Recombinant porcine alpha interferon and application thereof in preparing medicines for treating Porcine cytomegalovirus (PCMV)
A technology of alpha interferon and interferon, applied in the field of recombinant porcine alpha interferon and its application in the preparation of drugs for the treatment of porcine cytomegalovirus disease, can solve the problem of prevention and treatment of specific drugs that have not yet been developed, and have not yet been developed. PCMV vaccine and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method of embodiment 1 porcine alpha interferon
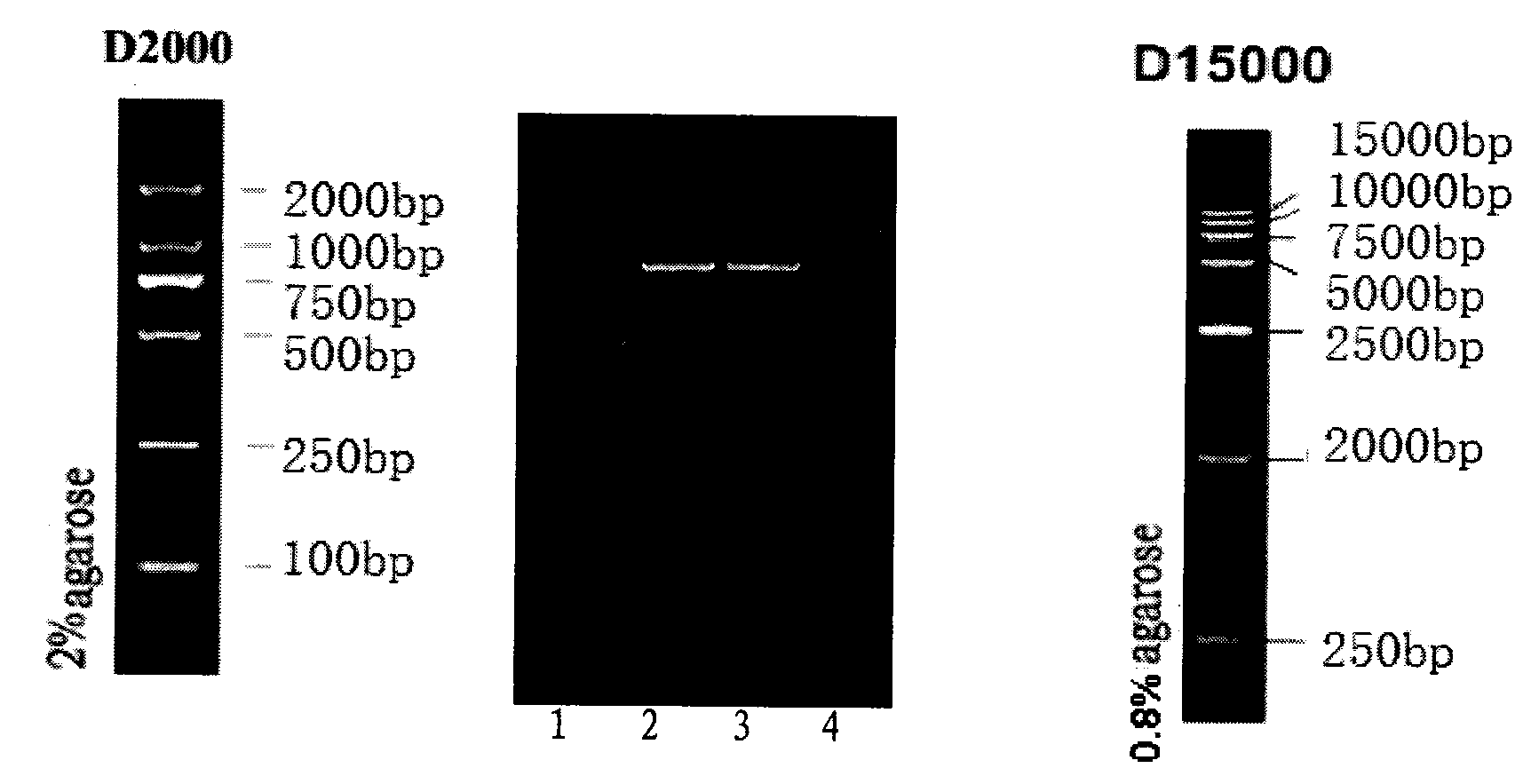
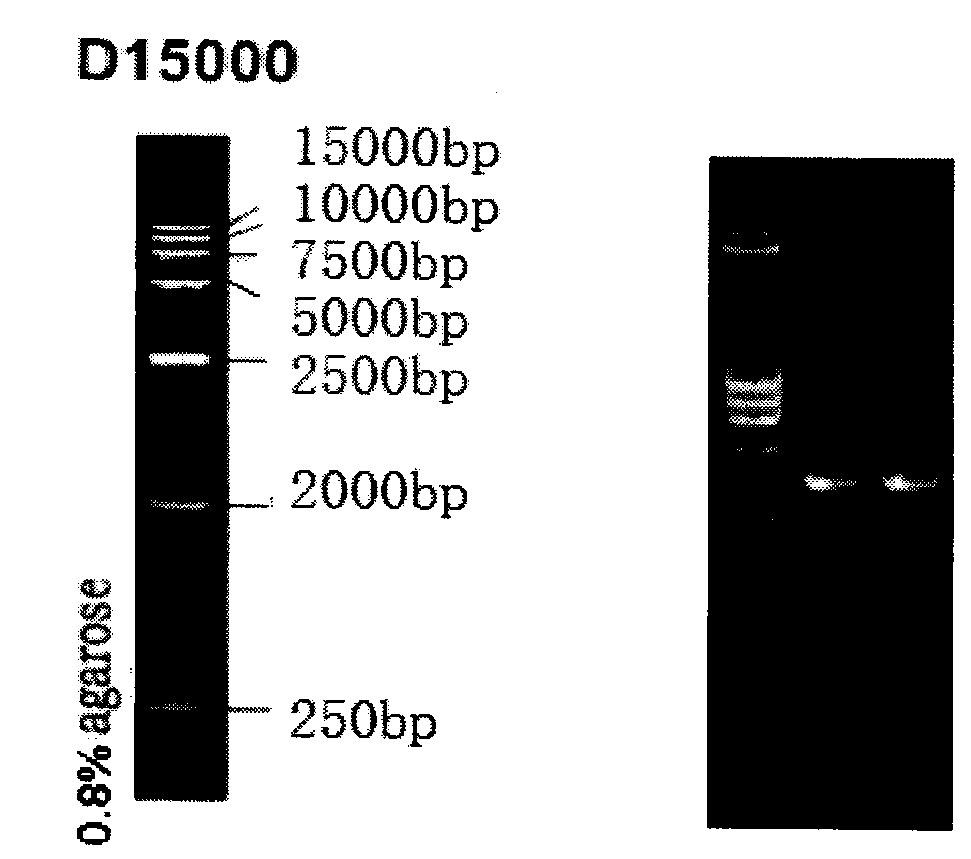
[0022] 1. Synthesis of artificially modified porcine interferon alpha gene
[0023] The 1-69 nucleotides of the signal peptide sequence in the pig natural IFNα nucleotide sequence are removed, and the XhoI and XbaI restriction sites and protective bases are added.
[0024] The specific operation is as follows: the 1-69 nucleotides of the signal peptide sequence in the natural IFNα nucleotide sequence are removed, and the XhoI and XbaI restriction site sequences and protective bases are added. The third base of the triplet codon of the 24th amino acid in the gene is subjected to a point mutation, that is, TGC is mutated into TGT; the second base of the 29th amino acid is subjected to a point mutation, that is, ACC is mutated into ATC; the 48th amino acid Carry out a point mutation at the third base of the amino acid at position 101, that is, mutate TCC to TCT; perform a point mutation at the first base of...
