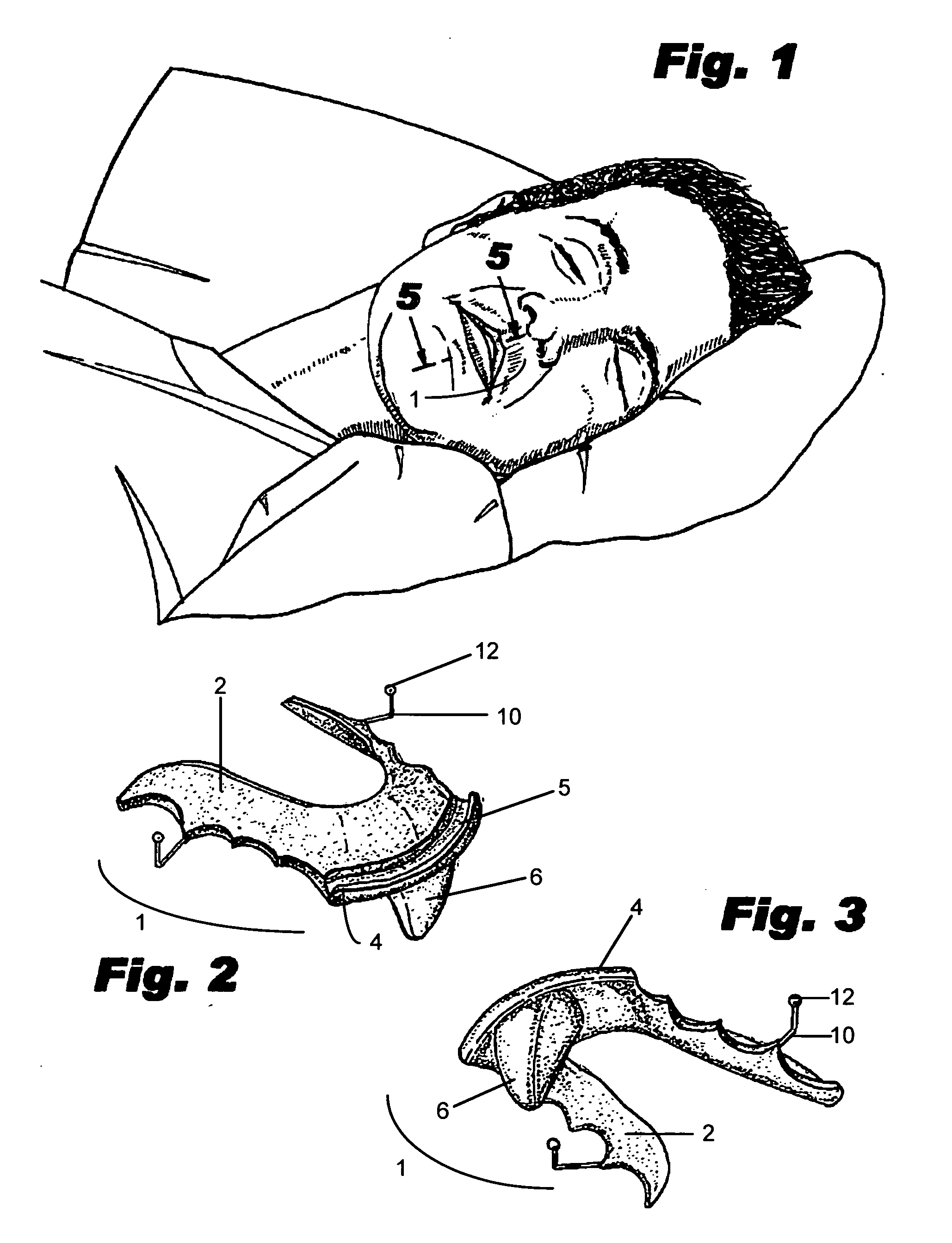
Intraoral mandibular advancement device for treatment of sleep disorders, including snoring, obstructive sleep apnea, and gastroesophageal reflux disease and method for delivering the same
InactiveUS20070079833A1Eliminates and reduces disadvantageReduce congestionSnoring preventionNon-surgical orthopedic devicesInitial treatmentTeeth clenching
An intraoral mandibular advancement device to treat problems associated with sleep disorders in a user having an obstructed airway, the disorders including, without limitation, snoring, obstructive sleep apnea, gastroesophageal reflux disease and / or bruxism having a main body for attachment to the user's mouth and having a central portion; a protrusive element distending from the central portion of the main body such that when worn by the user the element causes mandibular advancement sufficient to expand the oropharangeal space and reduce the obstruction; and a retainer extending from the main body for retention of the device in the user's mouth when worn during the user's sleep state. The main body is either complimentary with the user's palate and lingual surfaces of the maxillary teeth, rests against the palate and lingual surface of the user's anterior mandibular teeth when the device is inserted in the user's mouth, or customized to rest against two, four or six of the user's front teeth. The protrusive element is of distension between 5 and 15 mm, and optimally 10 mm downwardly from the main body, and can be adjusted such that the advancement ranges between 1.0 and 7.0 mm, and having an initial treatment protrusion of 3.0 to 4.0 mm, or ½ of the total potential maximum protrusive range. A method for diagnosis and delivery of the device is also shown.
Owner:LAMBERG STEVEN B
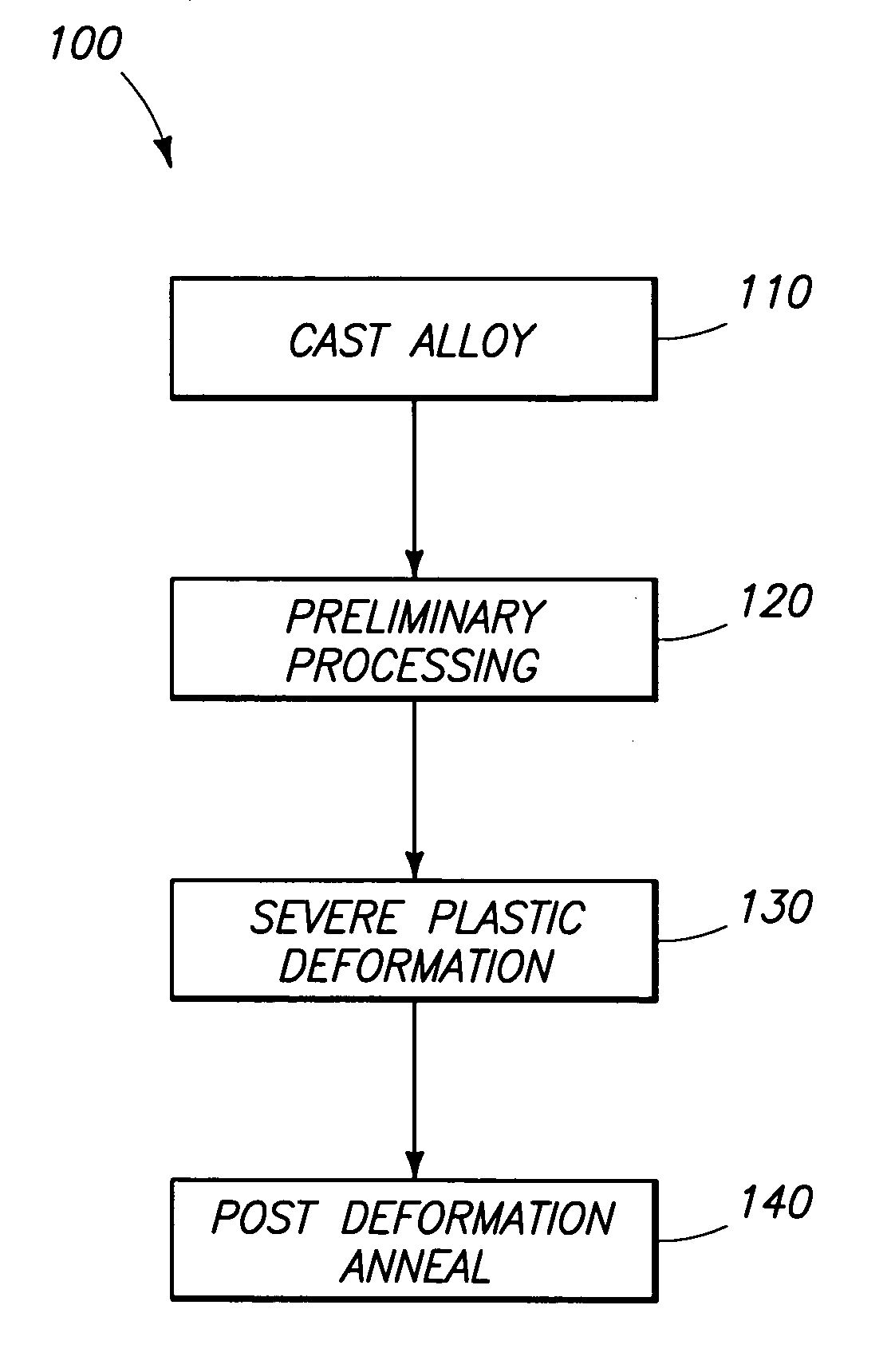
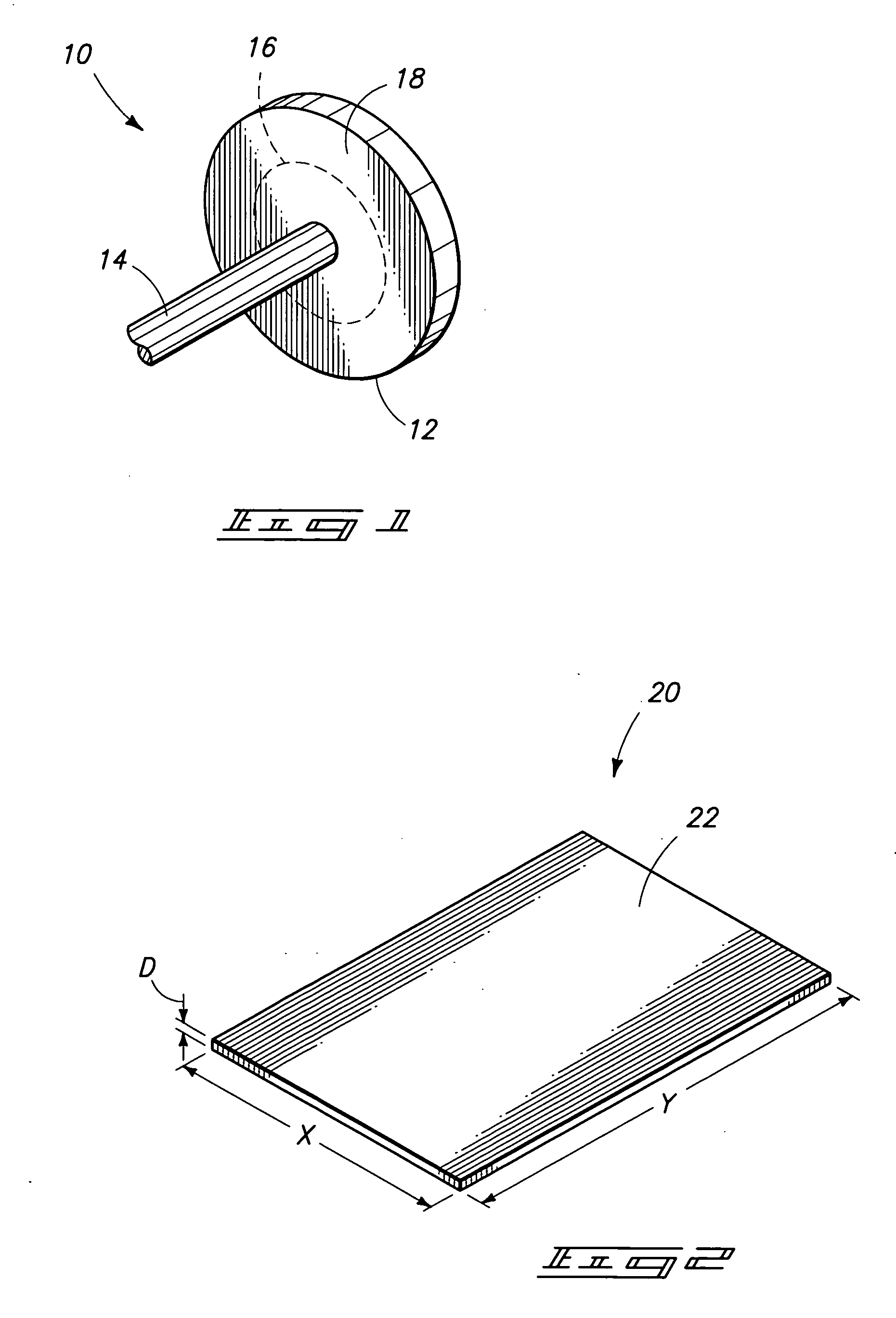
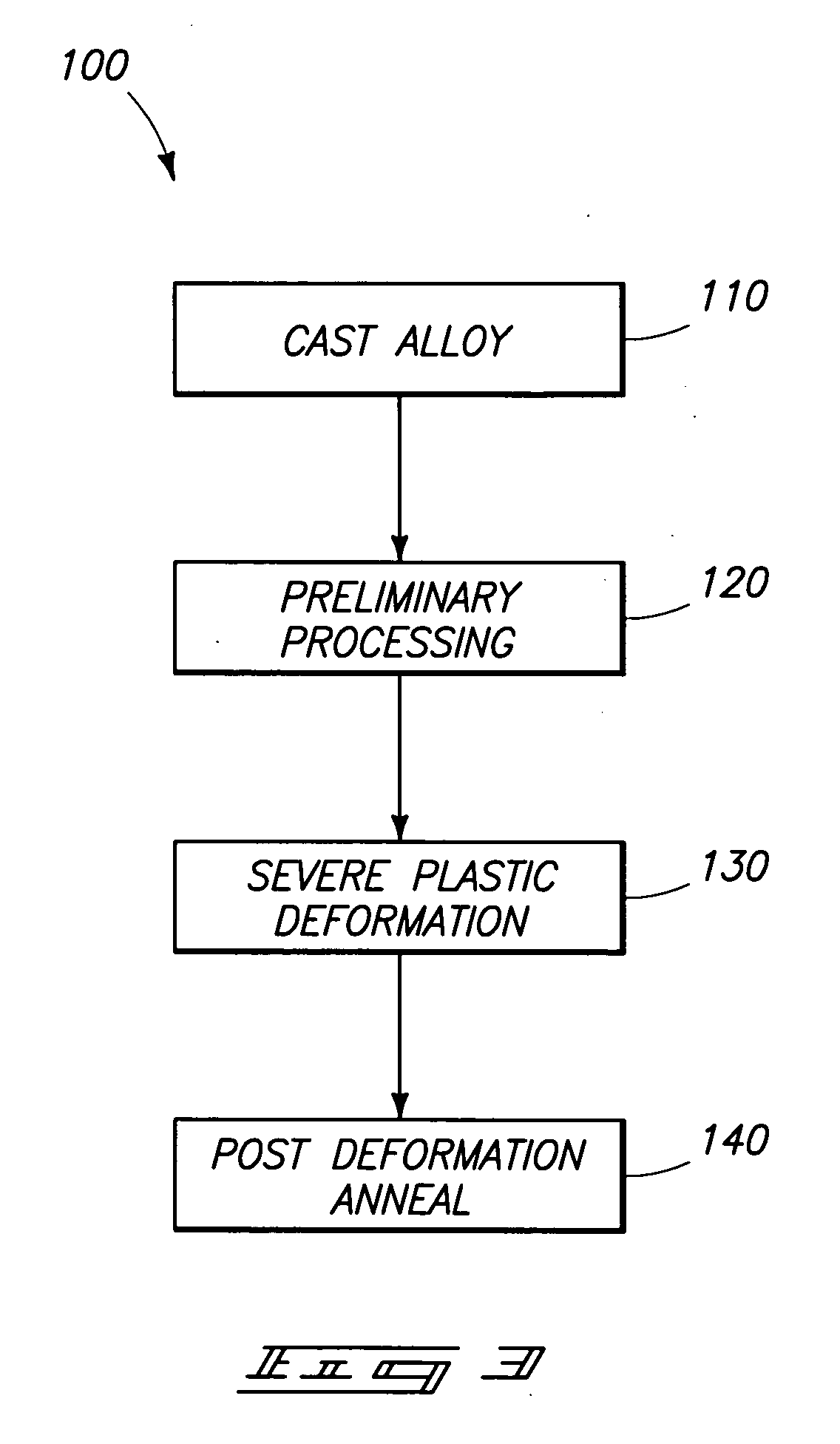
High-strength mechanical and structural components, and methods of making high-strength components
The invention includes components comprising an alloy containing a base metal and less than or equal to 30% alloying elements. The material has a grain size of less than or equal to about 30 microns and an absence of voids and inclusions of a size greater than 1 micron. The components have a yield strength at least 50% greater than the identical alloy composition in the 0 temper condition. Where the material is heat treatable, the yield strength is