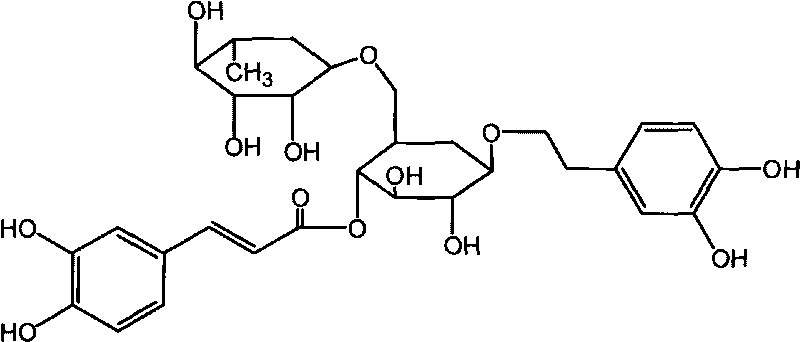
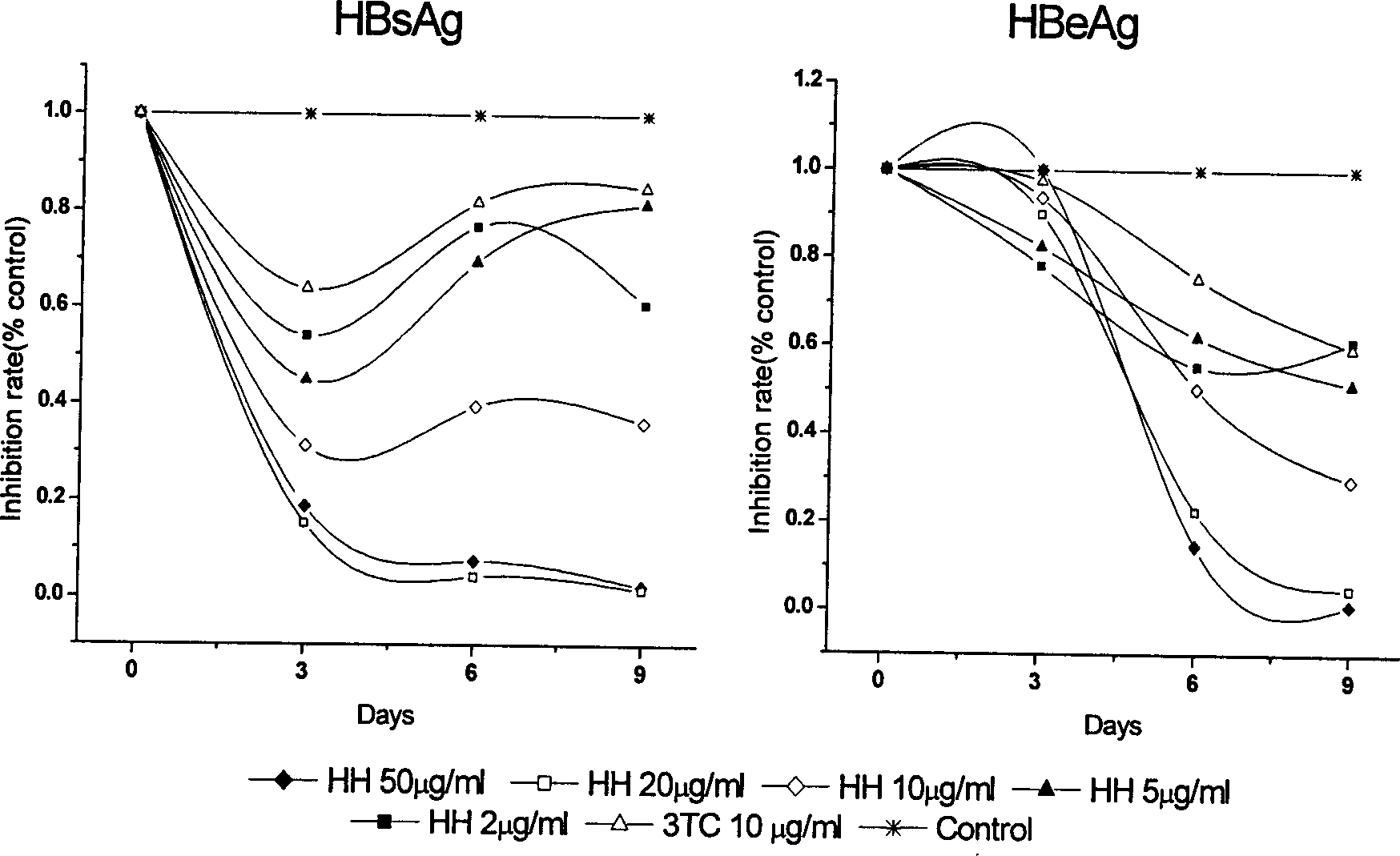
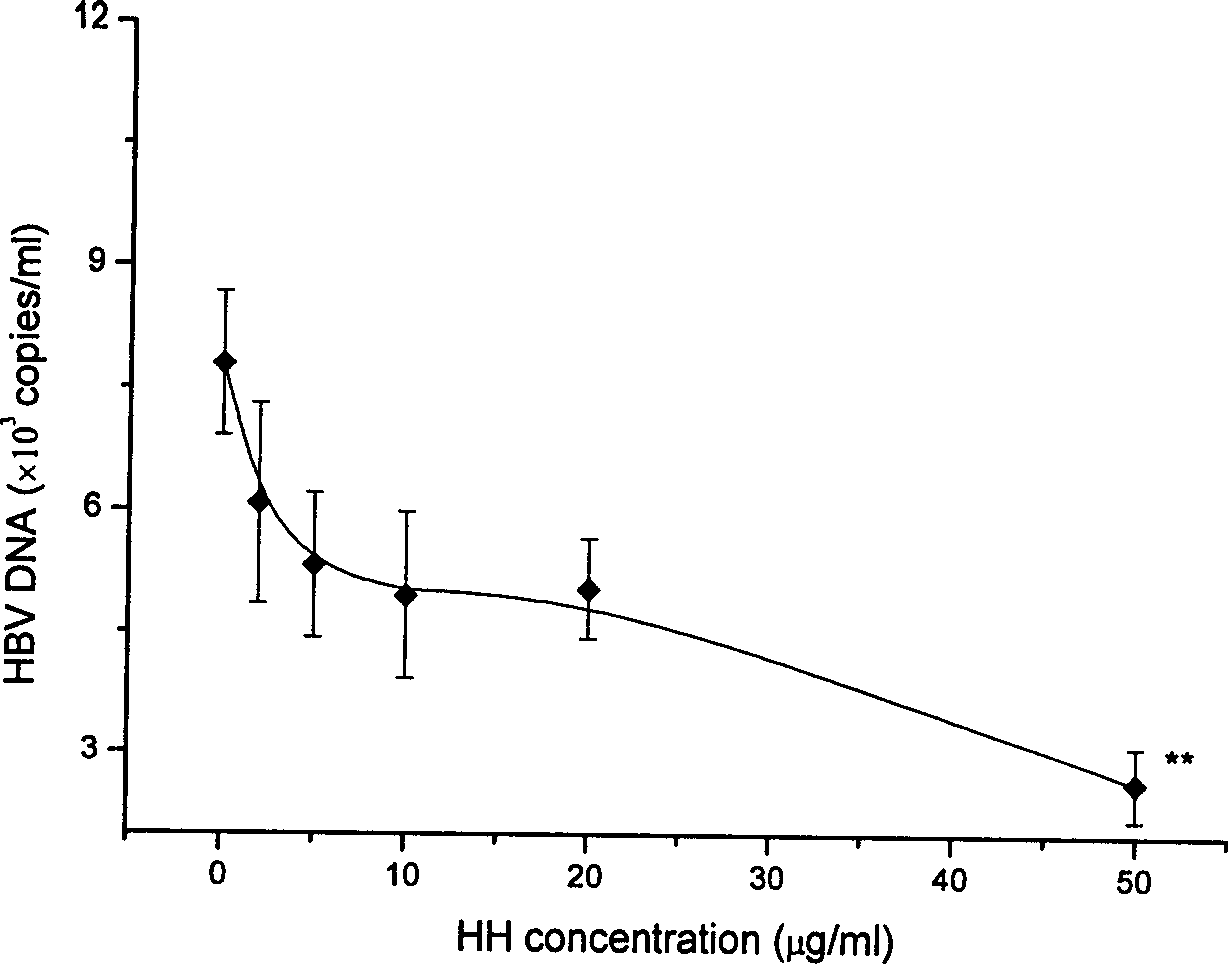
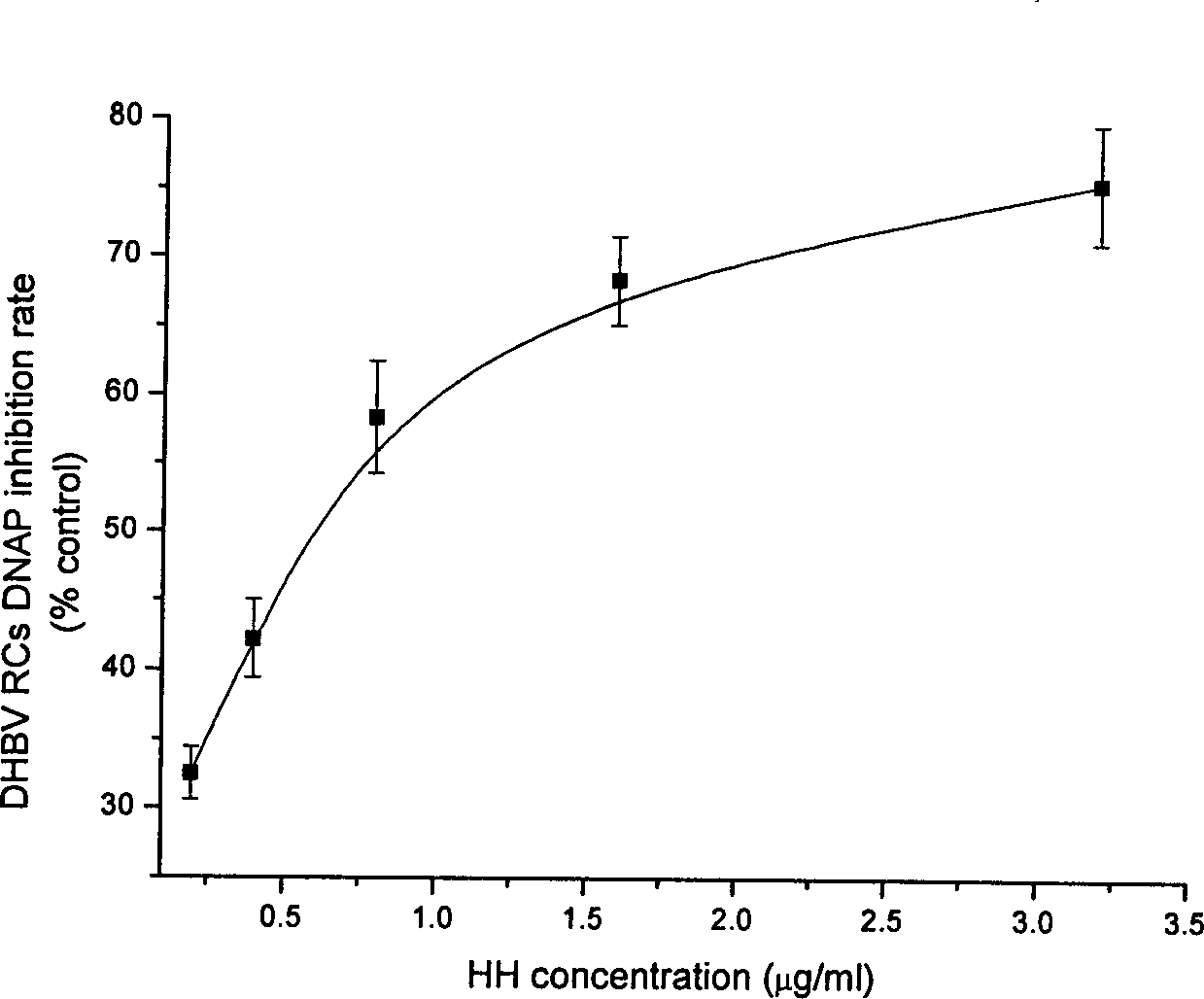
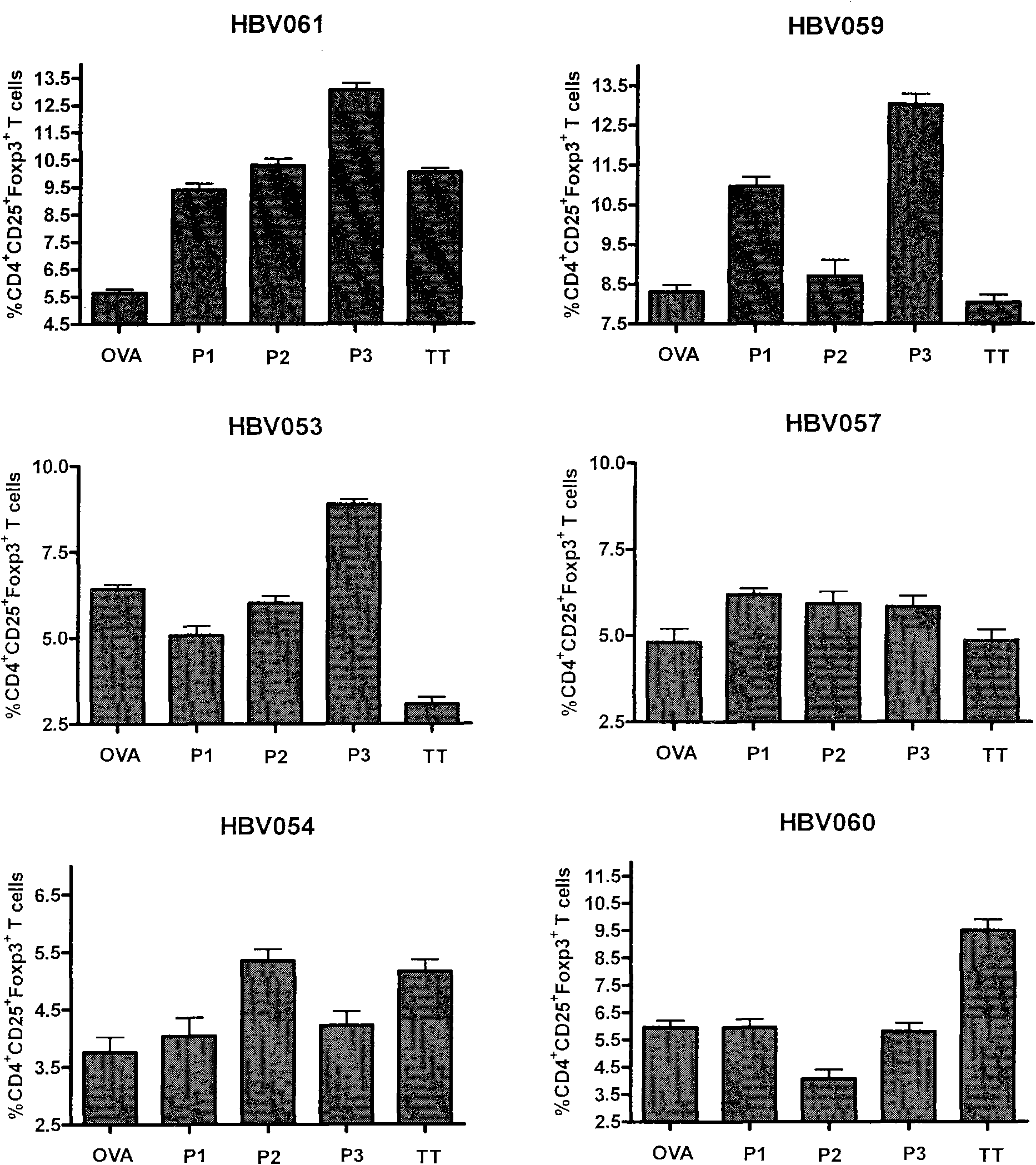
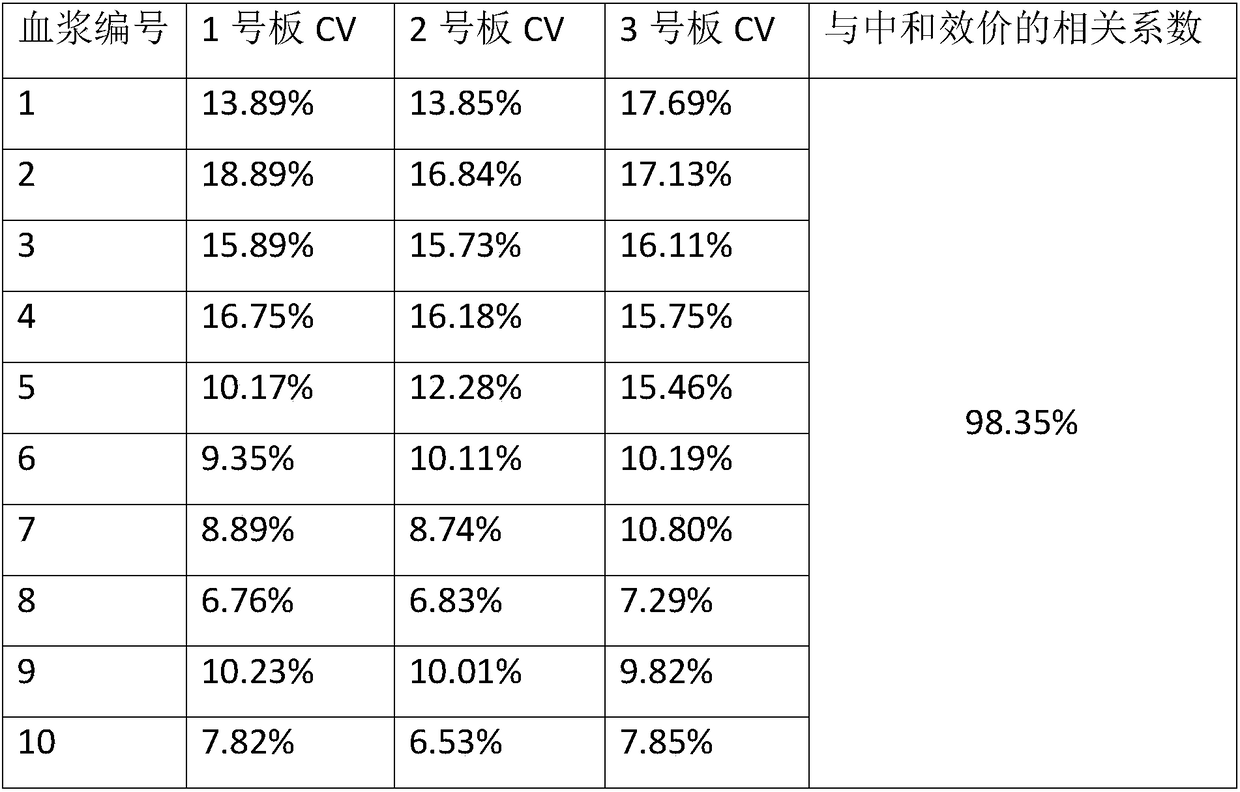
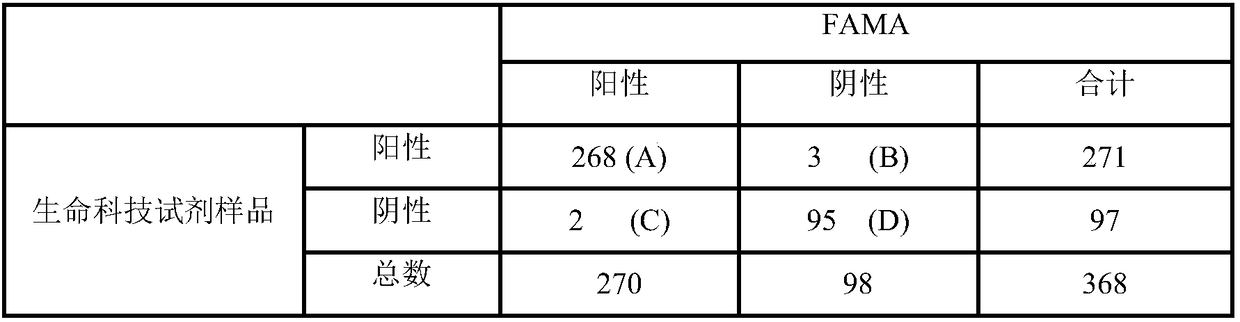
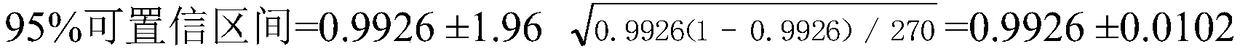Patents
Literature
61 results about "E Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
N a peptide present in blood infected with the hepatitis B virus. The e-antigen is indicative of an actively reproducing hepatitis B virus and probable liver damage.