Embodiment 2
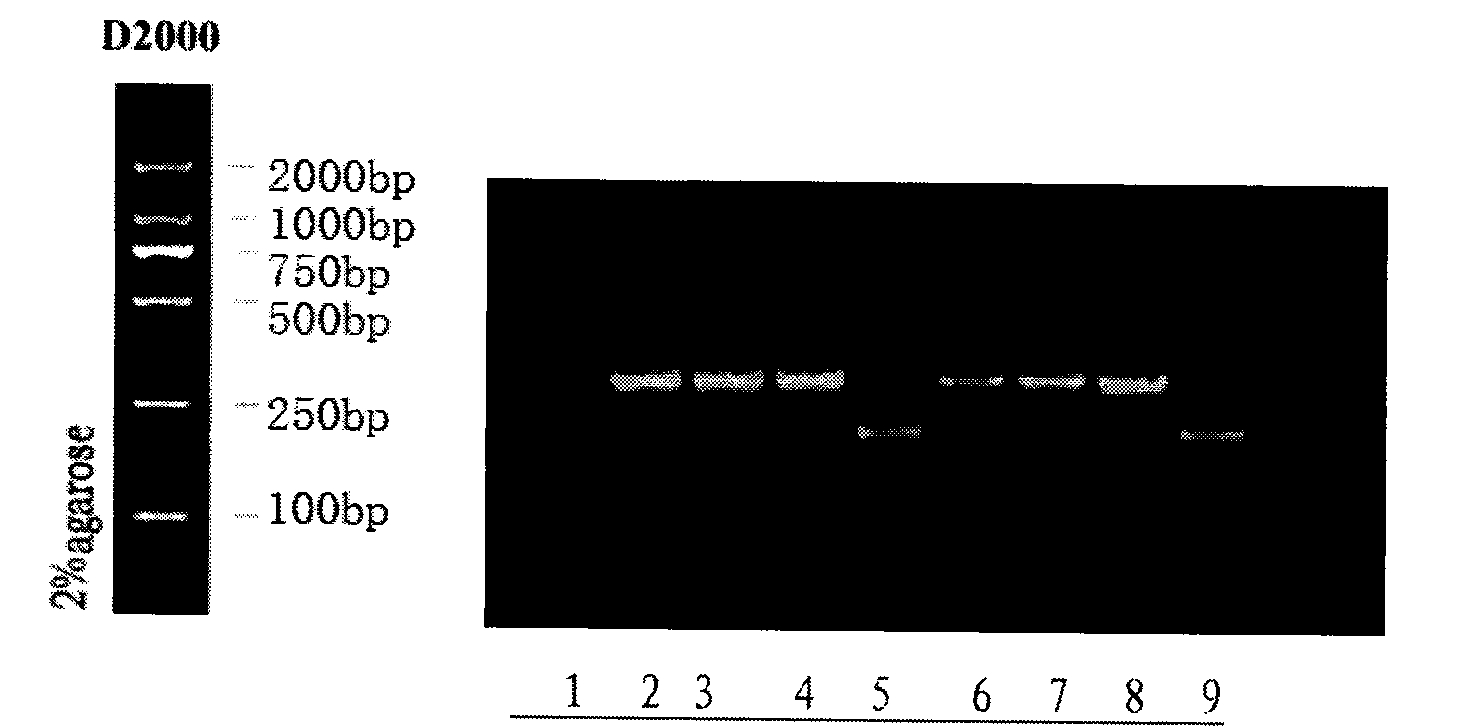
[0043] Embodiment two porcine alpha interferon biological activity assay
[0044] 1. Material method
[0045] Virus source: vesicular stomatitis virus VSV (preservation number: GDV-011, purchased from China Center for Type Culture Collection).
[0046] Cells: bovine kidney cells MDBK (NBL-1, Shanghai Biochemical Reagent Co., Ltd.).
[0047] Sample to be tested (porcine alpha interferon): prepared by genetic engineering methods.
[0048] Detection method: cytopathic inhibition method, the titer unit is calculated according to 50% pathological changes. The cells used in the determination are bovine kidney cells MDBK, and the challenge virus is VSV.
[0049] Biological activity calculation method:
[0050]
[0051] Pr is the biological activity of the standard, IU / ml;
[0052] Ds is the pre-dilution multiple of the test product;
[0053] Dr is the pre-dilution multiple of the standard;
[0054] Es is the dilution factor that the test product is equivalent to the half-ef...
Embodiment 3
[0060] Embodiment 3 Porcine alpha interferon clinical treatment of porcine cytomegalovirus disease
[0061] Select 120 diseased pigs as pig interferon treatment objects. According to the injection of 15,000 units per kilogram of body weight, once a day, a course of treatment for 3 days, after treatment, the clinical symptoms of most pigs were relieved.
[0062] Observation of Clinical Symptoms of Experimental Pigs
[0063]
[0064] Cured: 107; invalid: 13; the cure rate was 89.17%, and the ineffective rate was 10.83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com