at least 10% greater than the identical composition in the T6 temper condition. The invention includes a method of producing components by casting and initial treatment to form a billet. The billet is subjected to equal channel angular extrusion and subsequent annealing at a temperature of less than or equal to 0.85 times the minimum temperature for inducing growth of submicron grains to over 1 micron.
Owner:HONEYWELL INT INC
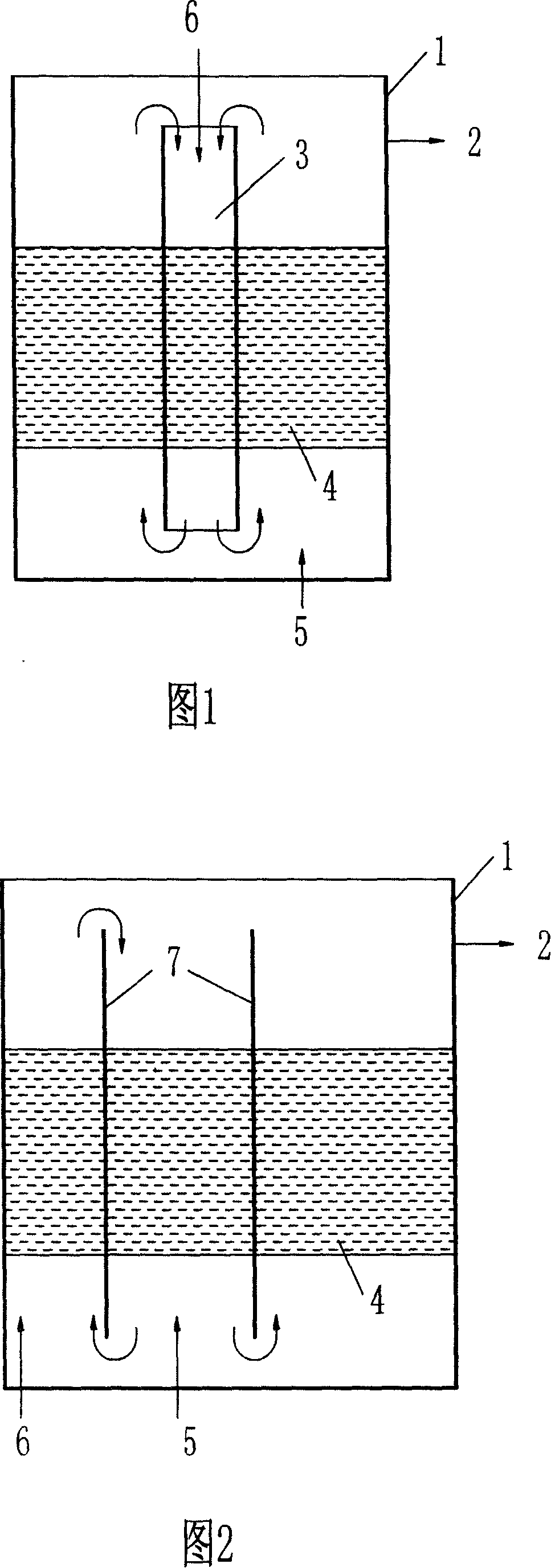
Microorganism advanced treatment for oil refining waste water
ActiveCN101148293AAvoid pollutionStable waterTreatment using aerobic processesBacteriaInitial treatmentBacillus megaterium
The present invention relates to deep treating process of refinery waste water after initial treatment. Refinery waste water is deeply treated biologically with microbial flora comprising Pseudomonas sp., Zoogloea sp., Plesiomonas sp., Bacillus, Bacillus megaterium and Rhodopseudomonas globiformis in a biological aeration filtering pond and / or a bioreaction pond while controlling the intake water pH value in 6-9. The deep treating process has simple path, high safety, low power consumption, low running cost and other advantages, and the water output can reach the reuse standard stably including CODcr not more than 60 mg / l, oil not more than 2 mg / l, NH3-N not more than 2 mg / l and SS not more than 10 mg / l.
Owner:JIANGSU BODA ENVIRONMENTAL PROTECTION
Systems, methods, and apparatus for correcting malocclusions of teeth
ActiveUS20190053876A1Additive manufacturing apparatusOthrodonticsInitial treatmentDental malocclusion
Methods and systems are provided for manufacturing an appliance for correcting malocclusions of a patient's teeth. The method may include measuring the positions of a patient's teeth and receiving tooth movement constraints. The method may also include generating an initial treatment plan based on the measured tooth positions and the tooth movement constraints and measuring the malocclusions of the patient's teeth for one or more types of dental malocclusions. The method may also include generating a plurality of treatment plans from the initial treatment plan based on the measured malocclusion and generating a model of an appliance for each stage of the treatment plan. The method may also include generating instructions for fabricating the appliance for each stage of the treatment plan based on the model of the appliance for each stage of the treatment plan.