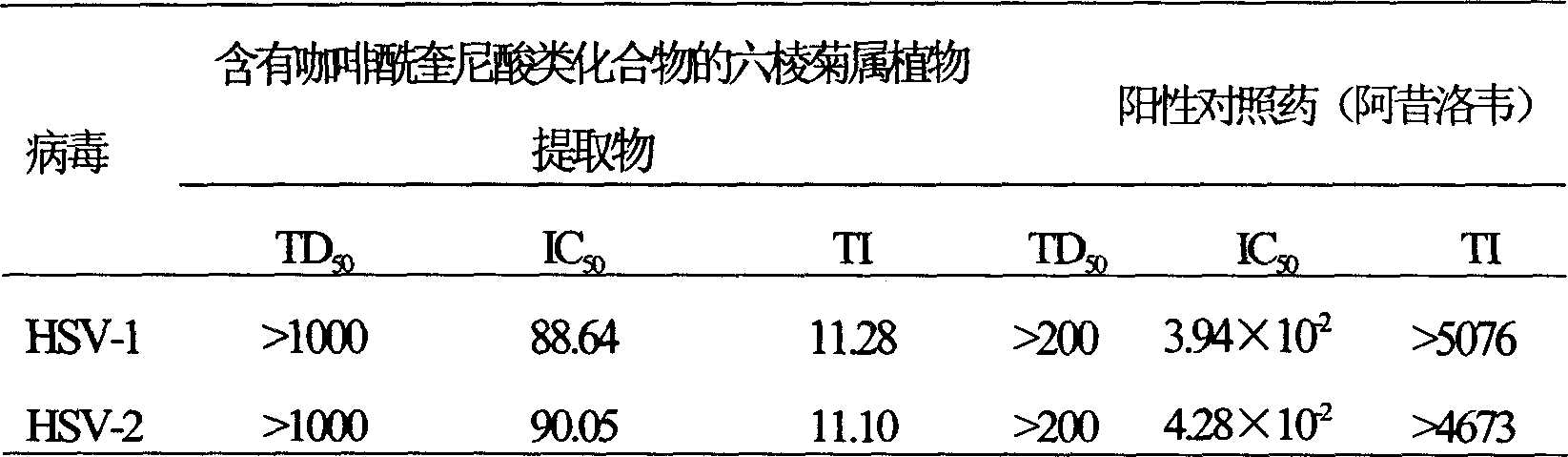
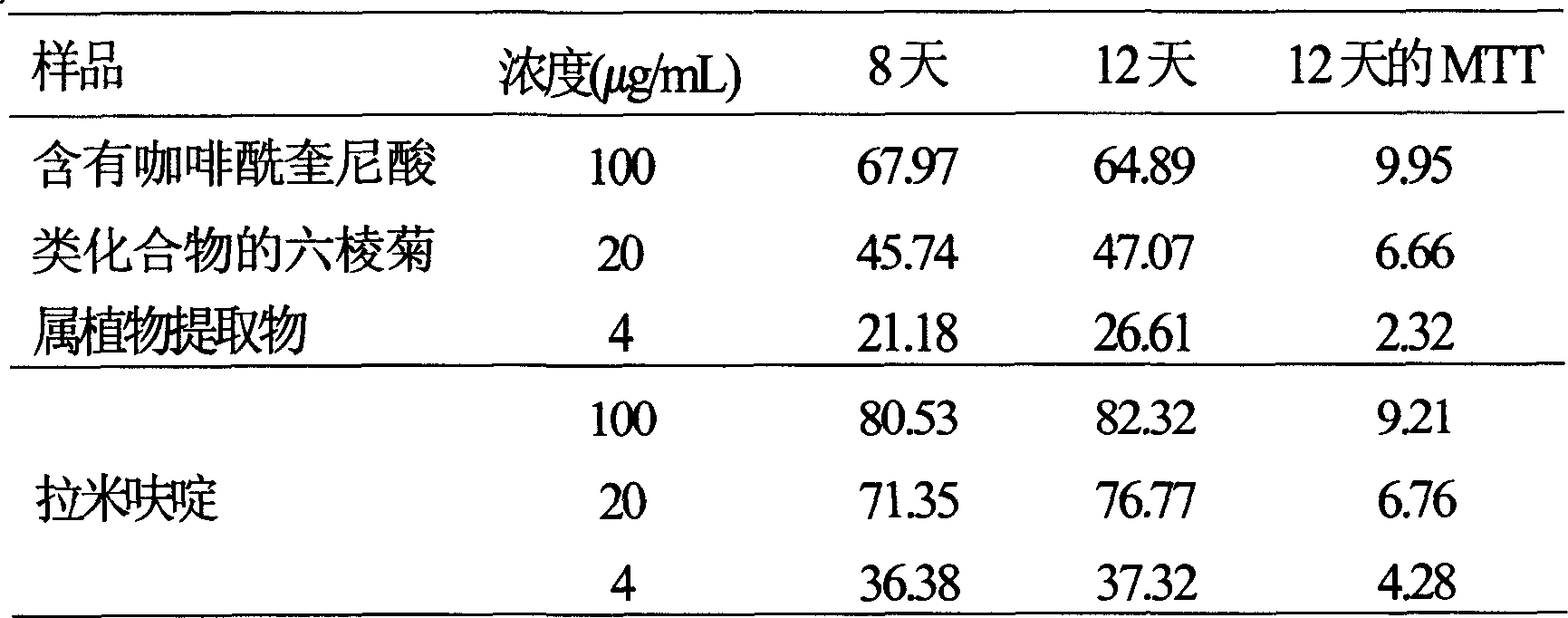
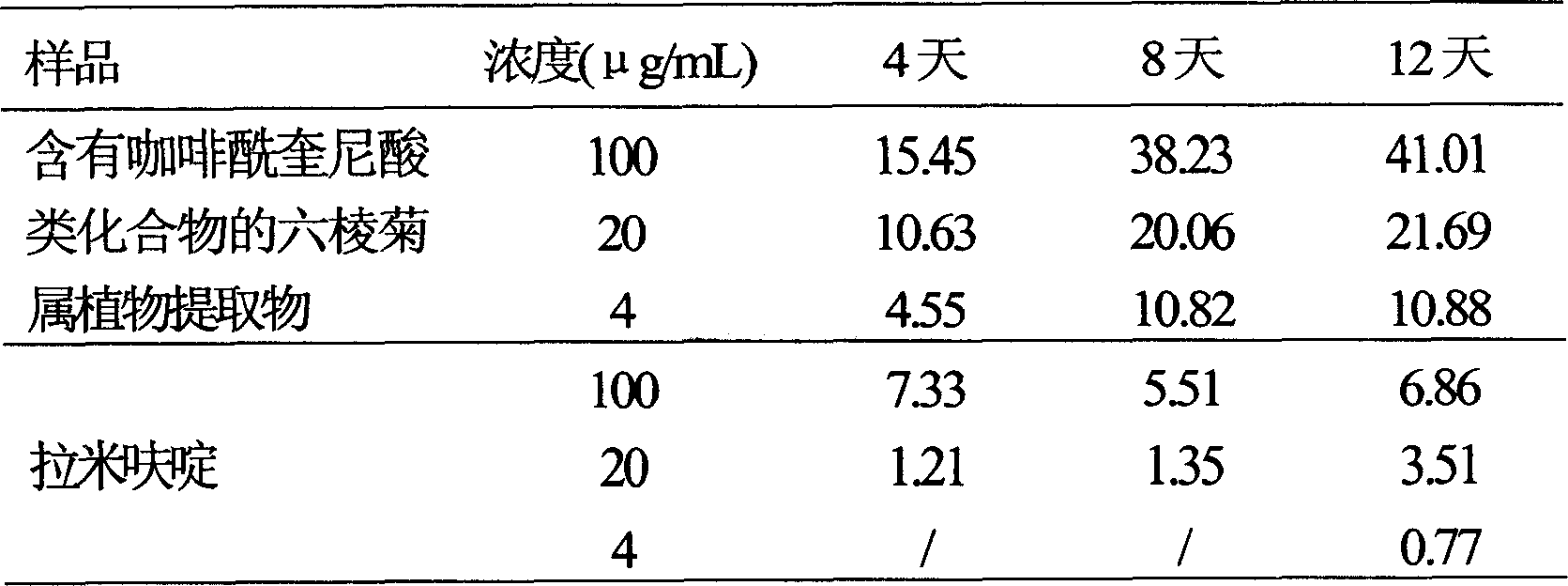
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
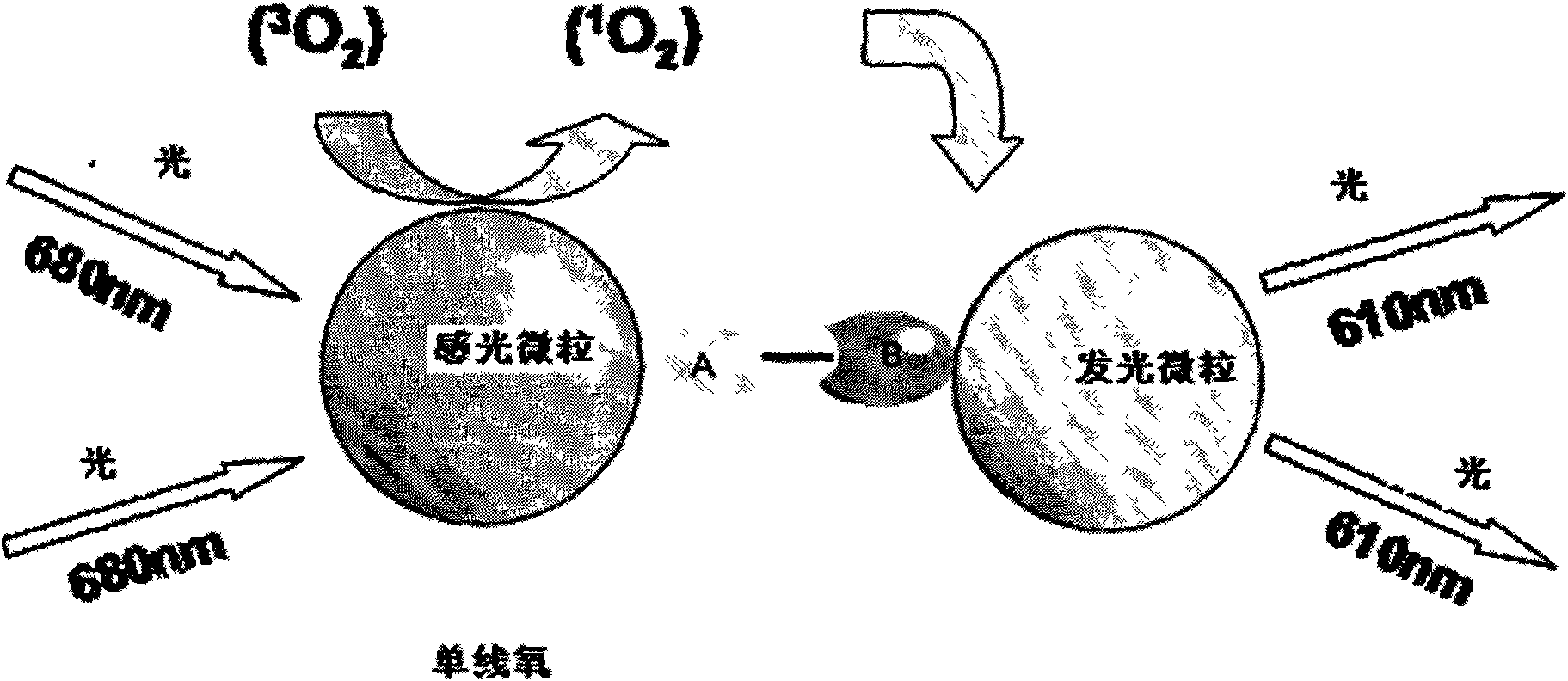
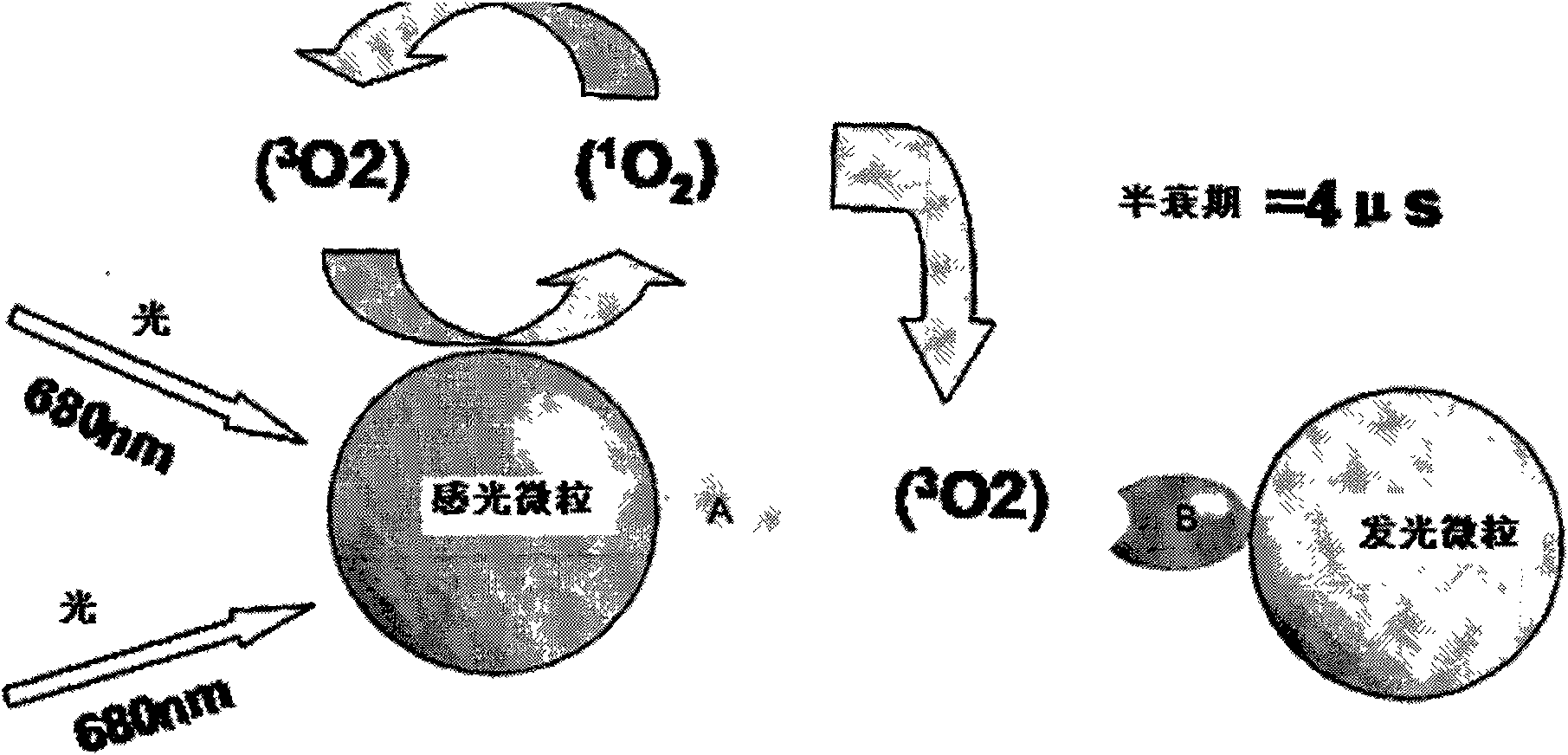
Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
ActiveCN101251540AWide detection rangeReduce sensitivityAnalysis by material excitationAntigenAnti hbe
The invention relates to a diagnosing reagent for hepatitis B, disclosing detection grains for e antigens of the hepatitis B virus, which are of the luminous grains coated by anti-HBE antibodies. The invention also discloses preparation and application for the detection grains for e antigens of the hepatitis B virus; moreover, the invention further discloses an outside-body diagnosis reagent box for detecting e antigens of the hepatitis B in a blood serum sample of human beings as well as a method for utilizing the light excitation chemiluminescence principle to quantitatively and qualitatively detect e antigens of the hepatitis B virus. The reagent box of the invention can be jointly used to diagnose the individual acute or chronic hepatitis B together with other blood serums and clinic information, and screen the hepatitis B for women in the perinatal period so as to judge the risk of newborn babies contaminating the hepatitis B.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
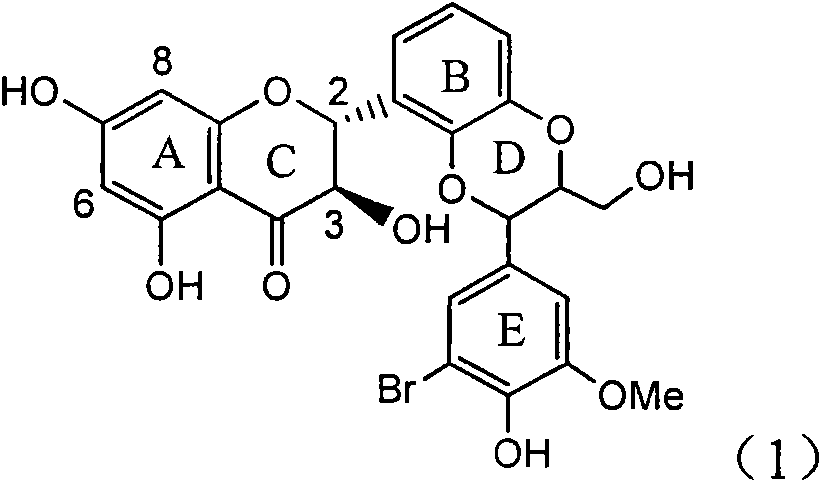
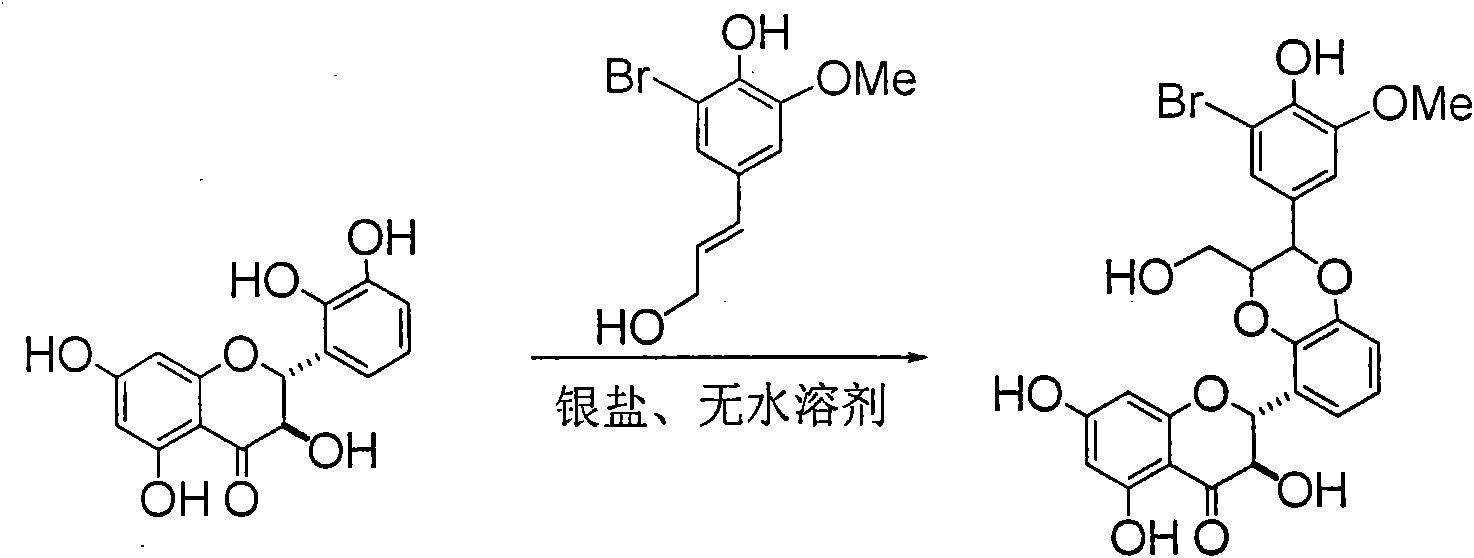
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Hepatits B virus e antibody assay kit and assay method thereof
The invention relates to a diagnostic reagent for hepatits B, and discloses a hepatits B virus e antibody assay kit and an assay method thereof. The invention adopts light initiated chemiluminescence assay technology, and discloses the hepatits B virus e antibody assay kit, which comprises luminescent particles coated by anti-HBe antibody, anti-HBe antibody marked by biotin and neutralized e antigen. The invention also discloses a method for qualitatively or quantitatively