Owner:ALIGN TECH
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
ActiveUS9603860B2Inhibit ovulationPowder deliveryOrganic active ingredientsInitial treatmentPharmaceutical drug
A pharmaceutical composition comprising an active contraceptive drug and one or more pharmaceutically-acceptable excipients. The pharmaceutical composition, when subjected to an in vitro dissolution test according to the USP XXIII Paddle Method, results in no more than 50% of said active drug initially present being dissolved within 30 minutes, and at least 50% of the active drug being dissolved in a time range from about 3 hours to about 4 hours. The pharmaceutical composition is administered daily to a patient having a BMI of about 25 kg / m2 or more for at least a portion of a treatment cycle. The pharmaceutical composition does not cause a number of days of bleeding events in the patient exceeding an average of 15% per treatment cycle in consecutive treatment cycles of administration after an initial treatment cycle of administration.
Owner:LAB LEON FARMA
Method for preparing ultra-metallurgical grade silicon
The invention provides a method for preparing ultra-metallurgical grade silicon, which comprises the following steps: initial treatment of a silicon source; acid leaching purification; acid cleaning purification; ultrasonic heating purification; solid-liquid separation; and sieve classification, wherein the acid leaching purification comprises the combined use of mechanical activation and acid cleaning. The method for preparing the ultra-metallurgical grade silicon has the advantages of simple process flow, less environmental pollution, low cost and easy large-scale production.
Owner:BYD CO LTD
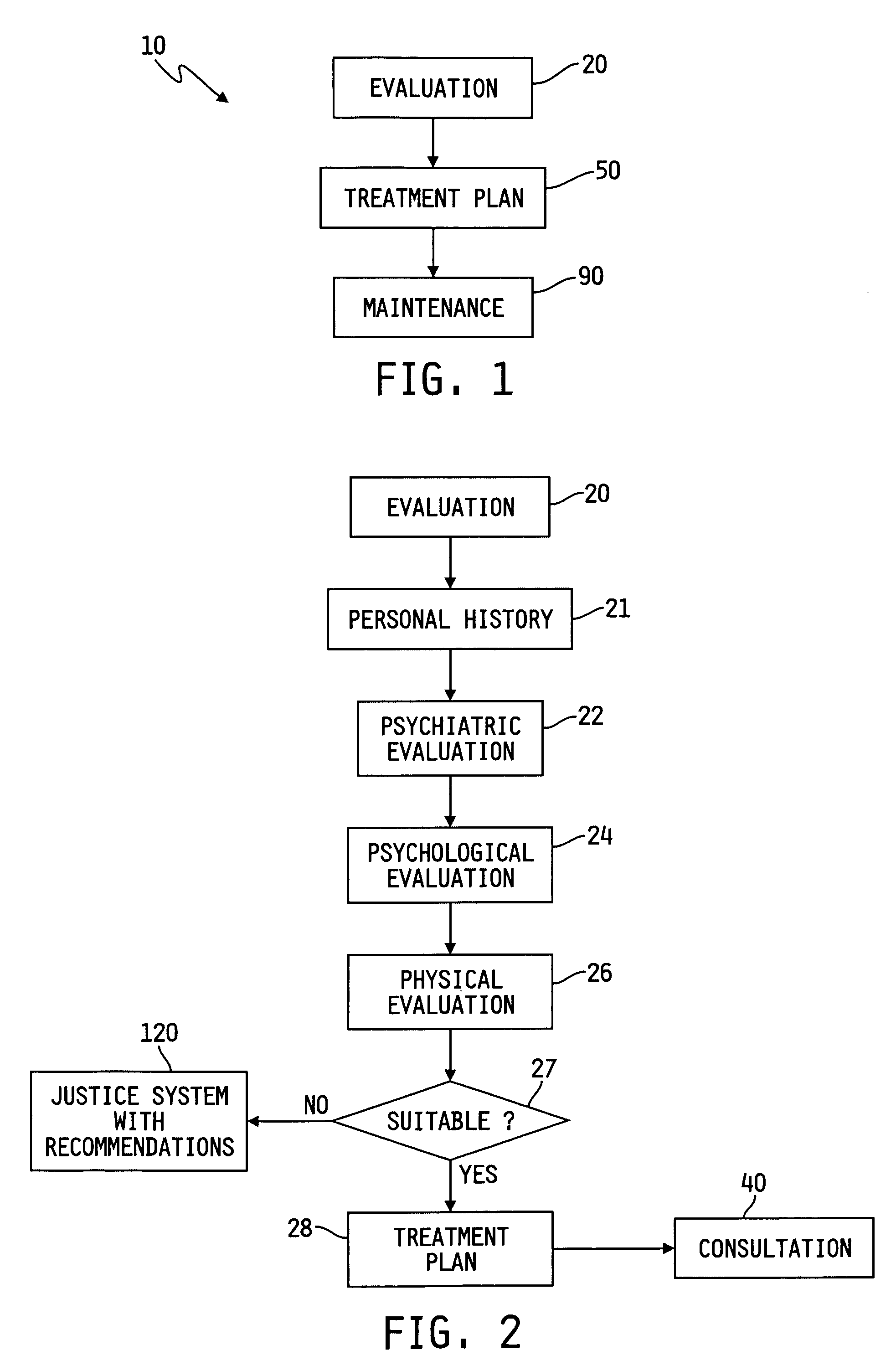
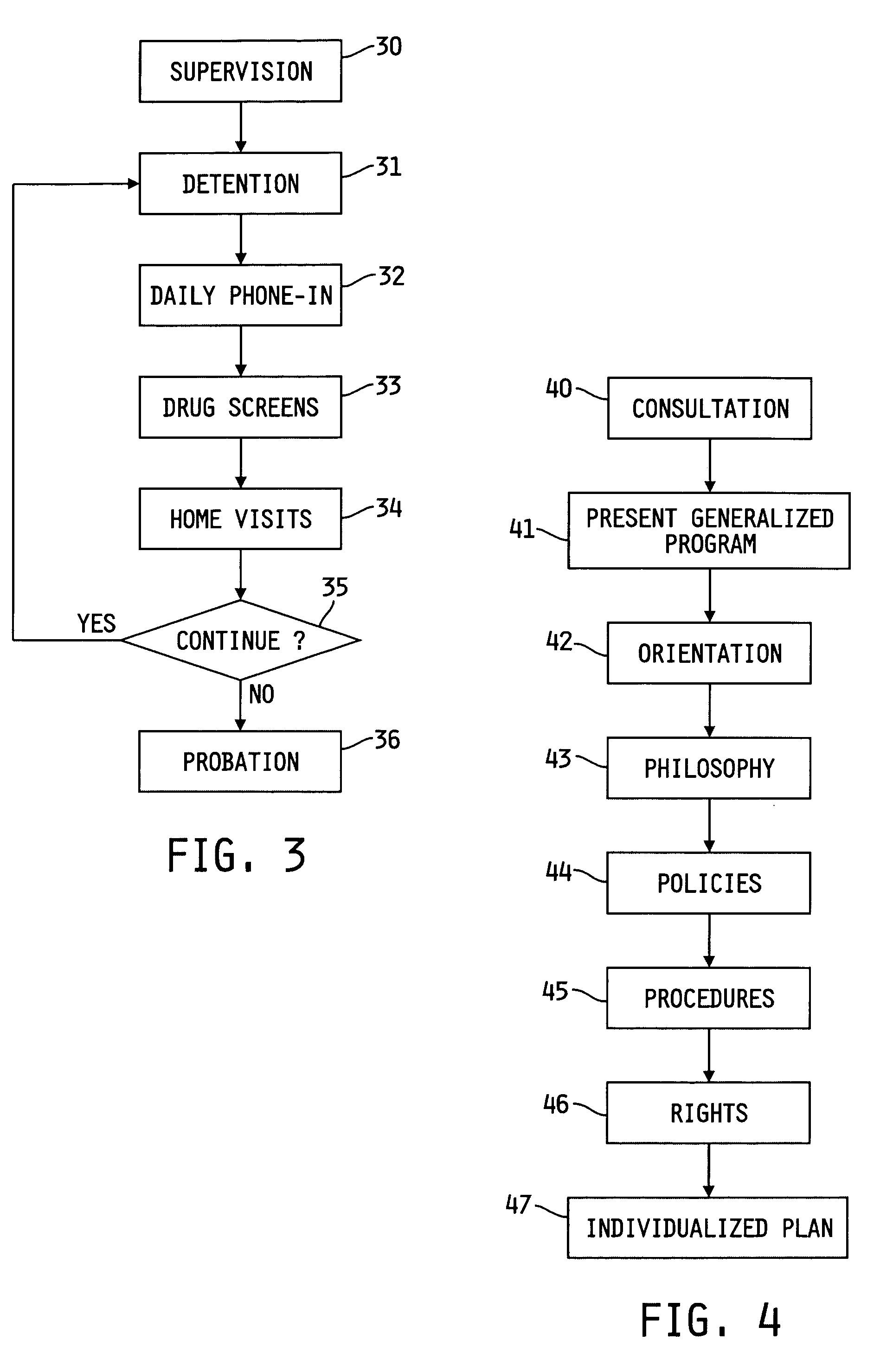
Method of treating dependencies
InactiveUS20060167723A1Data processing applicationsMental therapiesInitial treatmentRelapse prevention
A method and apparatus of treating an addiction is provided. The method includes evaluating the individual to determine the individual's needs. An individual admitted to the program undergoes a supervised treatment protocol that may include education and initial treatment, group therapy, and relapse prevention. There may also be a maintenance protocol following completion of the treatment protocol. In the case where the individual has been arrested for a crime, the supervision may include detention and probation aided by accountability devices.
Owner:BERG L MARTIN
Interactive dental restorative network
InactiveUS20050003329A1Save effortShorten the timeOptical radiation measurementTeeth fillingInitial treatmentDental laboratory
The invention relates to a computer readable medium that includes one or more programs for carrying out a method for restoration of a patient's tooth. The method includes the steps of generating an electronic image of a patient's tooth; providing a preliminary treatment plan for addressing the dental needs of the patient; and forwarding the electronic image and preliminary treatment plan to a dental laboratory so that technician can evaluate the image and treatment plan and in a manner such that the technician and dentist can review and discuss the preliminary treatment plan.
Owner:LEHMANN MARYANN
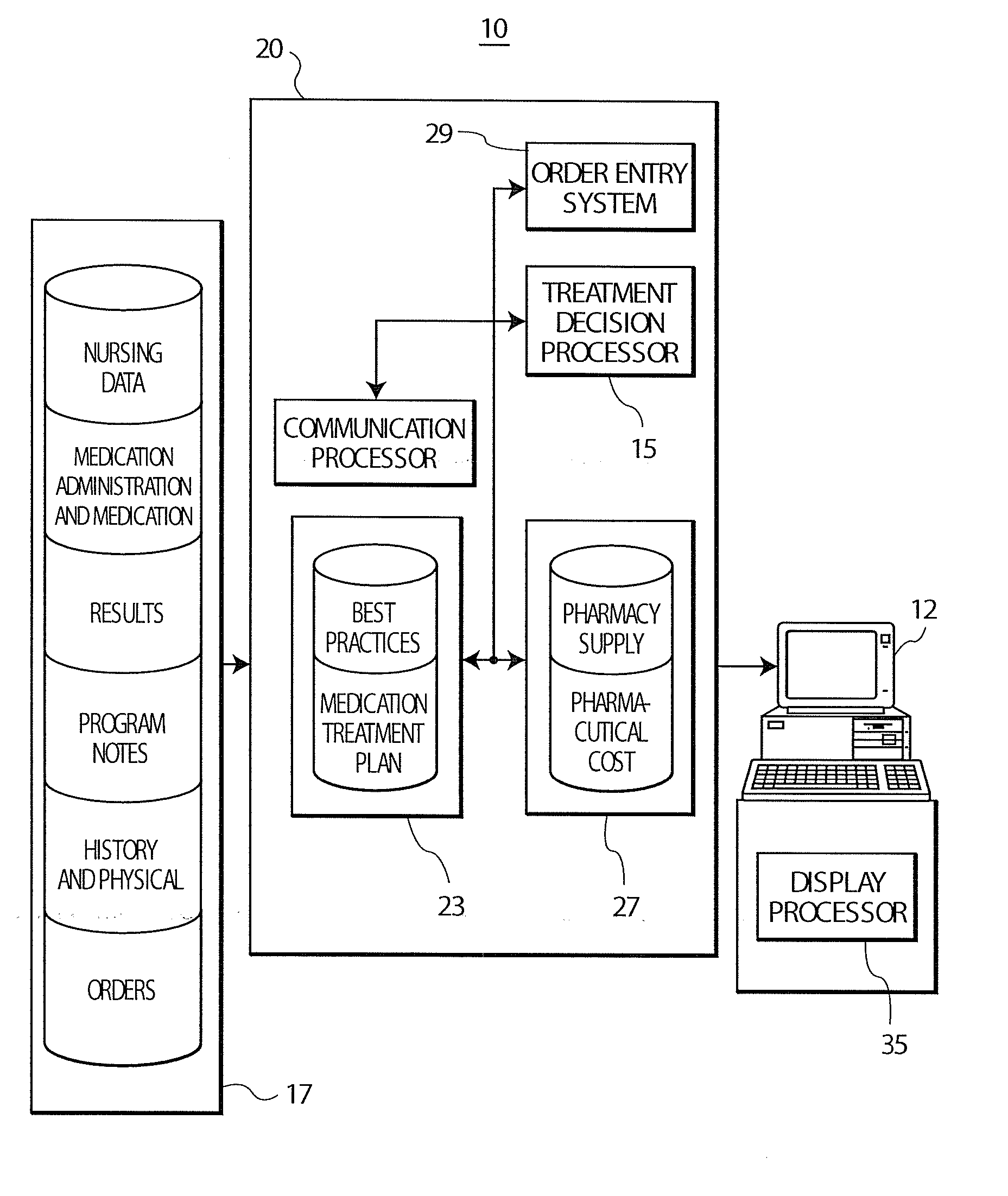
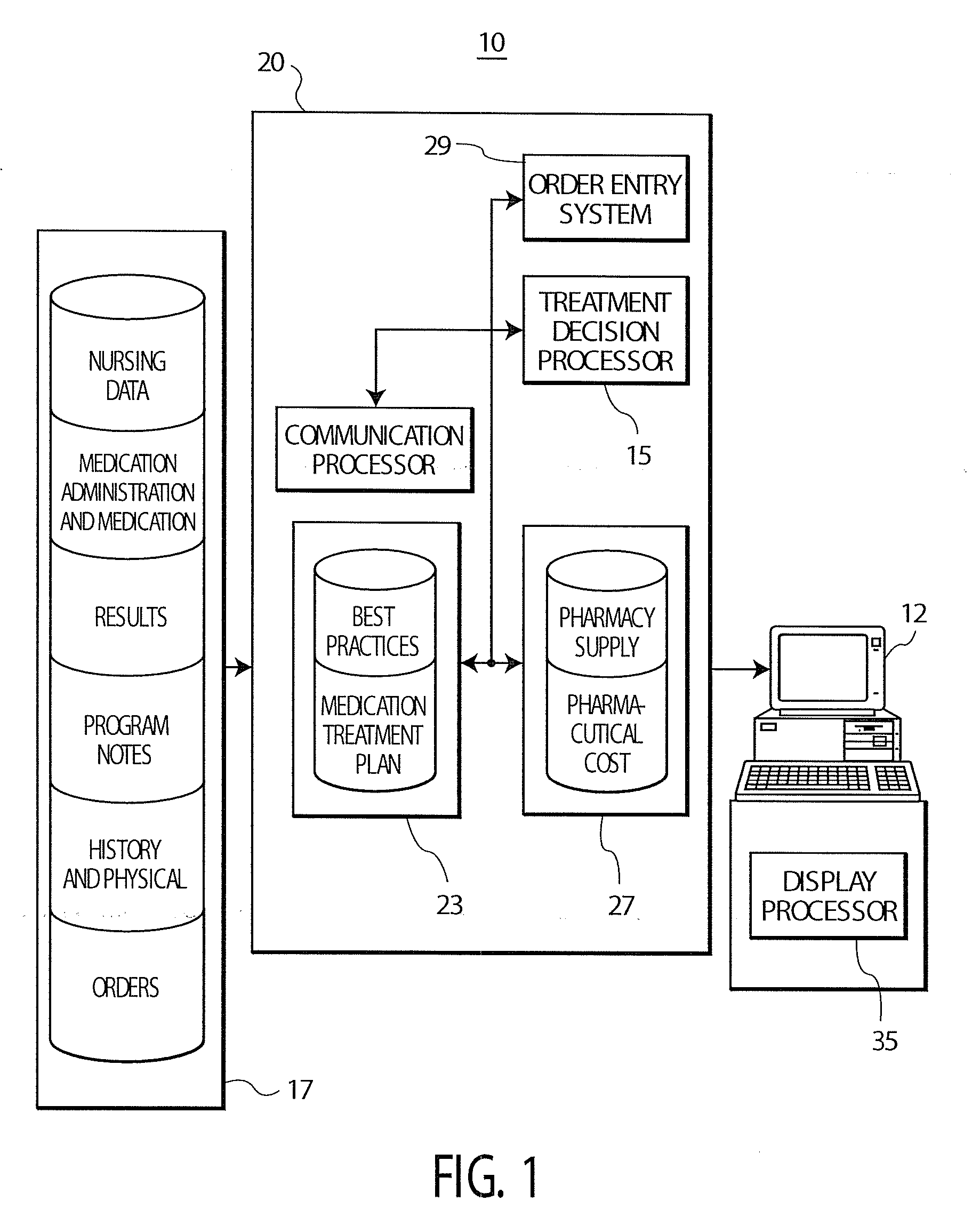
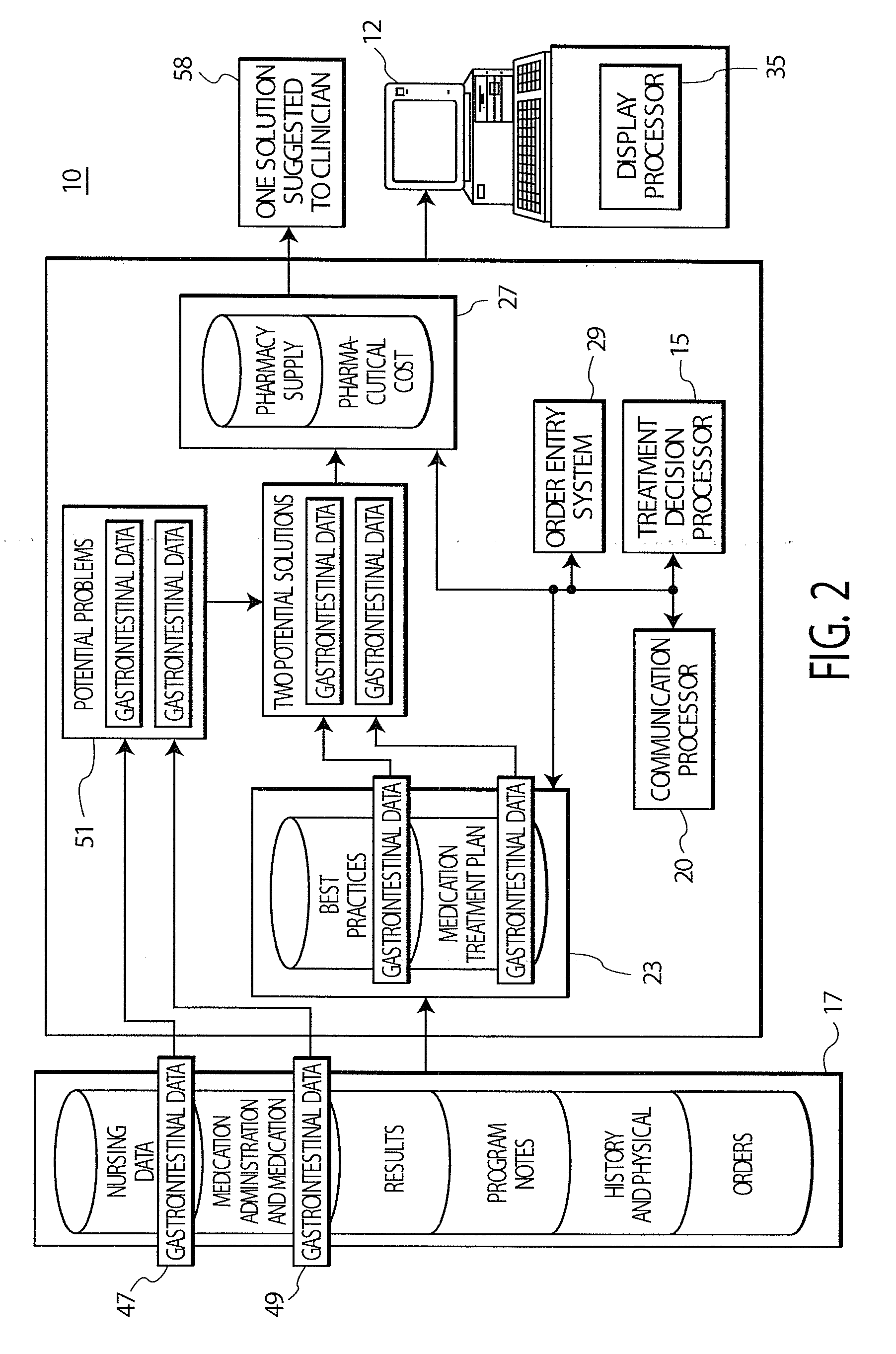
Treatment Decision Support System and User Interface
InactiveUS20080097792A1Low costData processing applicationsDrug and medicationsInitial treatmentGuideline
A system provides clinicians with realtime medication suggestions. A treatment decision support system comprises at least one repository including information associating candidate treatment items, treatment costs, treatment supply availability, patient specific medical information and corresponding related treatment parameters. An individual treatment item is associated with multiple related treatment parameters. A communication processor accesses the information in the at least one repository. A treatment decision processor uses accessed information provided by the communication processor for providing data representing candidate treatments in response to user entry of data identifying an initial treatment based on both clinical and non-clinical factors. The non-clinical factors include lowest cost and availability and the clinical factors include patient specific medical information and predetermined treatment guidelines for treating a particular medical condition. A display processor provides data representing at least one display image identifying the candidate treatments.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Preparation method of chicken bone meal and chicken essence containing same
InactiveCN104305271AIncrease profitImprove efficiencyFood preparationProtein food ingredientsFood additiveInitial treatment
The invention discloses a preparation method of chicken bone meal and a chicken essence containing the same. The preparation method comprises the steps of initial treatment of chicken skeletons, grinding, enzymolysis, enzyme deactivation, finish grinding and high-pressure spray drying, wherein a raw material adopted in the method is commercially available low-price fresh chicken bones, a lot of protein and minerals of calcium, phosphorus and the like are extracted from the chicken bones, and meanwhile, chicken bone marrows are fully extracted, so that the comprehensive utilization value of the chicken skeletons is developed; by using the preparation method, the product yield of the chicken bones can reach up to 98%; the preparation method is simple, has low technical requirements, and is low in equipment cost and high in efficiency; moreover, protease and flavor enzyme adopted in the step of enzymolysis can be flexibly added according to required tastes of customers, so that the chicken bone meal has an extremely wide application range, and not only can be used for preparing the chicken essence but also can be applied to other seasonings or food additive materials.