assaying hepatits B e antibody by the light initiated chemiluminescence assay technology. The assay kit can be combined with other serum and clinical information for diagnosing infectious conditions of acute or chronic hepatits B of an individual, and also can be used for screening hepatits B of female in perinatal period to judge the hazard that neonates are infected with the hepatits B. Besides, the kit has the characteristics of high sensitivity, wide assay range and the like, and the assay method has higher sensitivity and better assay range than an enzyme-immunoassay method methodologically.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
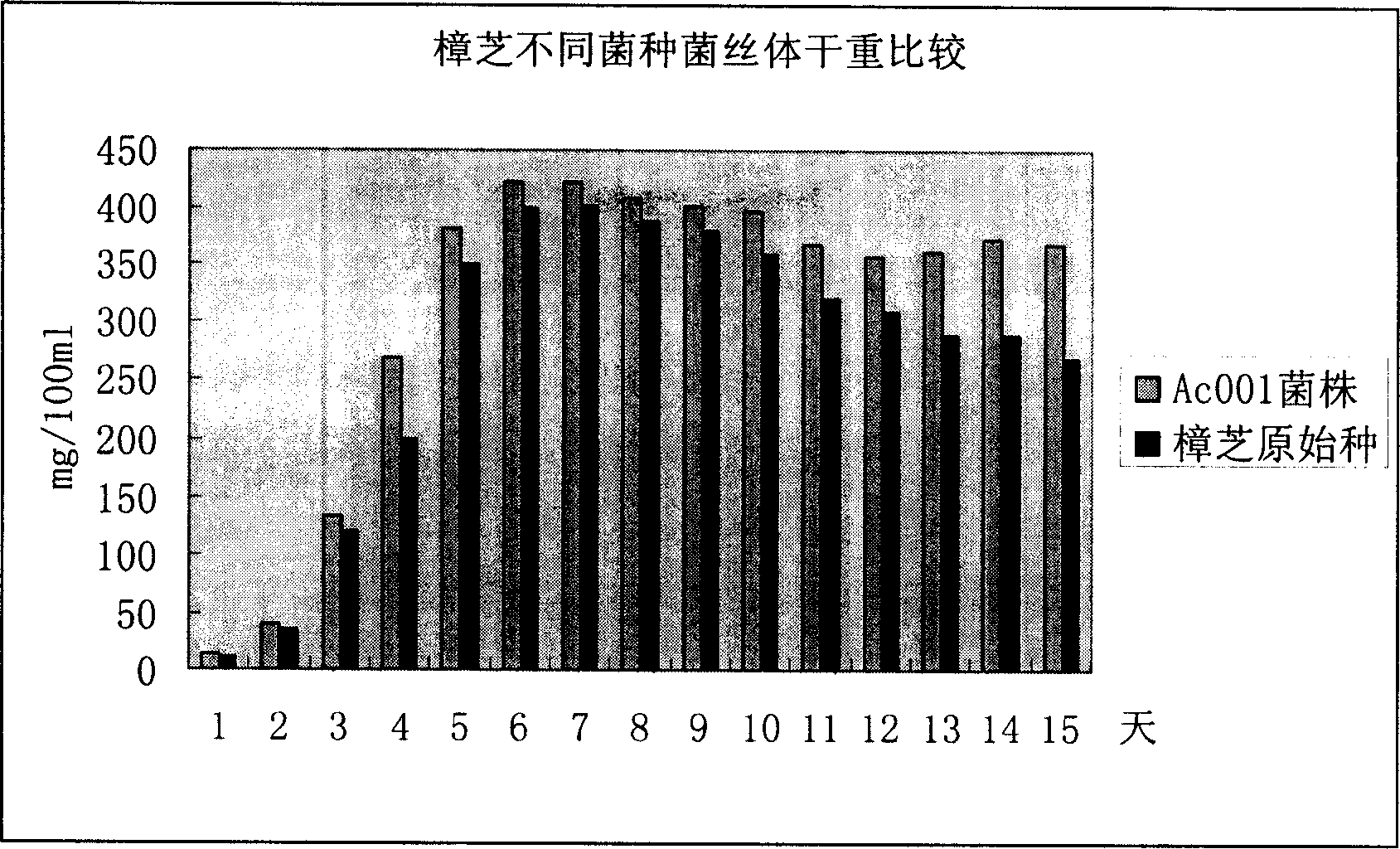
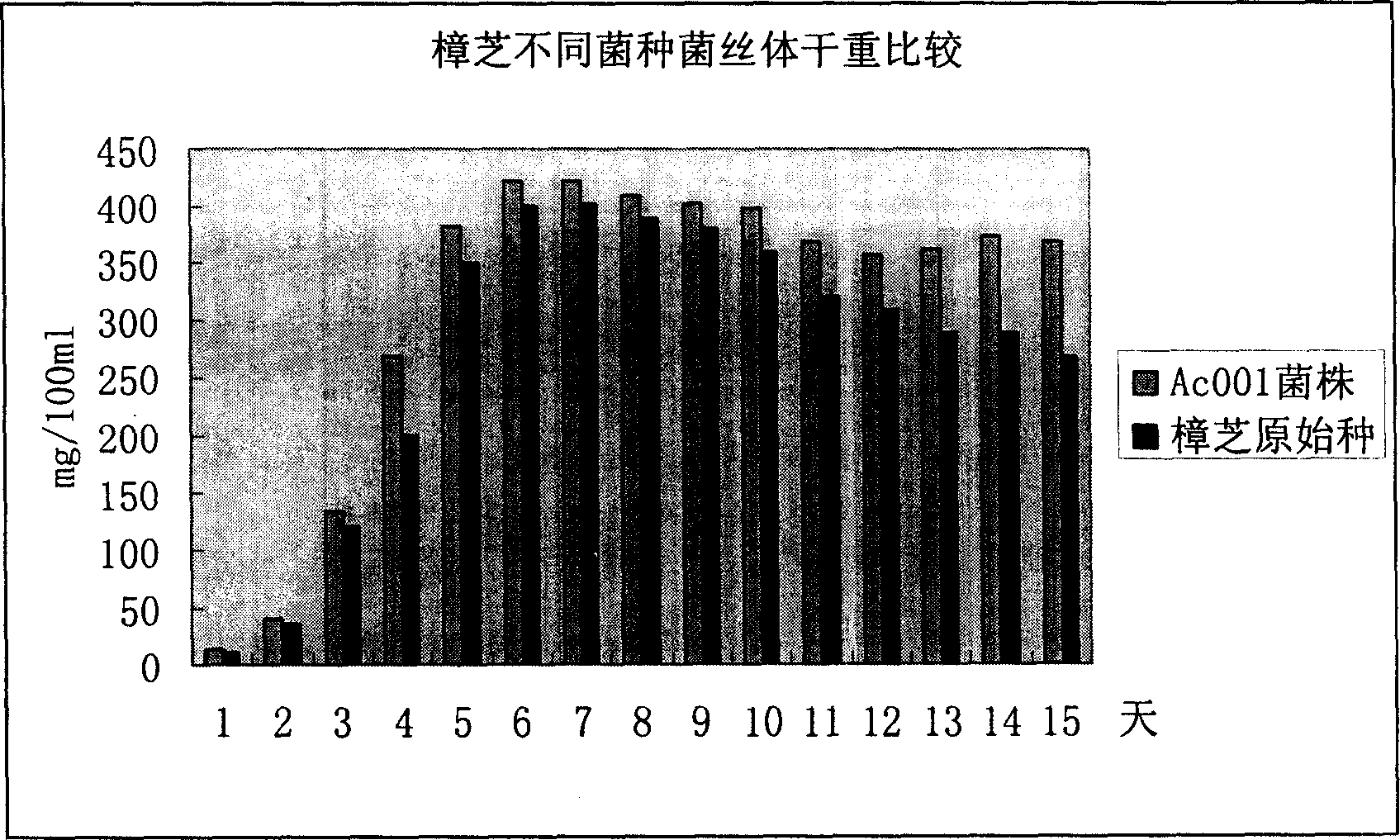
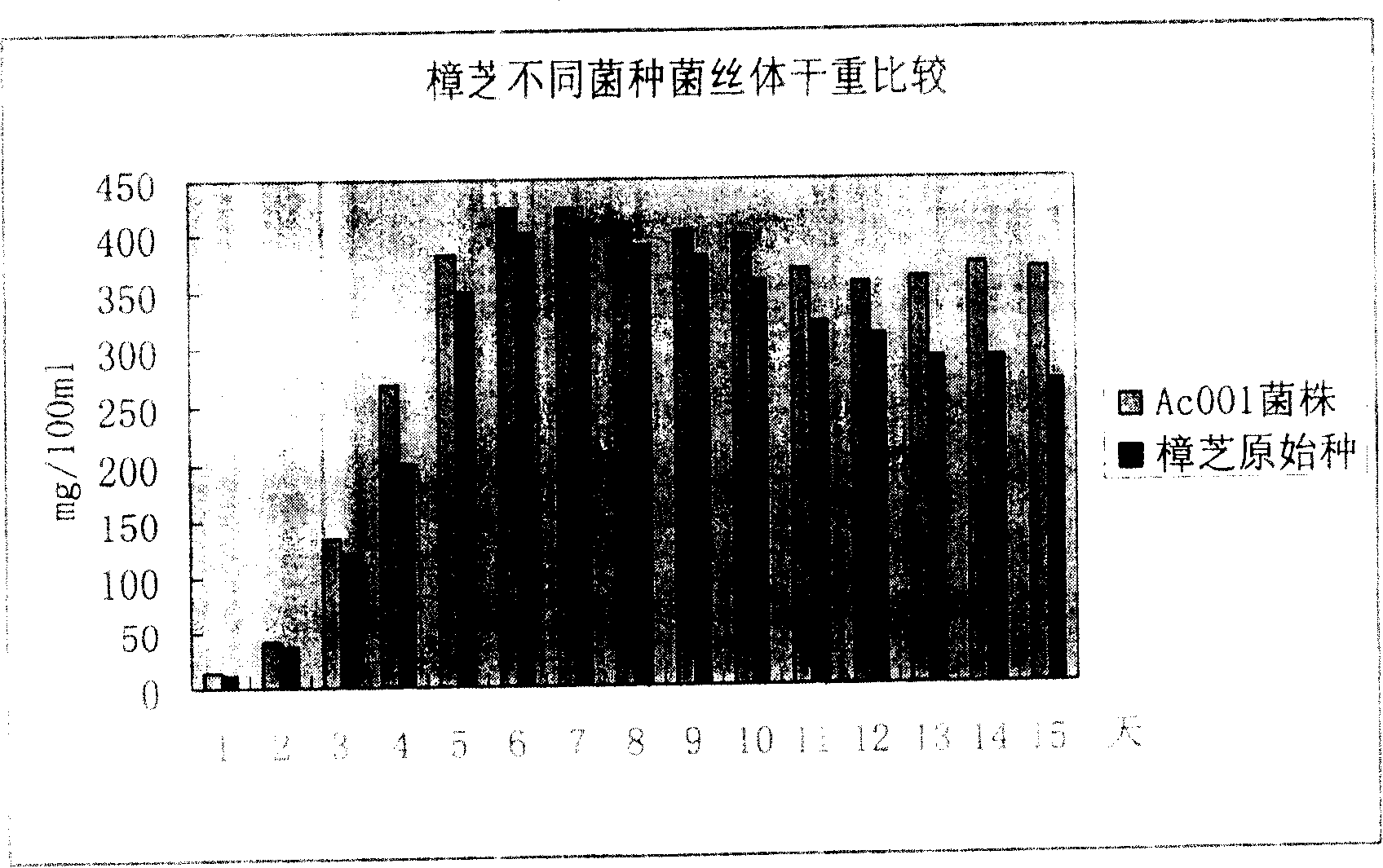
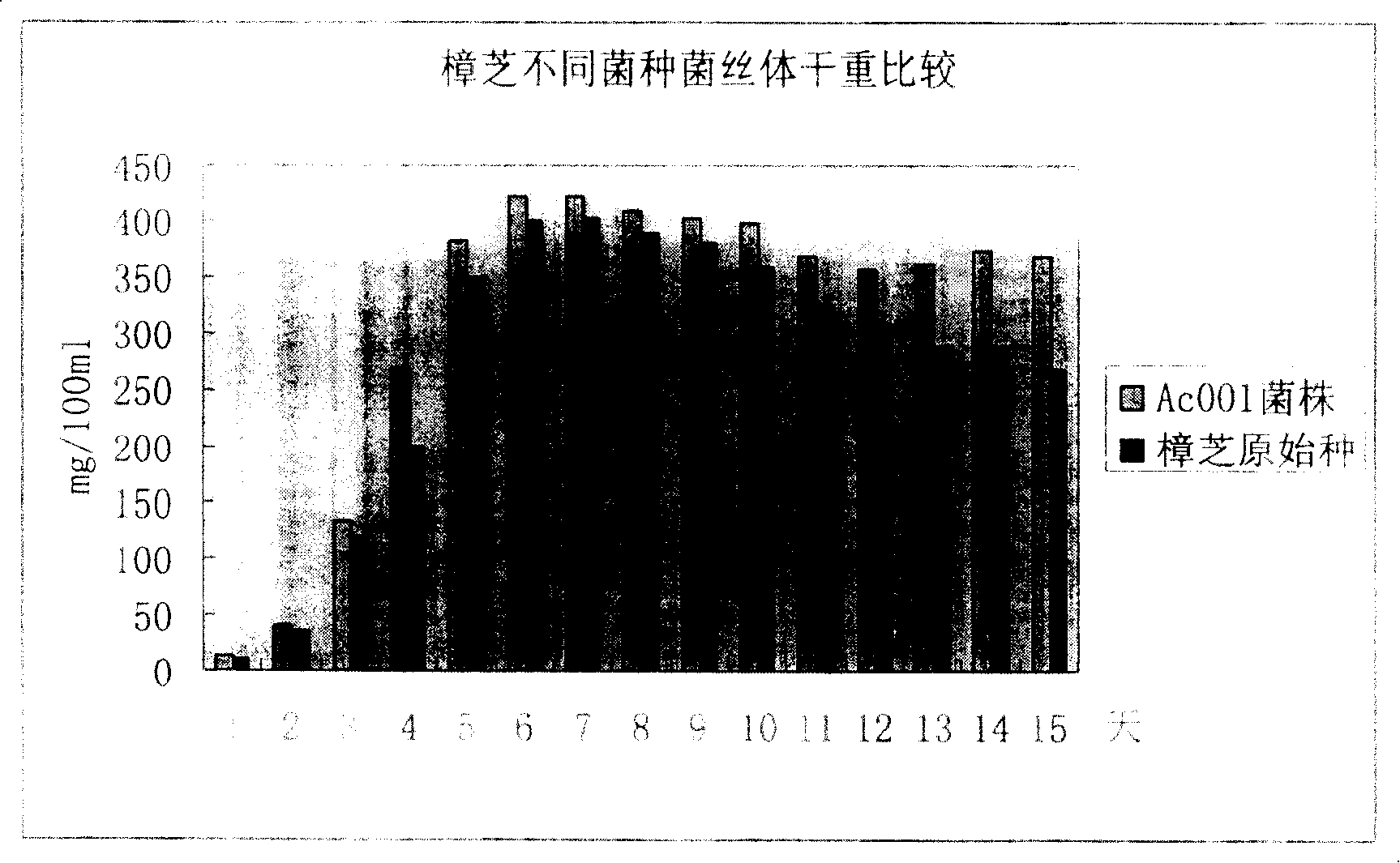
Antrodia camphorata mycelium fermented extract and application thereof
The invention discloses an Antrodia camphorate mycelium fermentation extract abstracted by ethanol, wherein the Antrodia camphorata bacterial strain Ac001 was preserved in the China General Microbiological Culture Collection Center with a docket number of CGMCC No.1460. The Antrodia camphorate mycelium fermentation extract has appreciable actions in inhibiting hepatitis B virus HbsAg, e antigen HbeAg and HBV DNA secretion and resisting cancers especially liver cancer.
Owner:LAIYANG AGRI COLLEGE
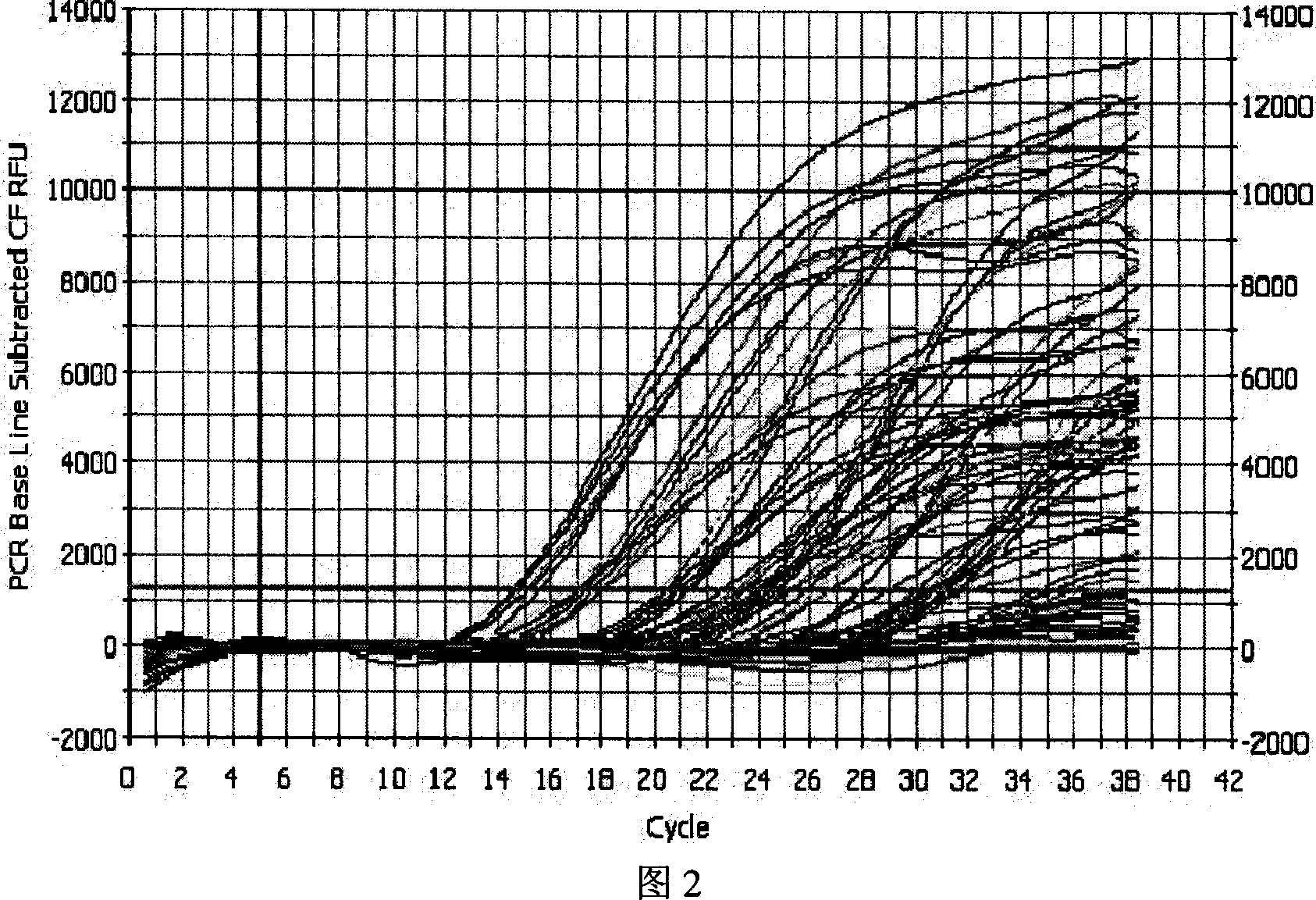
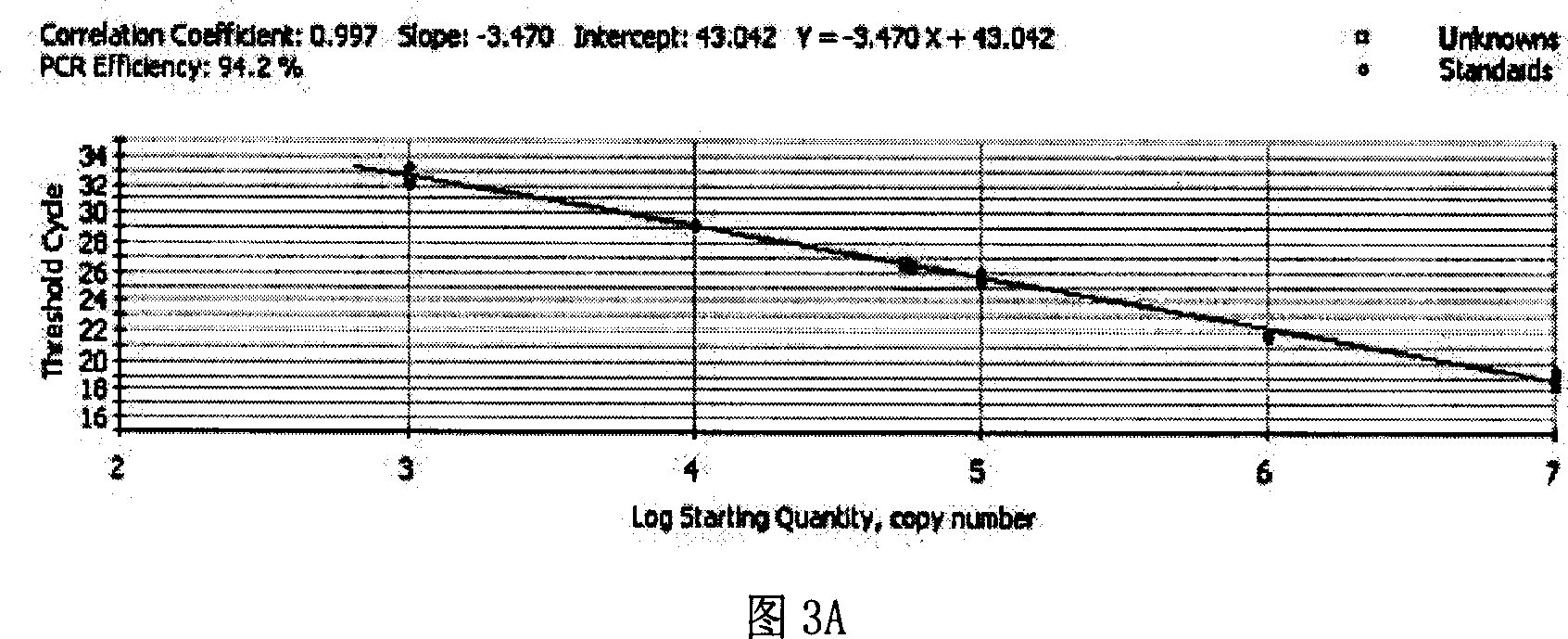
Fluorescent quantitative PCR detecting method for hepatitis B virus and special reagent kit
ActiveCN1944673AReduce extraction timeEasy to operateMicrobiological testing/measurementBiological testingBiotechnologyAntigen
The present invention is fluorescent quantitative HBV PCR detecting method and special reagent kit, and belongs to the field of biotechnology. The method includes comparing three pairs of primer and probe of HBV surface antigen, kernel antigen and E antigen region to screen out one pair of primer and probe with best proliferation effect; comparing the variation in HBV copy number during