Owner:安阳市兴厨调味品有限责任公司
Laser Device and Method for Decreasing the Size and/or Changing the Shape of Pelvic Tissues
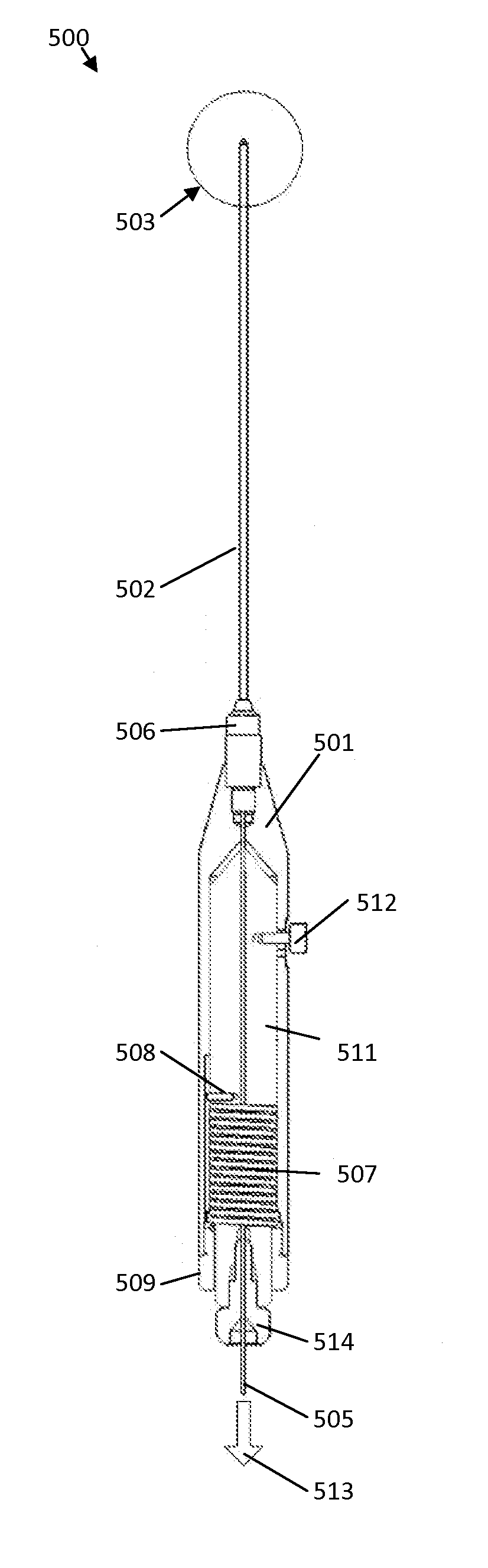
Methods for decreasing the size and / or changing the shape of pelvic tissues. In one embodiment a cannula needle having a laser fiber disposed therein is used to delivery energy to the surrounding pelvic tissue. The cannula needle may be advanced into the pelvic tissue, such as the vagina, with the laser fiber in a retracted position and retained within the distal tip of the cannula needle for protection. Upon reaching the initial treatment zone, the laser fiber may be advanced or motivated into an extended position where the distal end of the laser fiber extends beyond the distal tip of the cannula. Laser energy may then be applied as the device is withdrawn from the patient. The application of such energy may be constant, intermittent, increasing, and / or decreasing as needed. The energy application may also be deactivated if a maximum activation time, maximum temperature, or maximum energy level is reached.
Owner:ZIPPER RALPH
Bladder cancer specific ligand peptides
ActiveUS20120230994A1Peptide/protein ingredientsGeneral/multifunctional contrast agentsInitial treatmentCystectomy
The present invention is directed to bladder cancer specific ligand peptides, comprising the amino acid sequence X1DGRX5GF (SEQ ID NO:1), and methods of their use, e.g., for imaging detection for diagnosis of bladder, tumor localization to guide transurethral resection of bladder cancer, imaging detection of bladder cancer for follow-up after the initial treatment that can replace or complement costly cystoscopy, imaging detection of metastatic bladder cancer, and targeted therapy for superficial and metastatic bladder cancer.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERAN AFFAIRS WASHINGTON DC
System and method for detecting drug fraud and abuse
Owner:THE TRAVELERS INDEMNITY +1
Mobile station and methods for diagnosing and modeling site specific full-scale effluent treatment facility requirements
InactiveUS8790517B2Reduce financial riskHigh continuity of operationSolid sorbent liquid separationAnalogue computers for chemical processesInitial treatmentIon exchange
A mobile station and methods are disclosed for diagnosing and modeling site specific effluent treatment facility requirements to arrive at a treatment regimen and / or proposed commercial plant model idealized for the particular water / site requirements. The station includes a mobile platform having power intake, effluent intake and fluid outflow facilities and first and second suites of selectably actuatable effluent pre-treatment apparatus. An effluent polishing treatment array is housed at the station and includes at least one of nanofiltration, reverse osmosis and ion-exchange stages. A suite of selectively actuatable post-treatment apparatus is housed at the station. Controls are connected at the station for process control, monitoring and data accumulation. A plurality of improved water treatment technologies is also disclosed. The modeling methods include steps for analyzing raw effluent to be treated, providing a field of raw effluent condition entry values and a field of treated effluent condition goals entry values, and utilizing said fields to determine an initial treatment model including a selection of, and use parameters for, treatment technologies from the plurality of down-scaled treatment technologies at the facility, the model dynamically and continuously modifiable during treatment modeling.
Owner:ROCKWATER RESOURCE
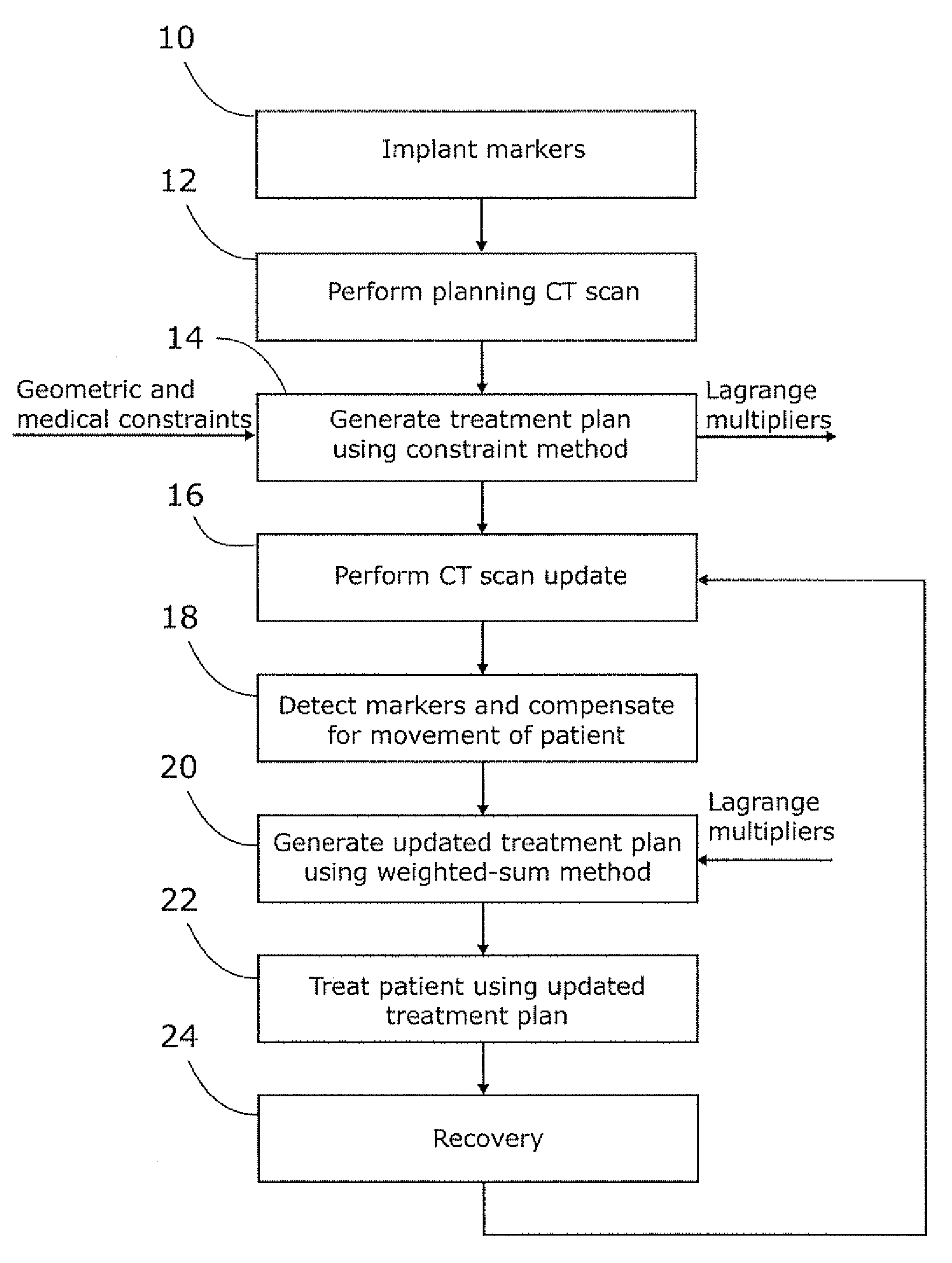
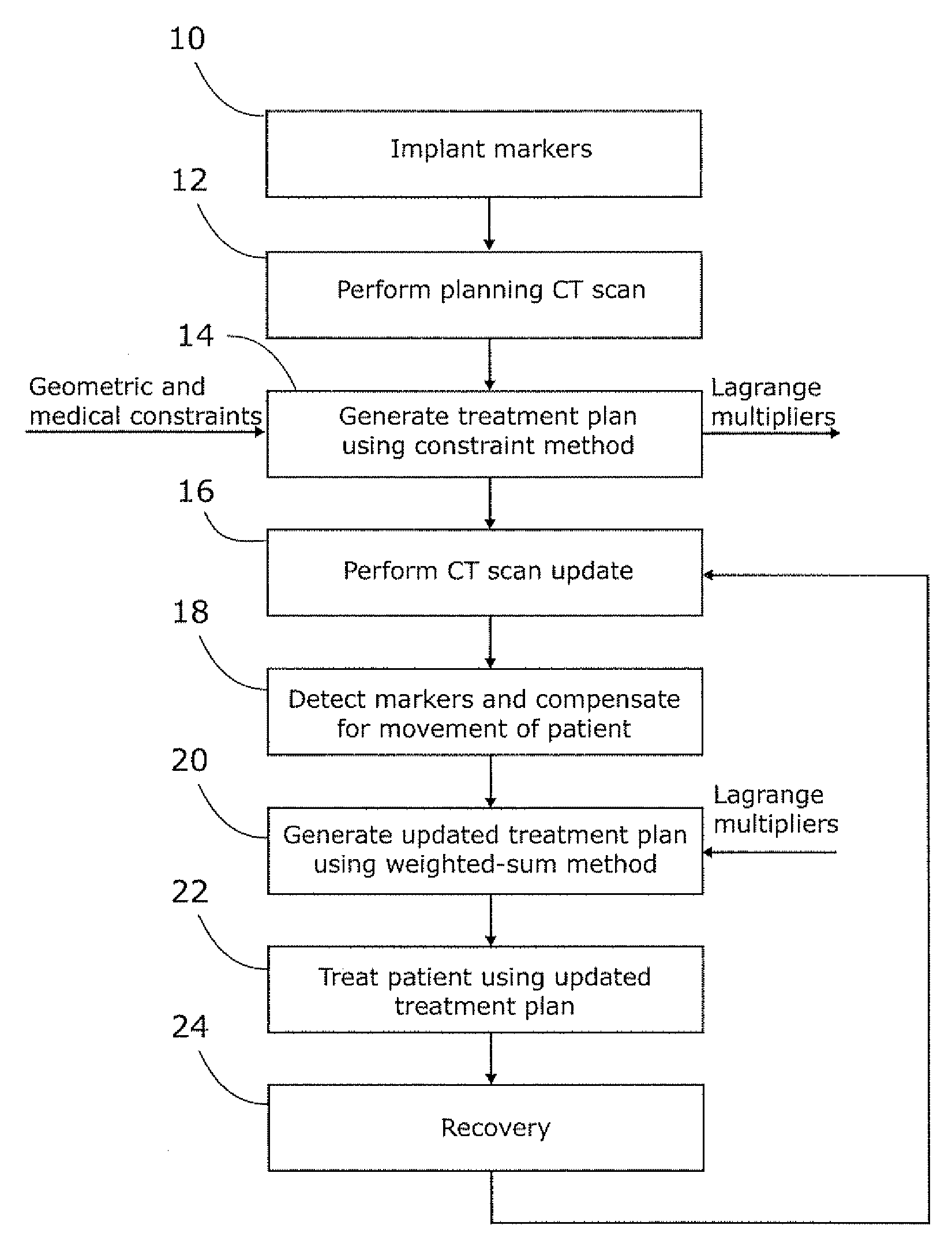
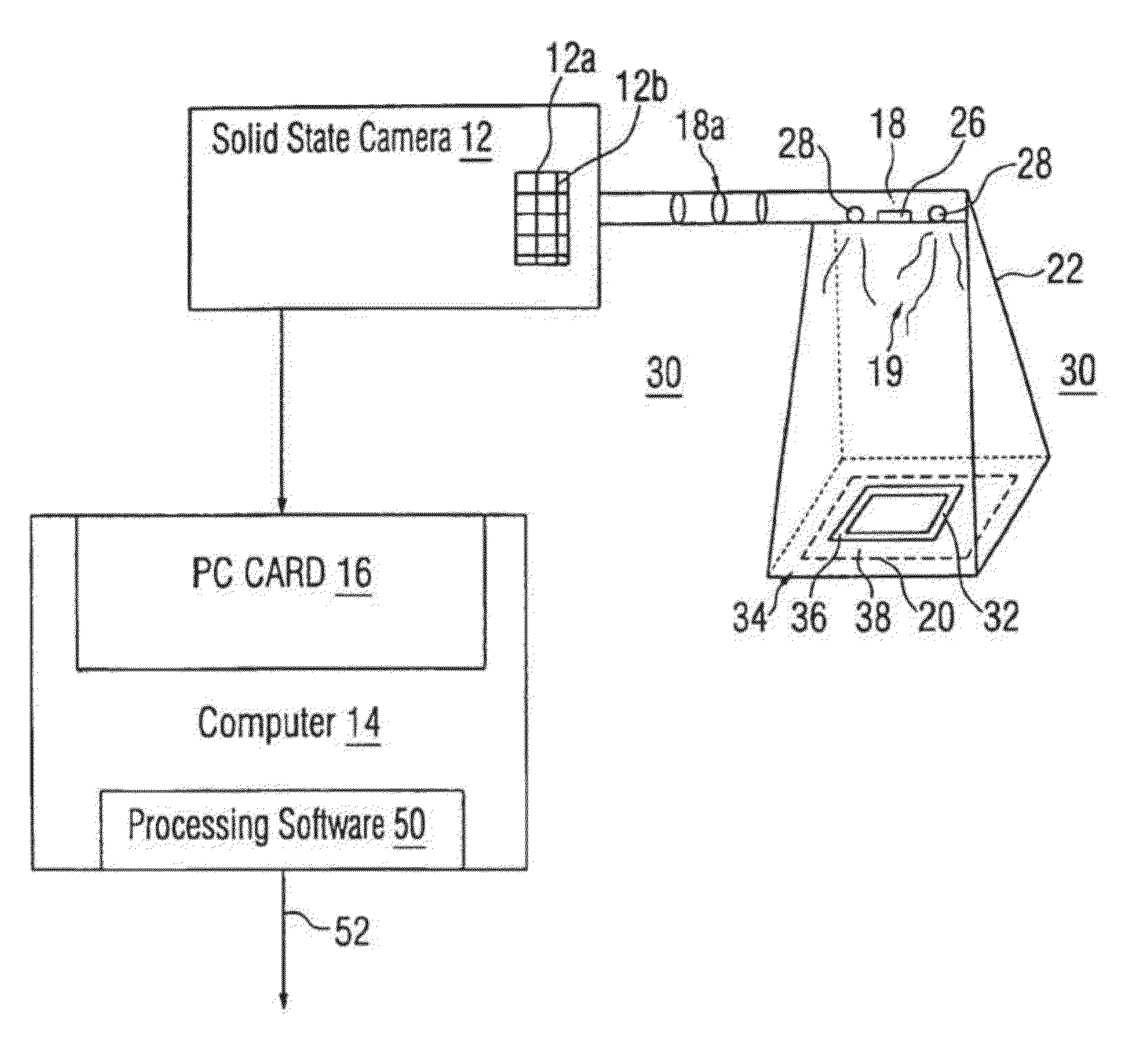
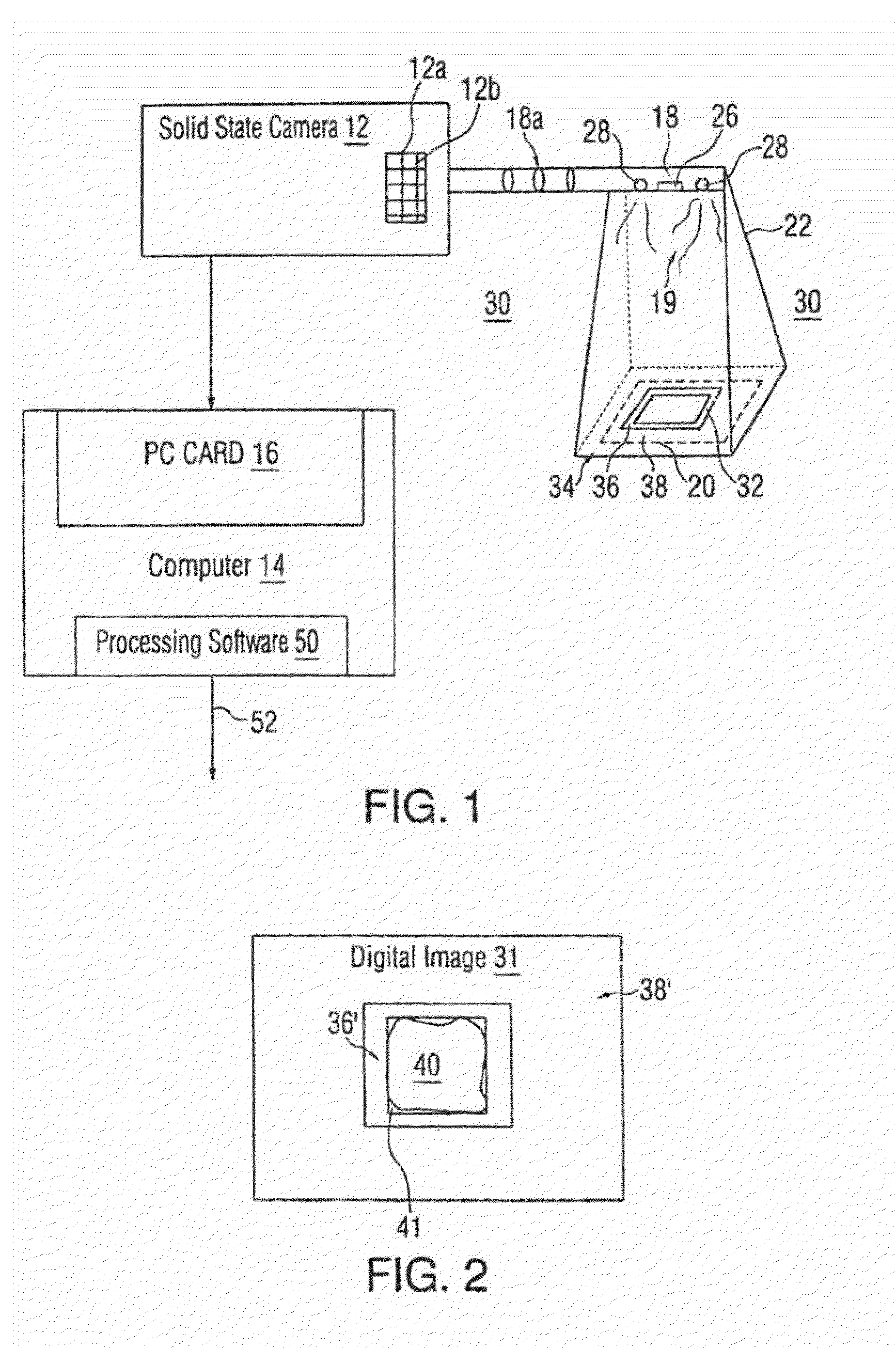
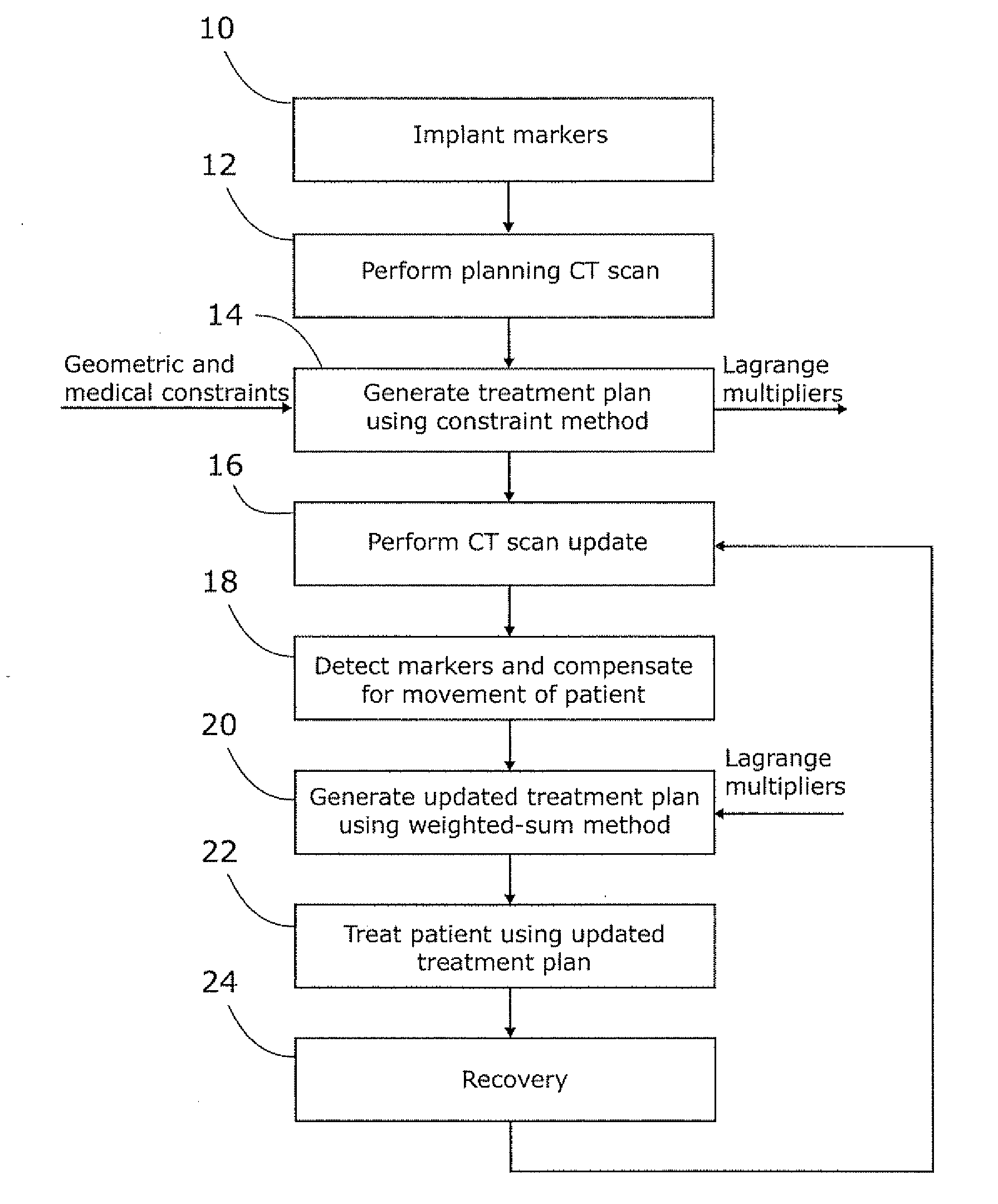
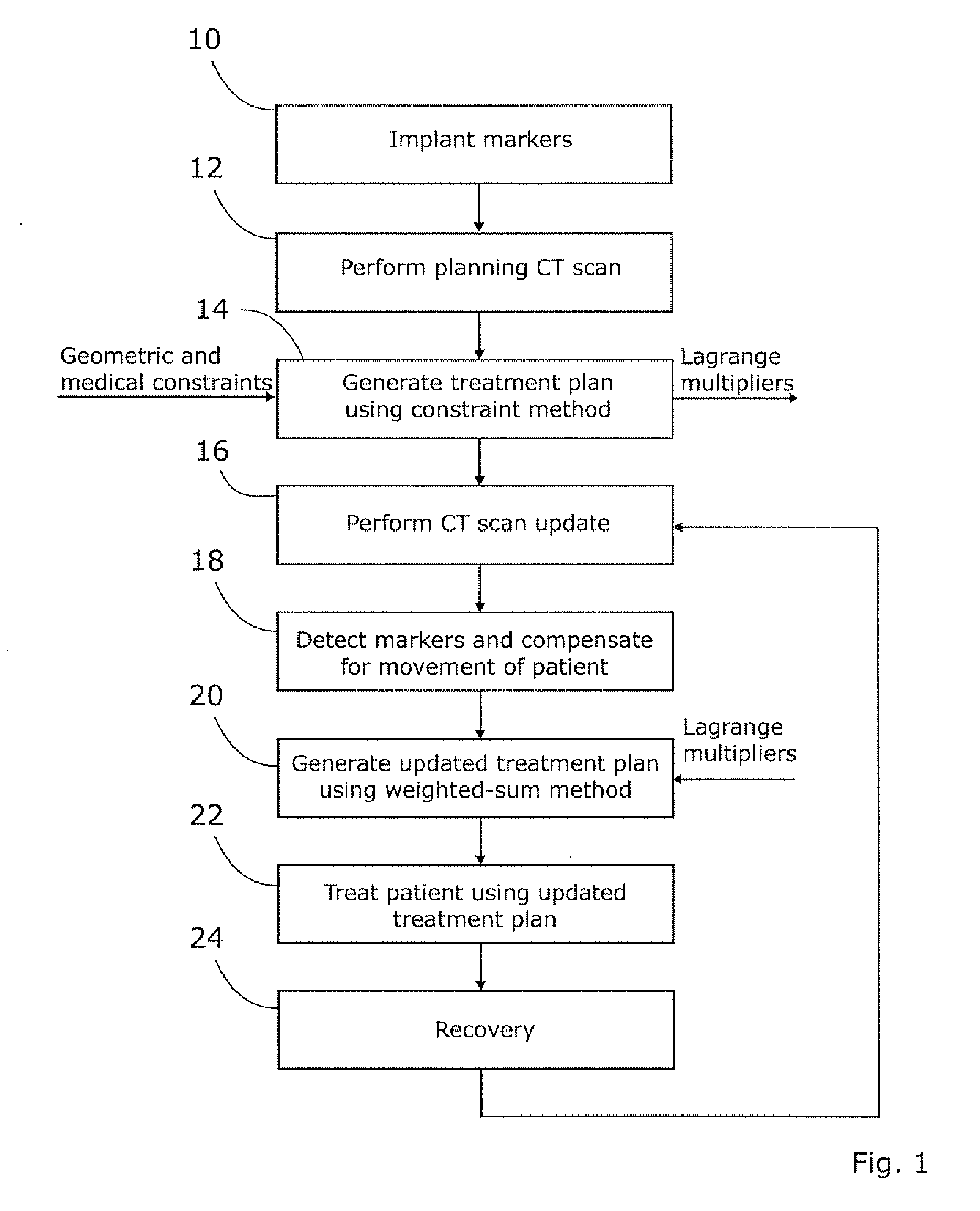
Planning for adaptive radiotherapy
ActiveUS8144833B2Shorten the timeX-ray/gamma-ray/particle-irradiation therapyInitial therapyInitial treatment