fluorescent quantitative PCR proliferation of the serum DNA samples extracted in different methods to optimize the HBV extracting method, and performing quantitative PCR detection in optimized primer and probe and in simple virus DNA extracting method. The present invention has high sensitivity, high specificity, low cost, short time, accurate detection and other advantages. The present invention may be used clinically and in scientific research.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Liver cancer resistant Antrodia camphorata and preparation method thererof
The invention discloses an Antrodia camphorata capsule for resisting hepatic carcinoma, which comprises the following constituents (by weight portions): Antrodia camphorate mycelium fermentation extract 20-100, protein-free maize starch or medicinal starch gum 200-480. The Antrodia camphorate mycelium fermentation extract is abstracted by ethanol and dried. The Antrodia camphorata bacterial strain Ac001 was preserved in the China General Microbiological Culture Collection Center with a docket number of CGMCC No.1460. The Antrodia camphorate mycelium fermentation extract in the hepatic carcinoma resisting Antrodia camphorate capsule has appreciable actions in inhibiting hepatitis B virus HbsAg, e antigen HbeAg and HBV DNA secretion and resisting cancers especially liver cancer.
Owner:LAIYANG AGRI COLLEGE
Application of forsythiaside A in preparing hepatitis B therapeutic drug
The invention discloses an application of forsythiaside A in preparing a hepatitis B therapeutic drug. An anti-hepatitis B virus test in vitro of the forsythiaside A shows that the forsythiaside A can effectively inhibit hepatitis B antigen and hepatitis B viruses, which is characterized by the following aspects: using a HepG2.2.2.15 cell model, TC50 cytotoxicity of the forsythiaside A in vitro is equal to 739mu g / mL; IC50 of inhabitation on S antigen is equal to 149.4mu g / mL; IC50 of inhabitation on e antigen is equal to 139.6mu g / mL; and IC50 of inhabitation on a cell HBV-DNA is equal to 56.3mu g / mL.
Owner:SHANDONG NEWTIME PHARMA
Application of wogonin for preparing medicine to treat or prevent hepatitis B
ActiveCN1785174AInhibition of replicationSignificant effectOrganic active ingredientsDigestive systemE AntigensSerum ige
Owner:CHINA PHARM UNIV +1
Zika virus (ZIKV) E antigen and application thereof in detecting anti-ZIKV antibody
The invention belongs to the field of immunity detection of an anti-zika virus (ZIKV) antibody, and particularly relates to a ZIKV E antigen and application thereof in detecting the anti-ZIKV antibody. After a ZIKV gene group and the expression protein thereof are analyzed, the E antigen for specifically detecting the anti-ZIKV antibody is found, the anti-ZIKV antibody in human blood is specifically detected by the founded E antigen, and a kit for detecting the anti-ZIKV antibody is prepared. The method for detecting the anti-ZIKV antibody has the advantages that the use of ZIKV with strong infection ability is effectively avoided in the detection process, so that the risk of a whole experiment is decreased; by adopting the technical scheme, the operation is simple and easy, the repeatability is good, and the like; the method can be easily popularized and applied.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
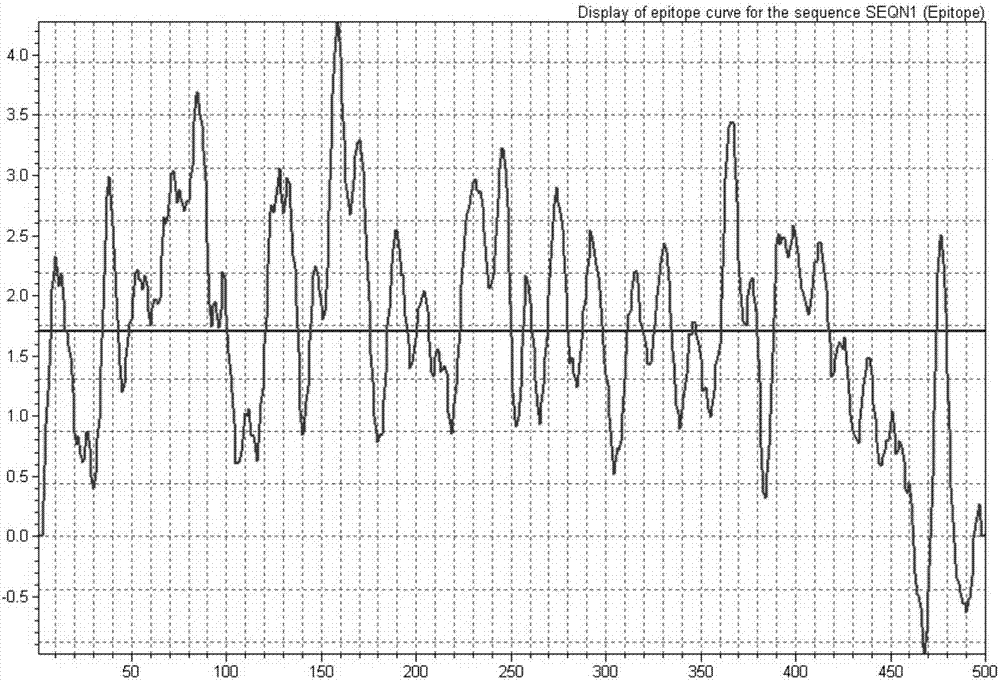
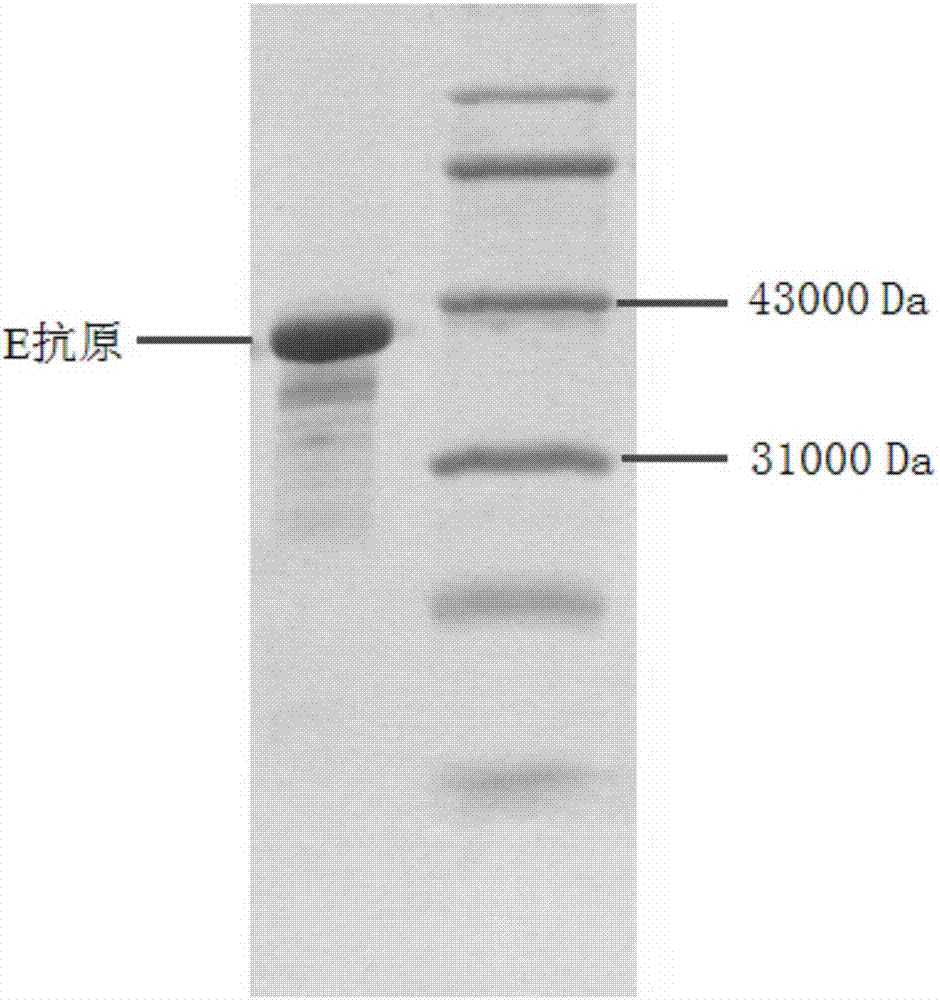
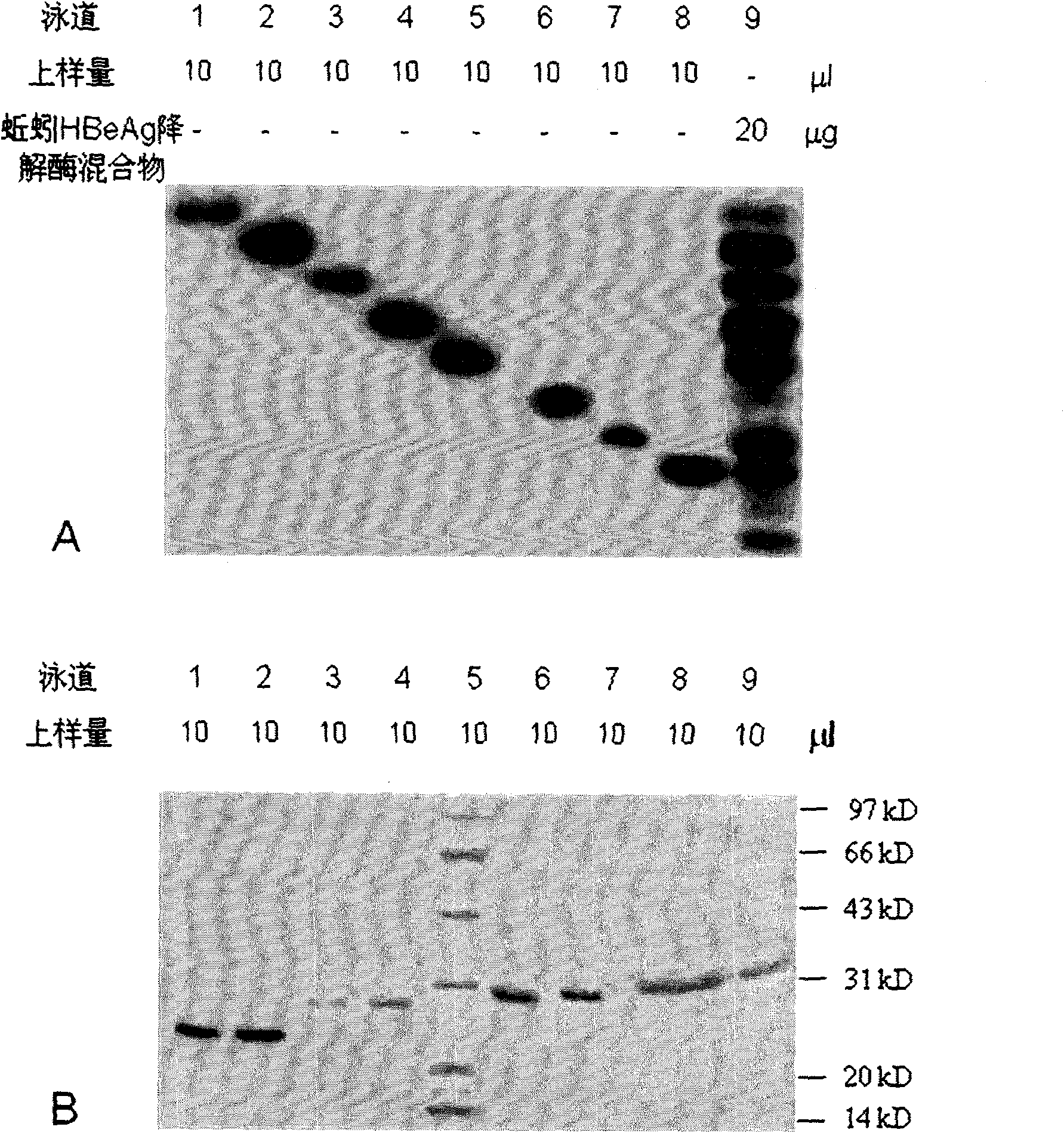
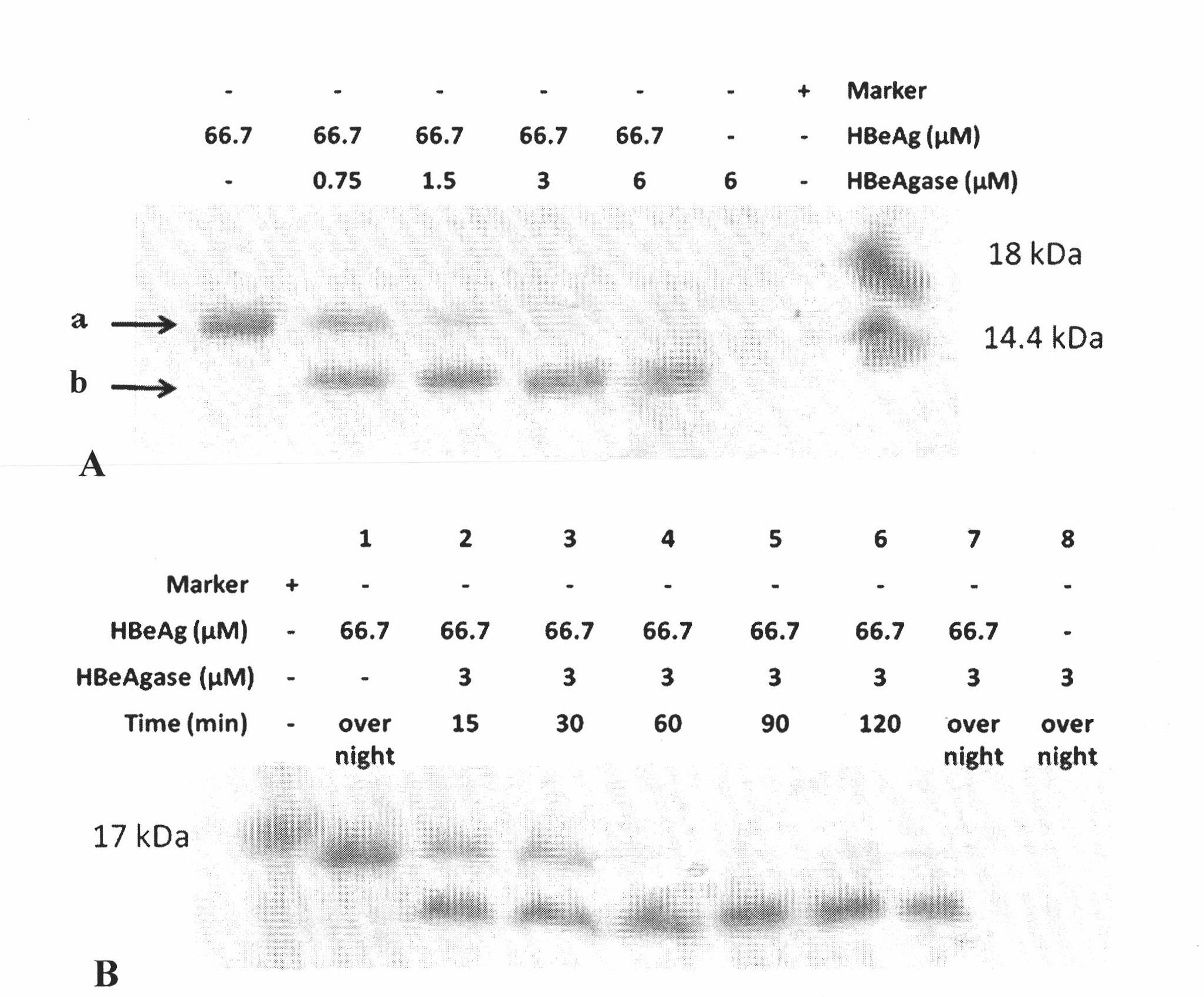
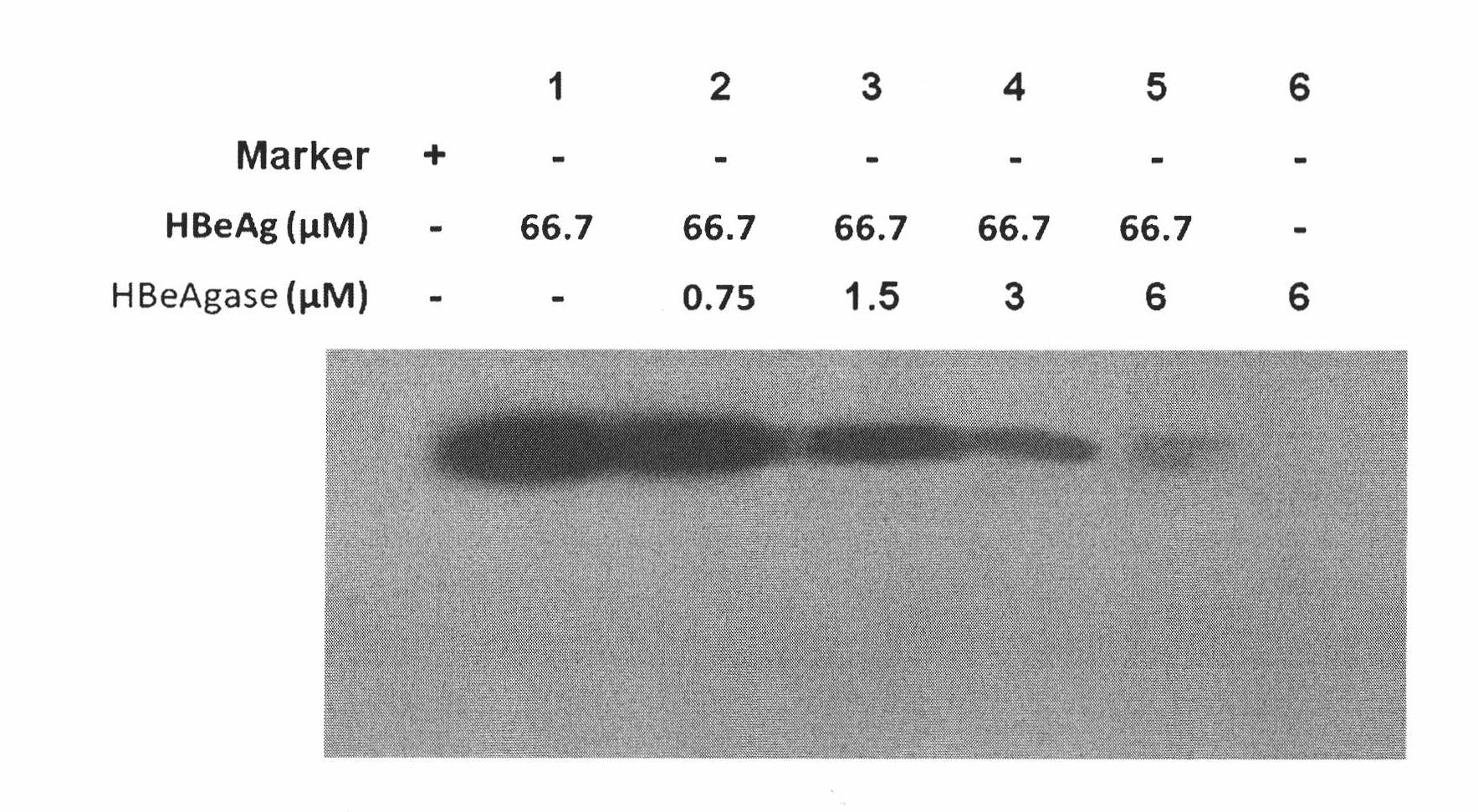
Earthworm protein with HBeAg degrading enzyme activity and application thereof
The invention provides earthworm protein with HBeAg degrading enzyme activity and a medicinal composition containing the earthworm protein. The invention further provides application of the earthworm protein to the preparation of medicines for treating HBeAg-related diseases. The HBeAg-related diseases comprise hepatitis B, cirrhosis caused by hepatitis B, hepatitis B virus vector with e antigen positive and surface antigen positive, and the like. In addition, the invention further provides a method for separating a crude enzyme extract containing the earthworm protein, an earthworm protein crude enzyme extract prepared by the method and application of the earthworm protein crude enzyme extract to the preparation of medicines for treating the HBeAg-related diseases.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
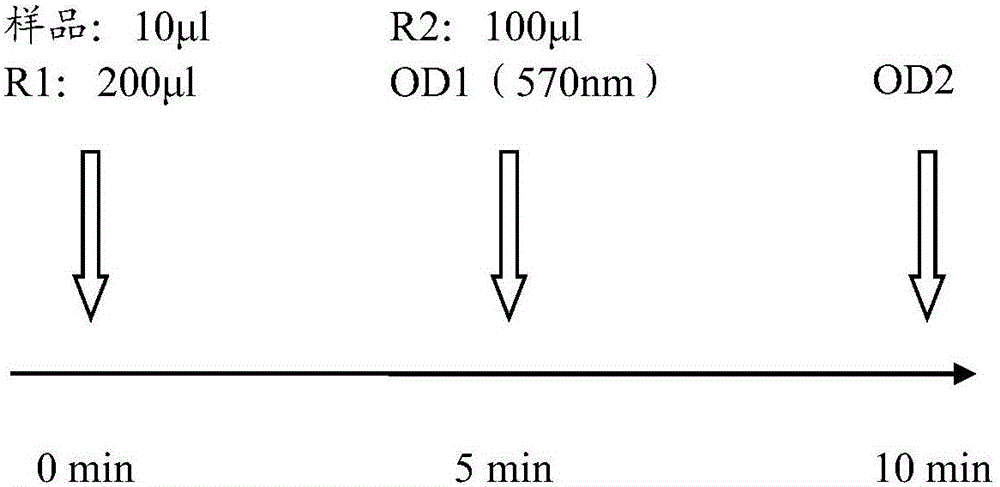
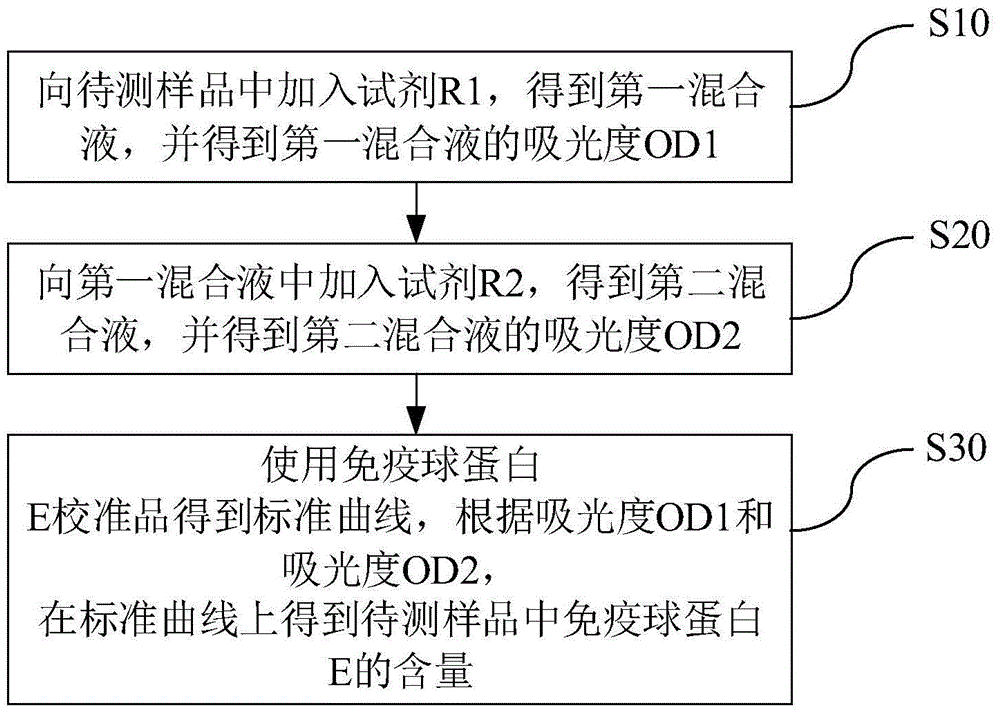
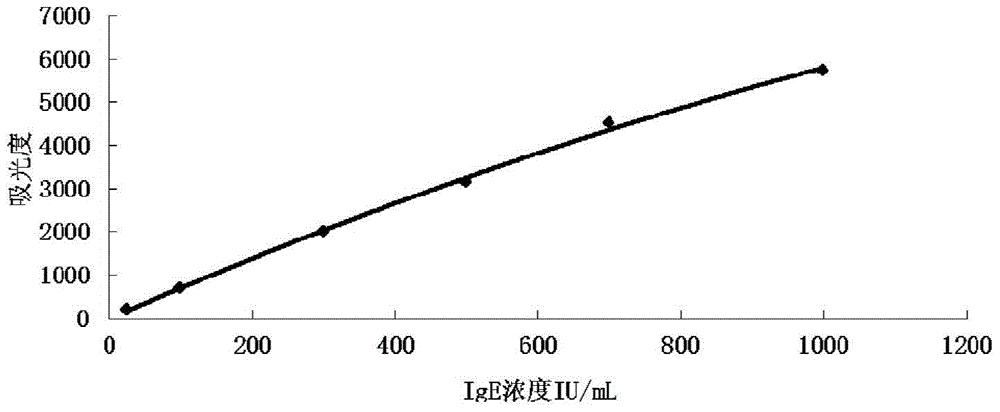
Kit and method for detecting content of immune globulin E and application of kit
The invention discloses a kit for detecting the content of immune globulin E.The kit comprises a reagent R1, a reagent R2 and an immune globulin E calibrator; the reagent R1 is prepared from a first buffer solution, a first electrolyte, surfactant, a first stabilizer, a macromolecular accelerant and first preservative; the reagent R2 is prepared from a second buffer solution, latex particles coated with an anti-human immunoglobulin E antibody, a second electrolyte, a second stabilizer and second preservative; the immune globulin E calibrator is prepared from a third buffer solution, a third stabilizer, third preservative, antioxidant and an anti-human immunoglobulin E antigen.The invention further discloses application of the kit for detecting the content of immune globulin E to detection of the content of immune globulin E and a method of adopting the kit for detecting the content of immune globulin E to detect the content of immune globulin E.The kit and method for detecting the content of immune globulin E are used for detecting the content of immune globulin E and can simply and fast detect the content of immune globulin E.