The present invention provides a method for updating and optimizing a treatment plan for radiotherapy. An initial treatment plan, calculated using a constraint-driven method, may be updated using a weighted-sum method, where Lagrange multipliers generated in the constraint method are reused as the weights for the weighted sum. This method results in acceptable updated treatment plans that are generated in a small fraction of the time taken to generate an entirely new treatment plan, reducing patient discomfort and ensuring the radiotherapy facility can treat more patients.
Owner:ELEKTA AB
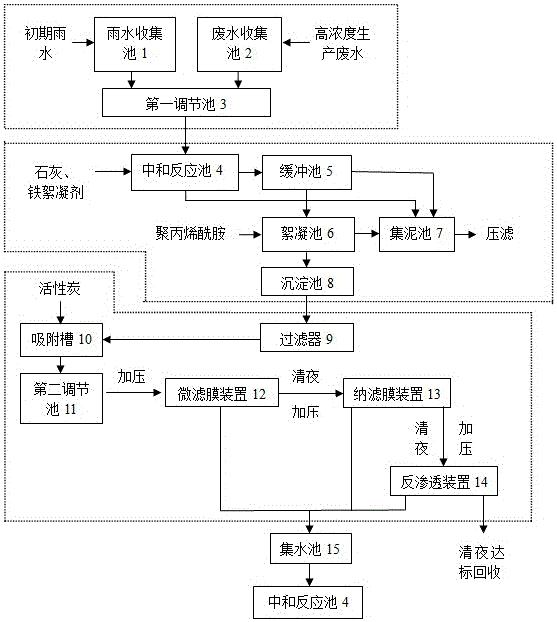
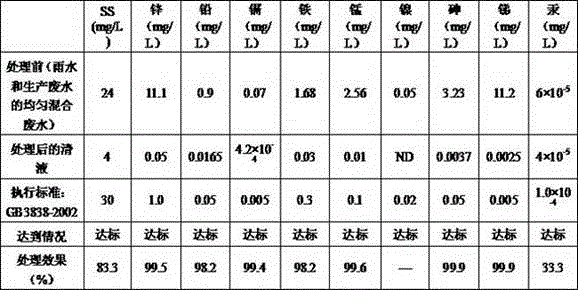
Lead-zinc smelting wastewater treatment method
InactiveCN106007074AReduce dosageReduce corrosionSludge treatmentWater contaminantsInitial treatmentFiltration membrane
The invention provides a lead-zinc smelting wastewater treatment method, which comprises the following steps: A, initial treatment of inflow; B, primary flocculation with an iron-based flocculating agent; C, secondary flocculation with polyacrylamide; D, filtration; E, adsorption with activated carbon; F, PH value regulation; G, membrane treatment: pumping water in a second regulation tank into a micro-filtration membrane device in a water pump pressurization form, pumping clear liquid filtered by the micro-filtration membrane device into a nanofiltration membrane device in the water pump pressurization form, pumping clear liquid filtered by the nanofiltration membrane device into a reverse osmosis device in the water pump pressurization form, and discharging or recycling clear liquid obtained by reverse osmosis of the reverse osmosis device after the clear liquid is detected to be qualified. According to the method, the iron-based flocculating agent and the polyacrylamide are adopted for performing flocculation twice, so that complete flocculation precipitation can be ensured; a filtration method combining micro-filtration, nanofiltration and reverse osmosis is adopted to deeply remove heavy metal ions such as lead and zinc ions in wastewater layer by layer, so that good removal effects are achieved.