Owner:潍坊三维生物工程集团有限公司
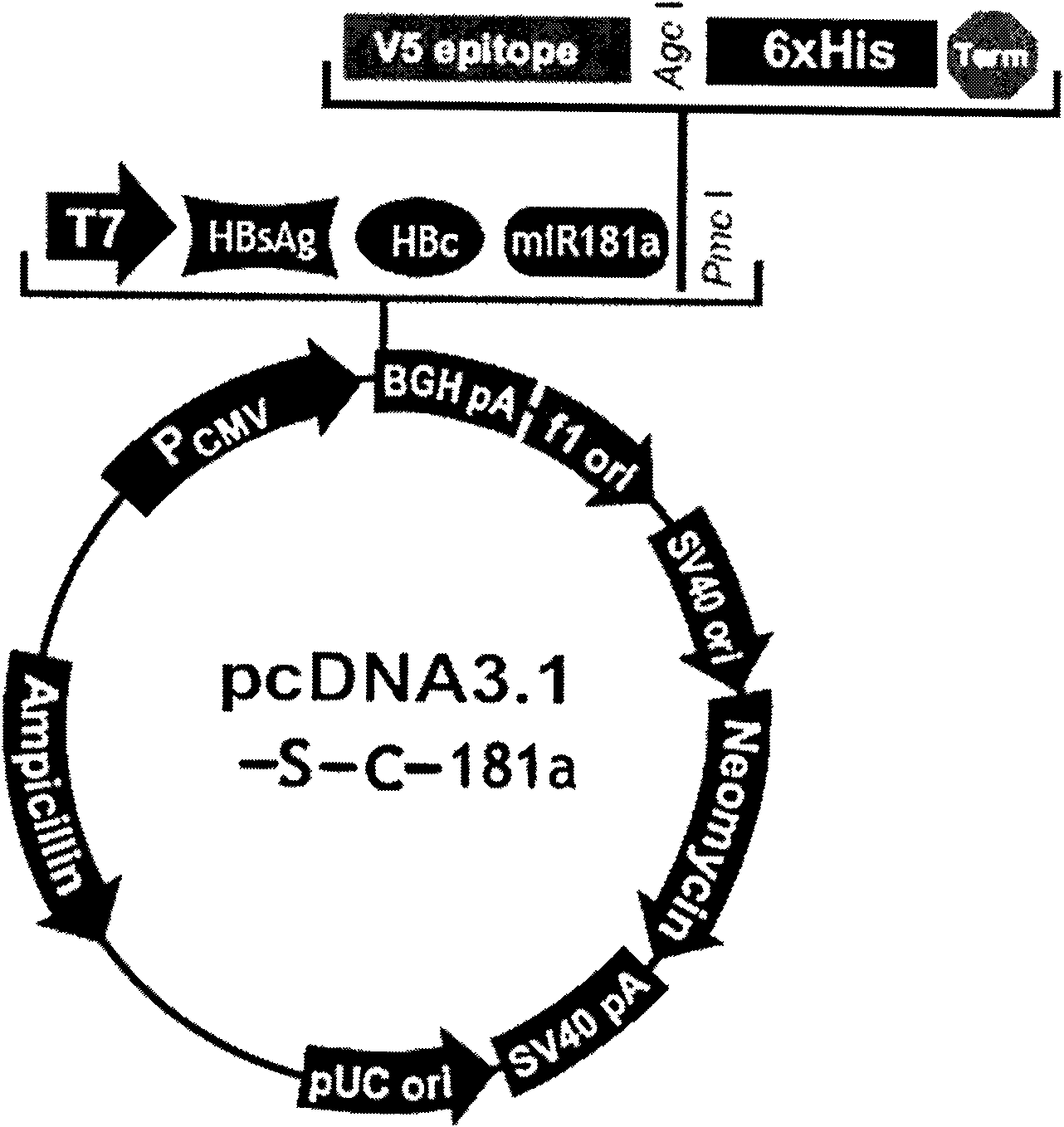
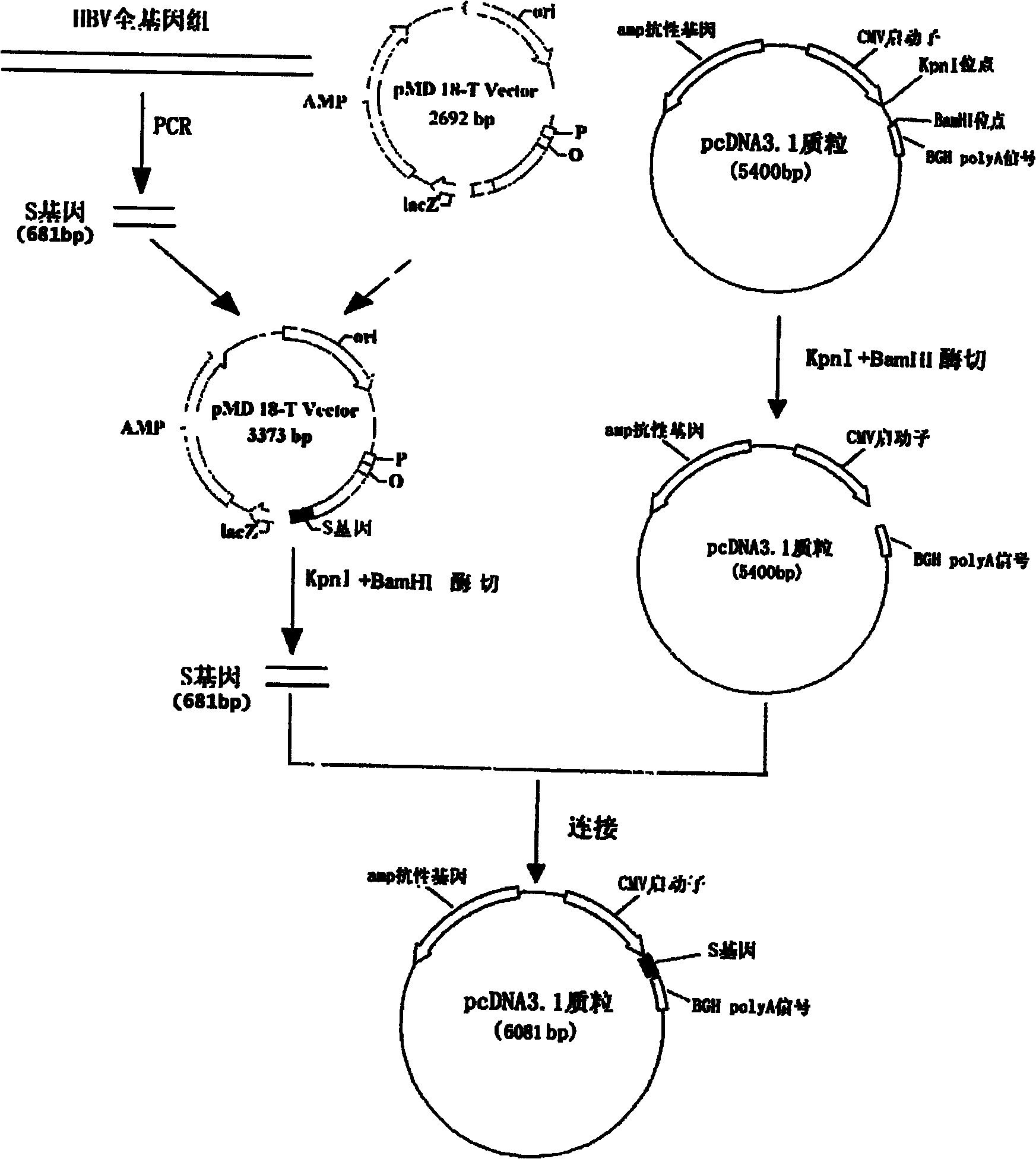
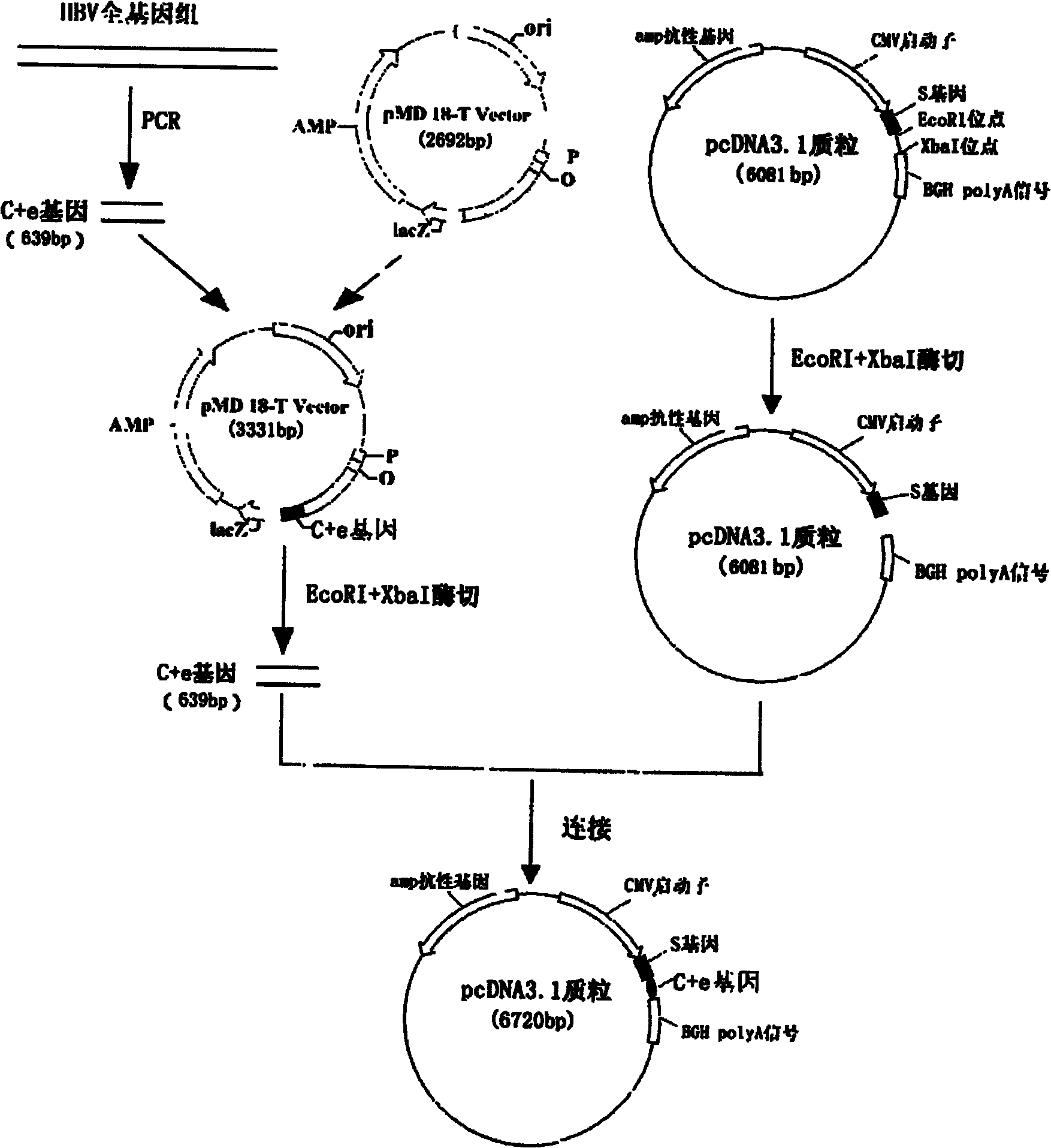
Hepatitis B nucleic acid vaccine and construction method thereof
InactiveCN101954093AImprove protectionGood immune effectGenetic material ingredientsDigestive systemTreatment effectHepatitis B virus DNA
The invention relates to the technical field of biomedicines. The currently reported hepatitis B virus vaccine mainly comprises one or several of HBsAg, preS 1, preS2 and HBcAg, some cell factor genes with immunological enhancement function or lymphocyte epitope genes and the like, however, the immunoprophylaxis effect and treatment effect of the vaccine are always unsatisfactory. The invention provides a hepatitis B vaccine which comprises hepatitis B virus surface antigen gene HBsAg, core protein gene HBc and e-antigen gene, also comprises a human microRNA181a precursor gene sequence, and can assist stimulating the body immunity pathway. The construction process of the vaccine relates to PCR, enzyme cutting, connection, conversion and other molecularly biological operating means. The hepatitis B vaccine has the advantages of effectively activating the body to produce a specific antibody against the hepatitis B virus, stimulating body cell immunity and secreting various cell factors. Therefore, the aim of treating chronic hepatitis B is fulfilled.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
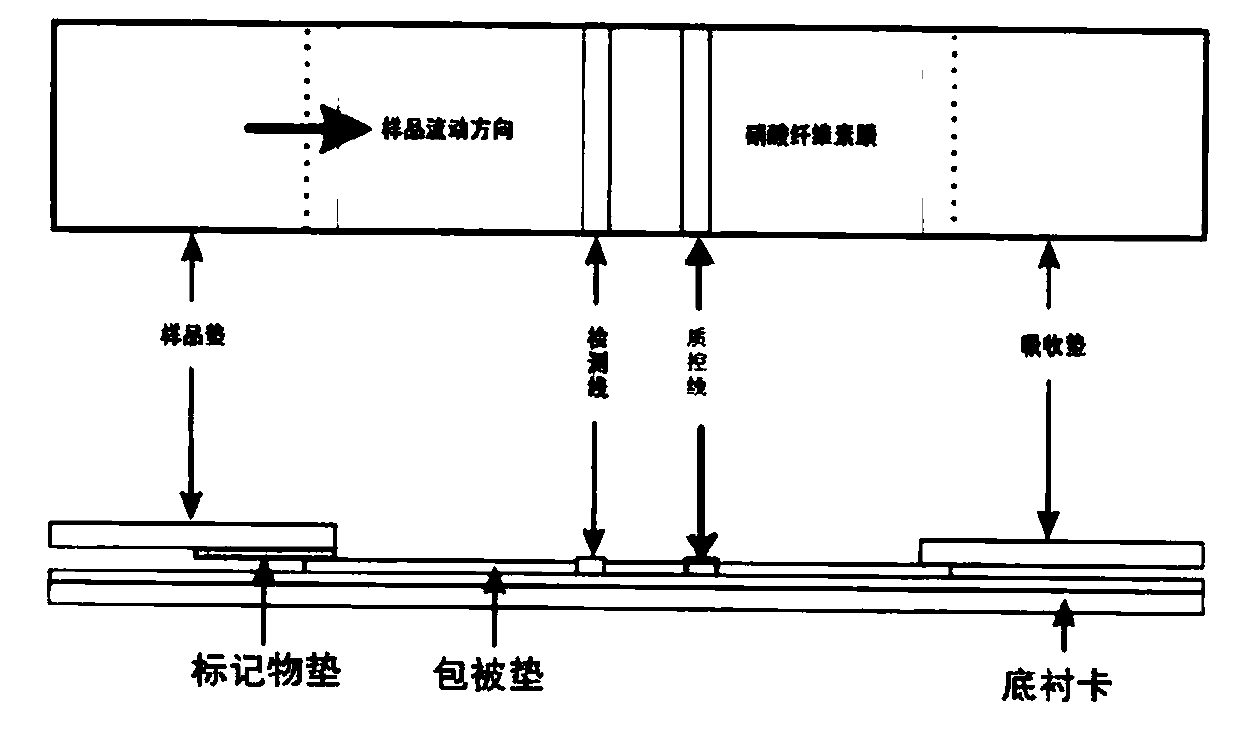
Zika virus E antigen and application of Zika virus E antigen in fluorescence immunochromatographic reagent
ActiveCN110568177ARealize intelligent computingEasy to operateBiological testingAgainst vector-borne diseasesAntigenZika virus
The invention relates to a Zika virus E antigen and application of the Zika virus E antigen in a fluorescence immunochromatography reagent. The amino acid sequence of the Zika virus E antigen is shownas SEQ ID NO.2. The invention also relates to the application of the Zika virus E antigen in the fluorescence immunochromatography reagent. According to the application, the fluorescence immunochromatographic reagent for detecting a Zika virus antibody is prepared by using the Zika virus E antigen, a sample pad, a marker pad, a coating pad and an absorption pad are sequentially overlapped and adhered to a bottom lining card, the marker pad is a glass fiber coated with a fluorescent microsphere labeled mouse anti-human IgG monoclonal antibody, and the coating pad is a nitrocellulose membrane which is coated with a goat anti-mouse IgG antibody as a quality control line and coated with a detection line of Zika virus E protein. The fluorescence immunochromatography reagent for detecting the Zika virus IgG antibody provided by the invention has the advantages of strong specificity, high detection sensitivity and simplicity and convenience in detection result judgment.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
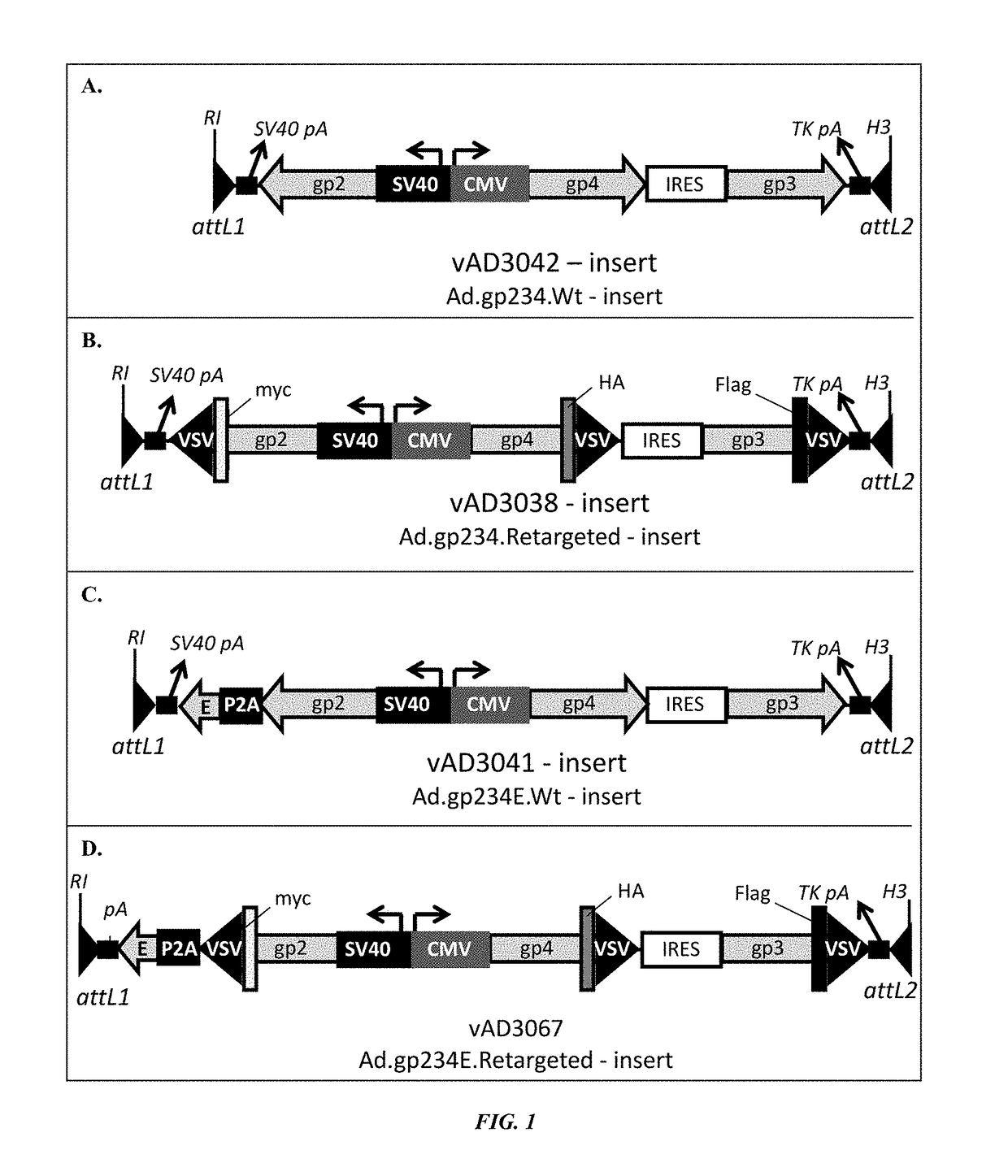
PRRSV minor protein-containing recombinant viral vectors and methods of making and use thereof
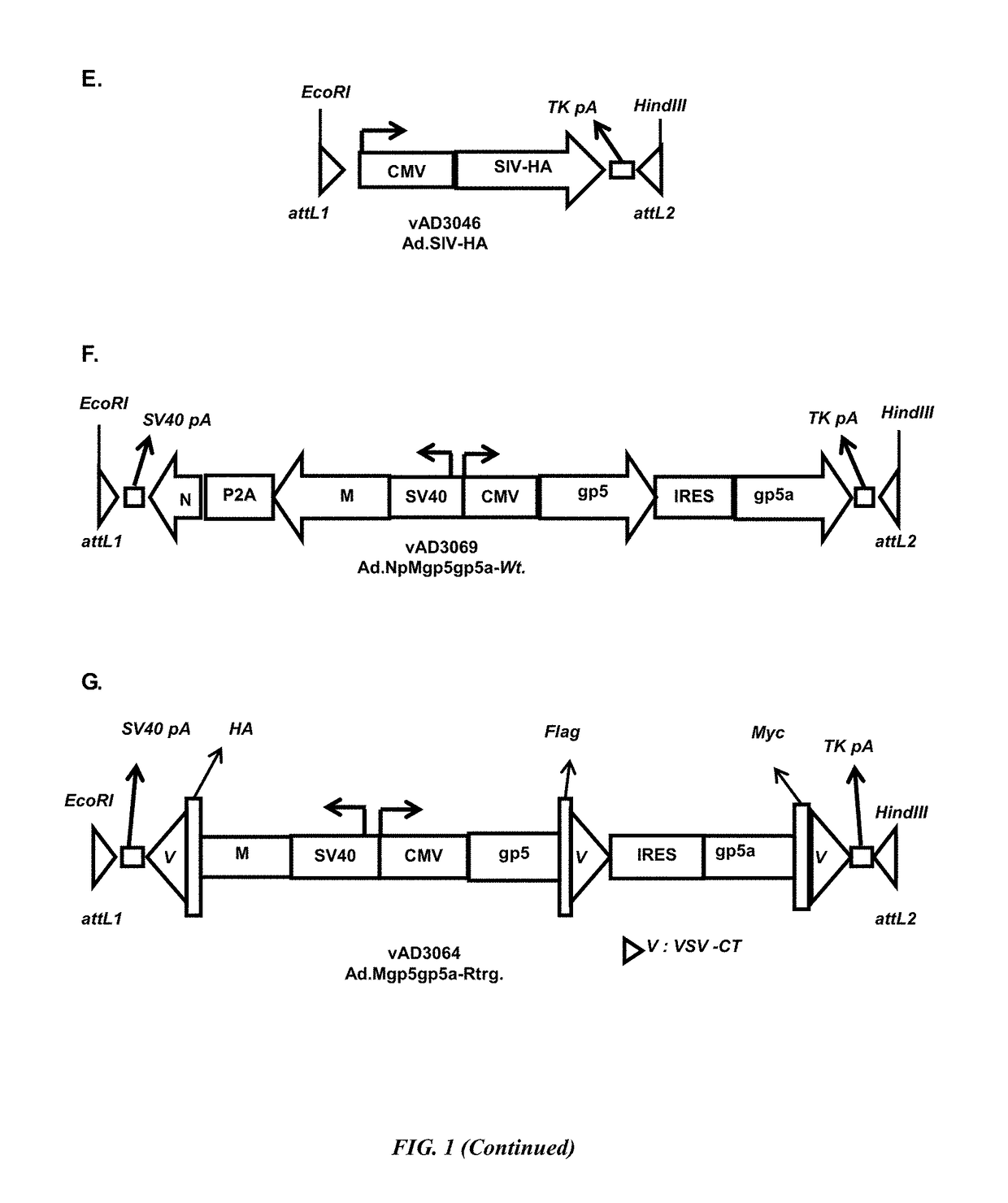
ActiveUS20160375122A1Avoid infectionStrong immune responsePolypeptide with localisation/targeting motifSsRNA viruses positive-senseEpitopeE Antigens
The present invention encompasses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) vaccines or compositions. In particular, the invention encompasses recombinant adenovirus vectors encoding and expressing PRRSV gp2, gp3, gp4, gp5a, gp5 and / or E antigens, proteins, epitopes or immunogens. Such vaccines or compositions can be used to protect animals from PRRSV.
Owner:MERIAL INC
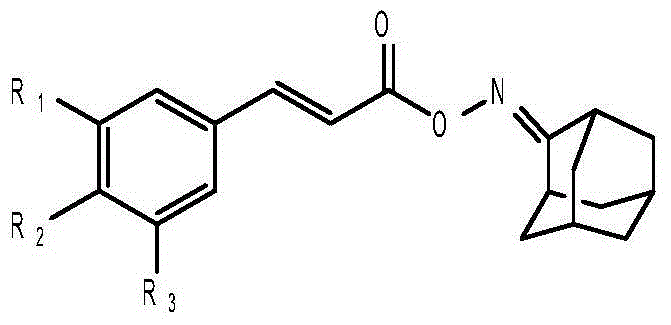
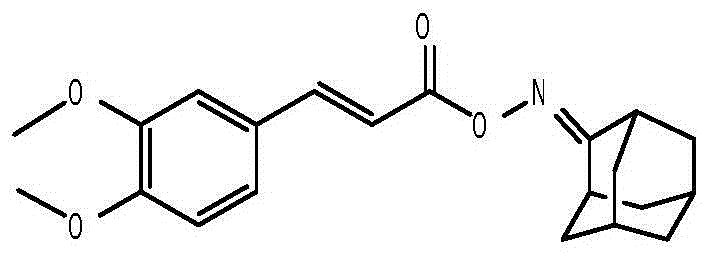
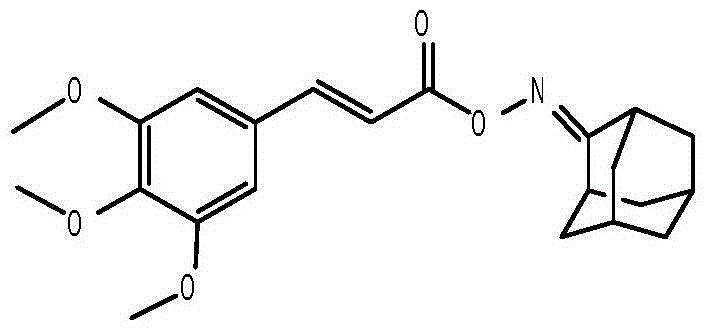
Anti-hepatitis B virus active phenylpropanoids adamantine ketoxime ester compound
InactiveCN104693066AHas inhibitory effectHigh inhibition rateAntiviralsOximes preparationPhenylpropanoidAntigen
The invention discloses an anti-hepatitis B virus active phenylpropanoids adamantine ketoxime ester compound. The structural formula of the phenylpropanoids adamantine ketoxime ester compound is shown in the description, and by conducting a HepG2.2.15 cell experiment, we find that the compound has certain inhabitation effect on HBV surface antigen (HBsAg) and e antigen (HBeAg) and is expected to prepare anti-hepatitis B virus medicines.
Owner:GUANGXI UNIV
Lignan compound and preparation method thereof
The invention discloses a lignan compound and a preparation method thereof. The chemical name of the lignan compound is (5R,6S,7R)-5-(3',4'-dimethoxyphenyl)-4-methoxy-6,7-di(methoxymethyl)-5,6,7,8-tetrahydronaphthalene[2,3-d][1',3']dioxole. The preparation method comprises the following steps of: crushing phyllanthus niruri linn which is dried in the shade and serves as a raw material; performing solvent extraction; filtering extracting solution and concentrating to obtain extractum; performing extraction separation on the extractum to obtain an active part of the (5R,6S,7R)-5-(3',4'-dimethoxyphenyl)-4-methoxy-6,7-di(methoxymethyl)-5,6,7,8-tetrahydronaphthalene[2,3-d][1',3'] dioxole; separating repeatedly by column chromatography; and crystallizing to obtain the new lignan compound, namely the (5R,6S,7R)-5-(3',4'-dimethoxyphenyl)-4-methoxy-6,7-di(methoxymethyl)-5,6,7,8-tetrahydronaphthalene[2,3-d][1',3'] dioxole. The lignan compound can inhibit secretion of hepatitis B virus surface antigen HbsAg and hepatitis B liver e antigen HbeAg in a human body.
Owner:GUANGXI UNIV
Antrodia camphorata mycelium fermented extract and application thereof
The invention discloses an Antrodia camphorate mycelium fermentation extract abstracted by ethanol, wherein the Antrodia camphorata bacterial strain Ac001 was preserved in the China General Microbiological Culture Collection Center with a docket number of CGMCC No.1460. The Antrodia camphorate mycelium fermentation extract has appreciable actions in inhibiting hepatitis B virus HbsAg, e antigen HbeAg and HBV DNA secretion and resisting cancers especially liver cancer.
Owner:LAIYANG AGRI COLLEGE
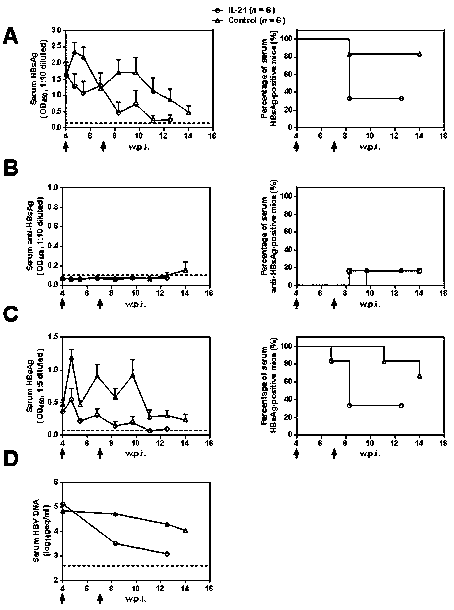
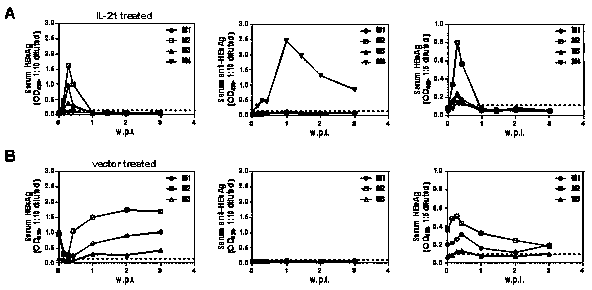
Application of interleukin 21 (IL-21) in preparation of anti-hepatitis B virus (HBV) medicine preparations
InactiveCN108324930AReduce DNA loadReduce loadPeptide/protein ingredientsDigestive systemSerum igeE Antigens
The invention belongs to the fields of genetic engineering and biomedicines and relates to a novel medicinal application of interleukin 21 (IL-21), and particularly relates to an application of the IL-21 in preparation of an anti-HBV preparation. Tests prove that by using the IL-21 for intervening in a HBV chronically infected mouse model, conversion rate of hepatitis B surface antigen (HBsAg) andhepatitis B e antigen (HBeAg) can be significantly increased, and meanwhile, DNA carrying quantity of HBV in serum is significantly reduced. The IL-21 can be used as a medicine precursor for developing a novel anti-hepatitis B virus medicine.