Owner:SCI RES ACADEMY OF GUANGXI ENVIRONMENTAL PROTECTION
Post-treatment breast cancer prognosis
InactiveUS20130281502A1Accurate assessmentBiocideMicrobiological testing/measurementInitial treatmentCancer metastasis
The disclosure includes the identification and use of gene expression profiles, or patterns, with clinical relevance to extended treatment and cancer-free survival in a patient. In particular, the disclosure includes the identities of genes that are expressed in correlation with benefit in a switch in endocrine therapy used to treat a patient. The levels of gene expression are disclosed as a molecular index for predicting clinical outcome, and so prognosis, for the patient. The disclosure further includes methods for predicting cancer recurrence, and / or predicting occurrence of metastatic cancer, after initial treatment with an anti-estrogen agent. The disclosure further includes methods for determining or selecting the treatment of a subject based upon the likelihood of life expectancy, cancer recurrence, and / or cancer metastasis.
Owner:THE GENERAL HOSPITAL CORP +1
Interactive dental restorative network
The invention relates to a method for assisting a dental practitioner in the dental restoration of a patient treatment area. The method includes forming a three-dimensional (3D) model represented in digital form of the treatment area to be restored, the model being formed during a first visit by the patient to a dentist's office; developing a preliminary treatment plan including a prostheses specification that is based, at least in part, on the 3D digital model of the treatment area; storing the 3D digital model and the preliminary treatment plan in a database along with the patient's record; and conferencing with other practitioners in order to determine whether the preliminary treatment plan is appropriate for the patient based on the dental data.
Owner:IVOCLAR VIVADENT AG +1
Planning for adaptive radiotherapy
ActiveUS20110255665A1Shorten the timeX-ray/gamma-ray/particle-irradiation therapyInitial therapyInitial treatment
The present invention provides a method for updating and optimizing a treatment plan for radiotherapy. An initial treatment plan, calculated using a constraint-driven method, may be updated using a weighted-sum method, where Lagrange multipliers generated in the constraint method are reused as the weights for the weighted sum. This method results in acceptable updated treatment plans that are generated in a small fraction of the time taken to generate an entirely new treatment plan, reducing patient discomfort and ensuring the radiotherapy facility can treat more patients.
Owner:ELEKTA AB
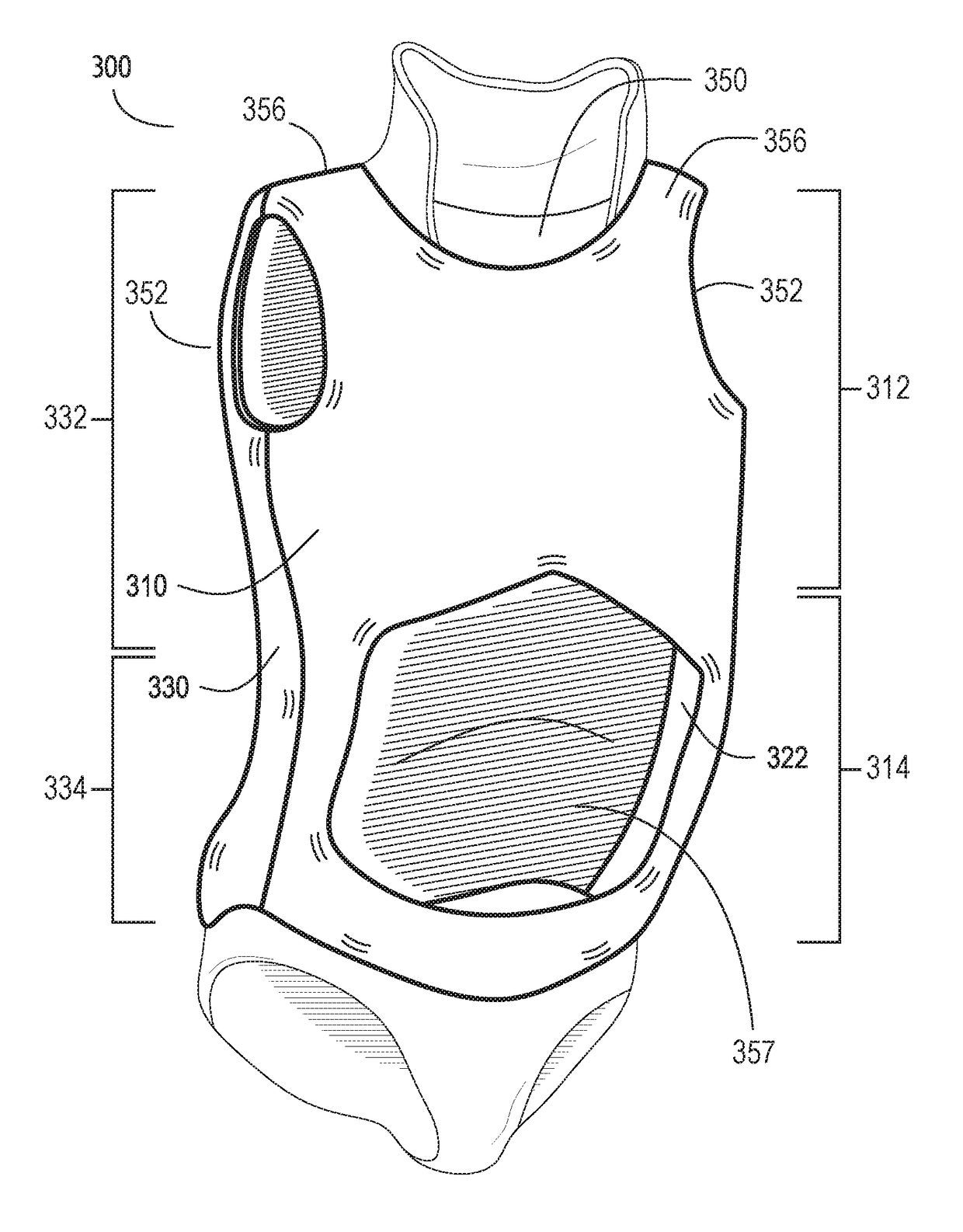
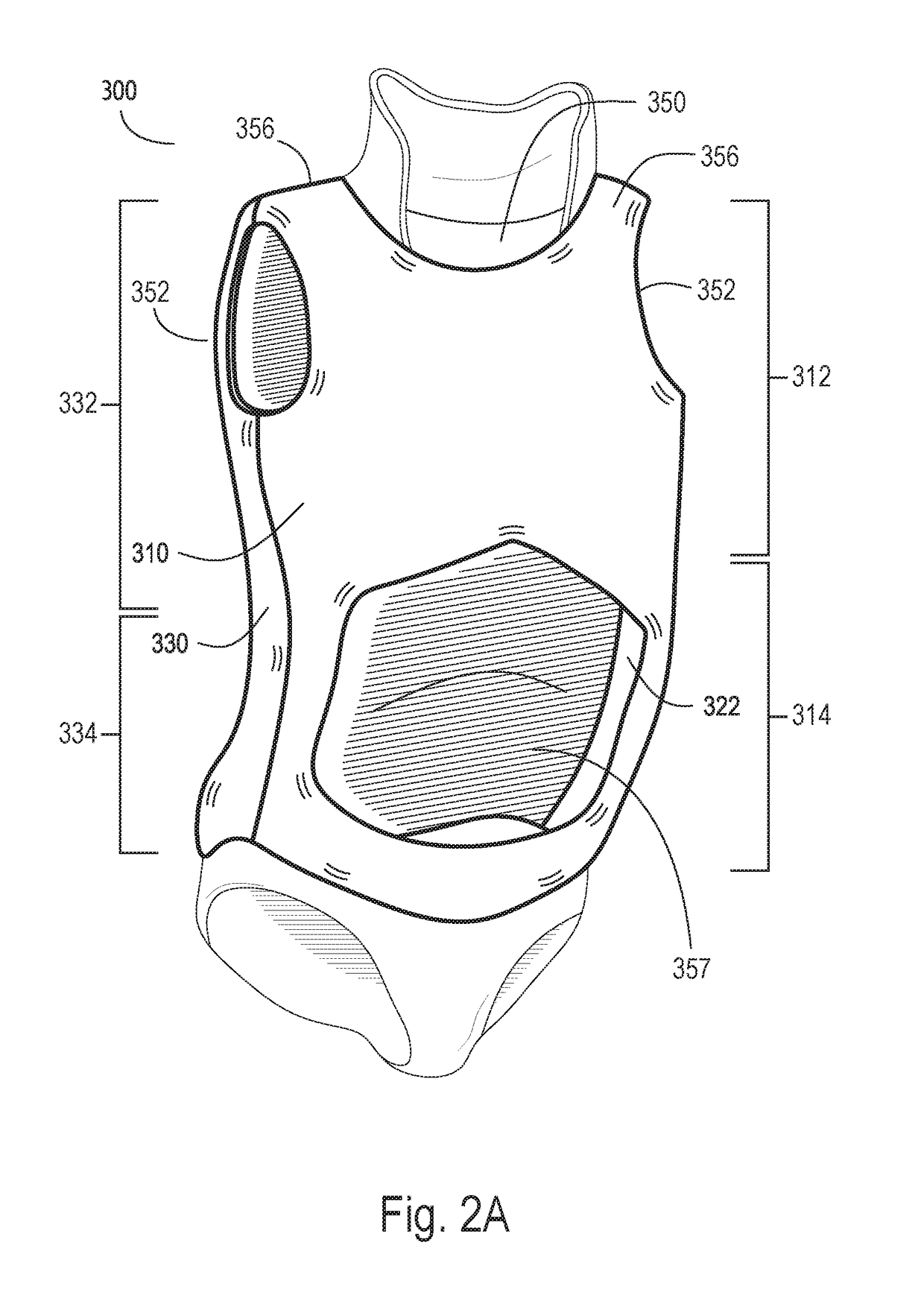
Scoliosis treatment system and method
A system of multiple scoliosis treatment braces is custom fitted for a torso of a patient and organized in a sequential series, with each of the braces varying from a previous brace in the series by at least one shape characteristic. The sequential series of braces includes an initial treatment brace with an initial configuration that conforms to an initial shape of the torso, a final treatment brace with a final configuration that approximates a desired shape of the torso, and at least one intermediate treatment brace with an intermediate configuration that approximates a torso shape between the initial configuration and the final configuration. The initial configuration, the intermediate configuration and the final configuration may be determined, at least in part, by a single digital profile of the torso of the patient.
Owner:LIM INNOVATIONS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com