Owner:FUDAN UNIV +1
Liver cancer resistant Antrodia camphorata and preparation method thererof
Owner:LAIYANG AGRI COLLEGE
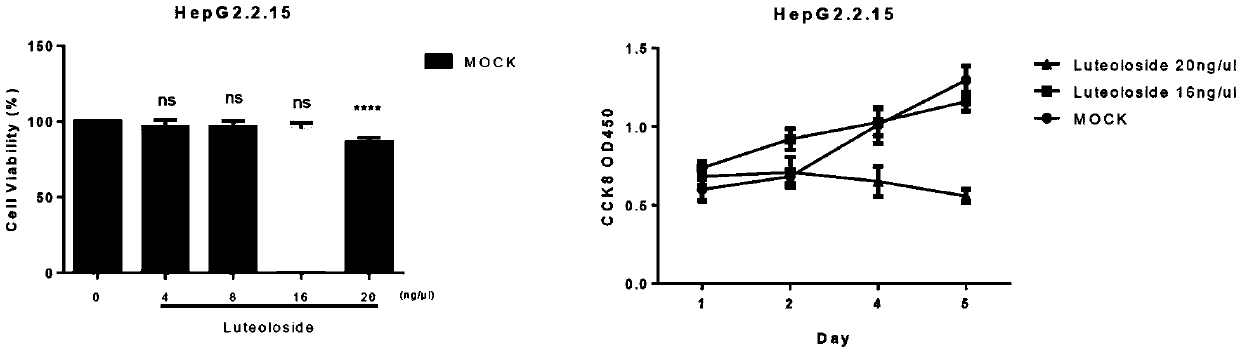
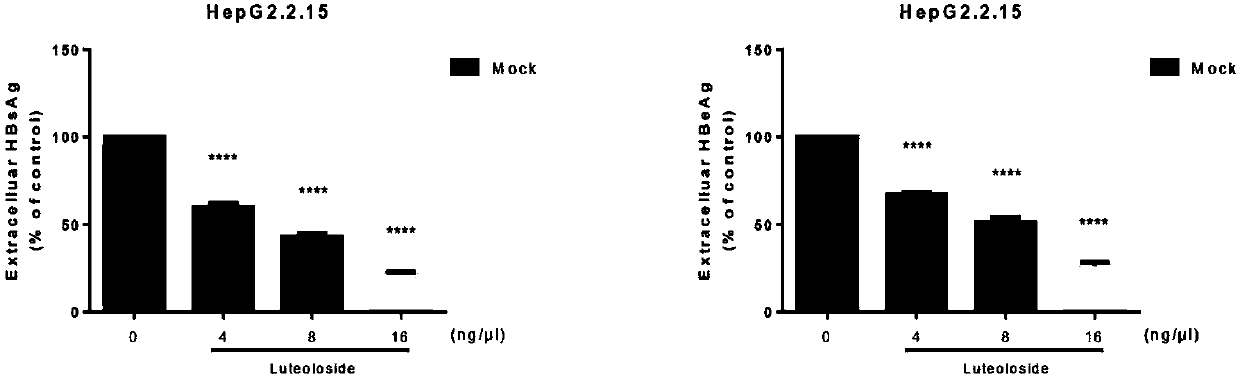
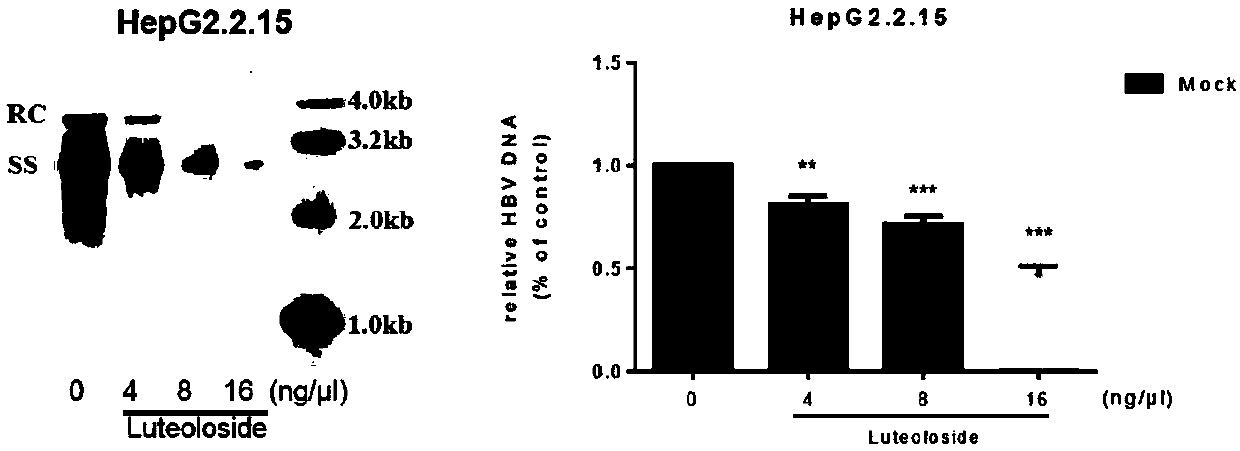
Application of luteolin 7-O-glucoside in preparing anti-HBV drug
The invention belongs to the field of chemicals and relates to an application of luteolin 7-O-glucoside (the molecular formula is C21H20O11, CAS: 5373-11-5) in preparing an anti-HBV drug. The compoundis free of obvious toxic and side effects and anti-proliferative effect to HepG2.2.15 cells which express and secrete hepatitis B virus. The concentration of the maximum non-toxic effect reaches 16 [mu]g / ml. Hatched in 48 hours at the concentration, the inhibiting rates of the compound on a hepatitis b surface antigen HBsAg and an e antigen HBeAg secreted by the HepG2.2.15 cells can separately reach 77.4% and 73.3%, the copy inhibiting effect of hepatitis B virus in the HepG2.2.15 cells reaches 45%, and the transcription inhibiting effect to hepatitis B virus in the HepG2.2.15 cells can reachabout 50%. The formula is as shown in the description.
Owner:FUDAN UNIV +1
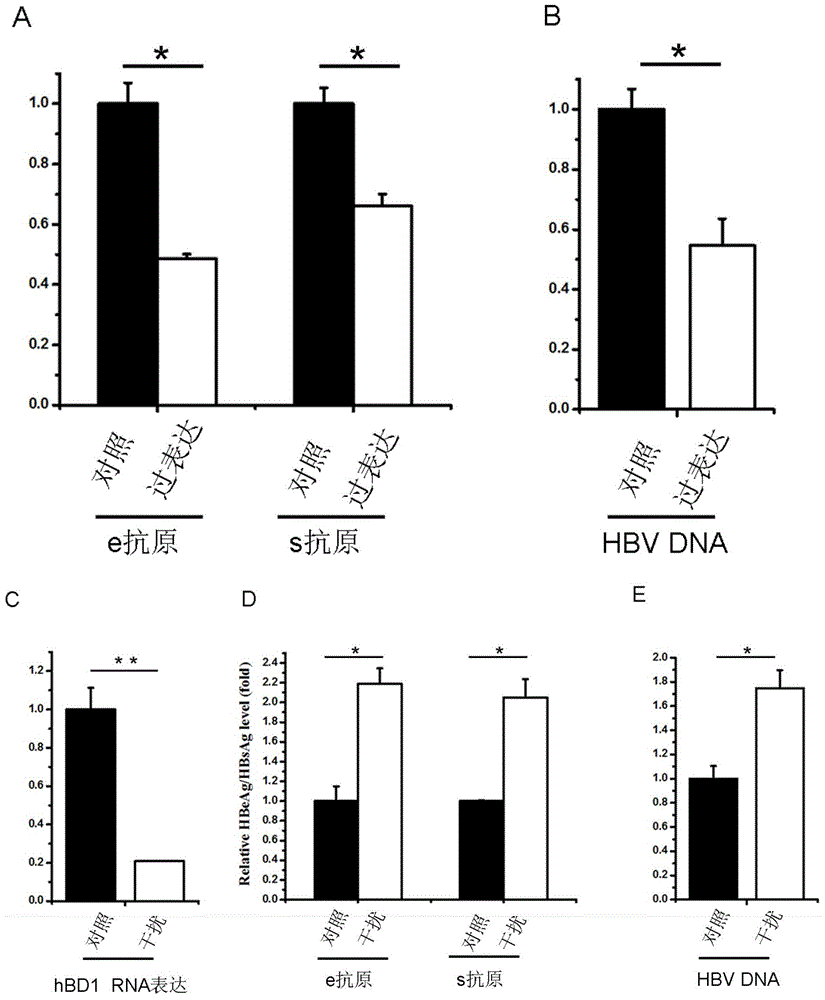
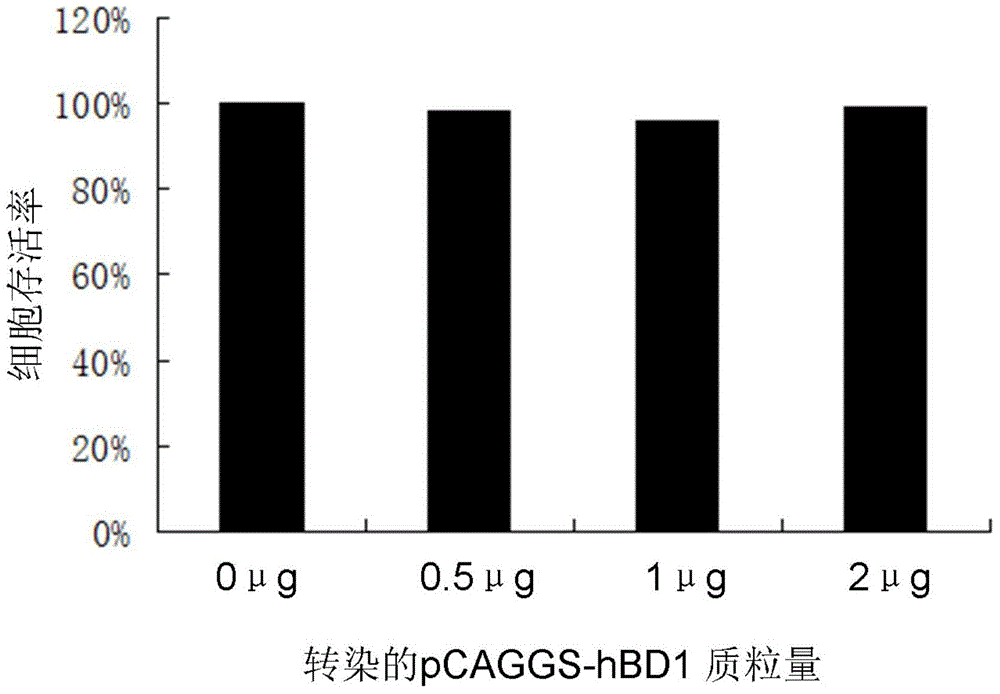
Use of human beta-defensin-1 in preparation of drugs for treating or preventing hepatitis B virus infection
ActiveCN105031613ANo side effectsLower resistancePeptide/protein ingredientsAntiviralsAntigenHepatitis B virus
The invention discloses a use of human beta-defensin-1 in preparation of drugs for treating or preventing hepatitis B virus infection. Through cotransfection of beta-defensin-1 overexpression plasmids or small interfering RNAs targeting beta-defensin-1 and HBV 1.3 ploid plasmids into liver cells, beta-defensin-1-caused influence on hepatitis B virus replication is researched, and surface antigen and e antigen secreted by viruses and an intracellular virus nucleocapsid-related DNA level are detected, and the detection result shows that replication of hepatitis B viruses in liver cells are inhibited by beta-defensin-1. Beta-defensin-1 can influence HBV activity or influence HBV infection or replication after infection. Through individual use or combination with other anti-HBV drugs, beta-defensin-1 can produce important effects in prevention or treatment on hepatitis B virus infection. Human beta-defensin-1 is an anti-microbe polypeptide constitutively expressed in the human body and has no toxic and side effect on the human body at a physiological concentration.
Owner:广东龙帆生物科技有限公司
HLA-DR9 restrictive regulatory T cell epitope of Hepatitis B virus core antigen and e antigen and application thereof
InactiveCN101942013ABreak immune toleranceClear specificityDigestive systemVirus peptidesHepatitis B virus core AntigenRegulatory T cell
The invention discloses an HLA-DR9 restrictive regulatory T cell epitope of Hepatitis B virus core antigen and e antigen, which is composed of the following amino acid sequence: SRDLVVNYVNTNMGLKIRQLLWFHI. The invention also discloses an application of a regulatory T cell epitope in preparing hepatitis B therapeutic vaccine containing the Hepatitis B virus core antigen and e antigen, namely when the hepatitis B therapeutic vaccine containing the Hepatitis B virus core antigen and / or e antigen is prepared, the regulatory T cell epitope with immunosuppressive action in the Hepatitis B virus coreantigen and / or e antigen is eliminated. The invention provides a new strategy and a method for development of the high-efficient hepatitis B therapeutic vaccine, is expected to break hepatitis B immune tolerance, rebuilds cellular immune function and effectively inhibits and eliminates the hepatitis B virus.
Owner:ARMY MEDICAL UNIV
Detection kit for varicella-herpes zoster virus neutralizing antibody and detection method for varicella-herpes zoster virus neutralizing antibody
The invention relates to a detection kit for a varicella-herpes zoster virus neutralizing antibody and a detection method for the varicella-herpes zoster virus neutralizing antibody. The detection kitcomprises a recombination expression varicella-herpes zoster virus glycoprotein E antigen, a sample dilution solution, 20-times concentrated washing solution, an enzyme-labelled second antibody, a substrate, a stop solution, standard serum and negative control. The recombination expression varicella-herpes zoster virus glycoprotein E antigen is a recombination expression plasmid transformation escherichia coli which is constructed by connecting a VZV-gE gene and a recombination expression vector pET-32a(+) vector, wherein the VZV-gE gene is optimized to be a escherichia coli preferred codon,and expressed recombinant protein is cloned, cultured and induced. The antigen used in the detection kit is the recombination expression varicella-herpes zoster virus glycoprotein E antigen, the safety is good, and pollution to the environment is not caused. The recombination expression varicella-herpes zoster virus glycoprotein E antigen is prepared by genetic engineering, the specificity is high, the quality is pure, and the cost is relatively low; and antigen site selection is accurate, coupling is good, nonspecific binding can be effectively excluded, the measurement error is reduced, andthe appearance of false negative and false positive is reduced.
Owner:WUHAN LIFE TECH
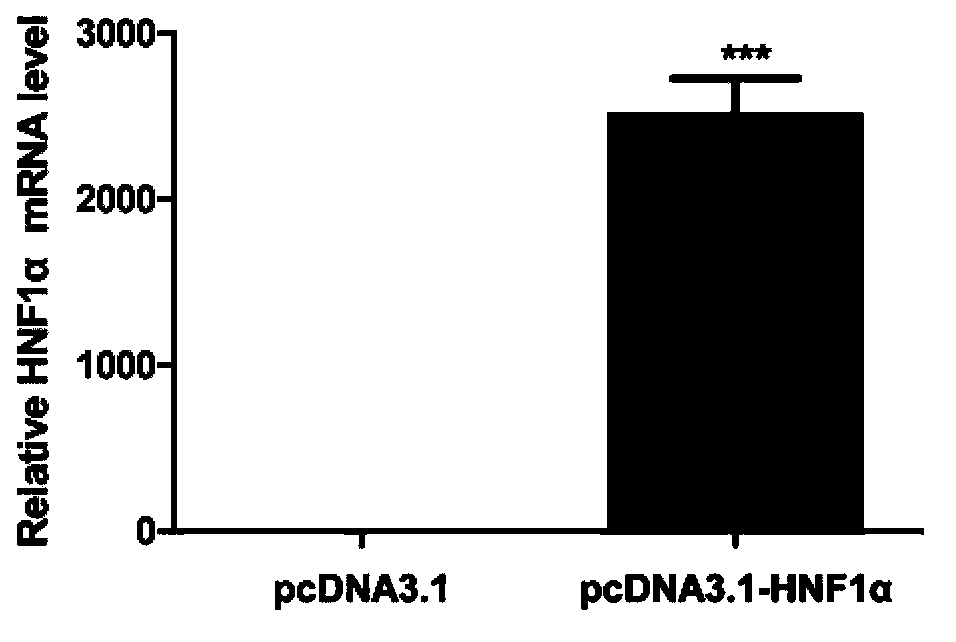
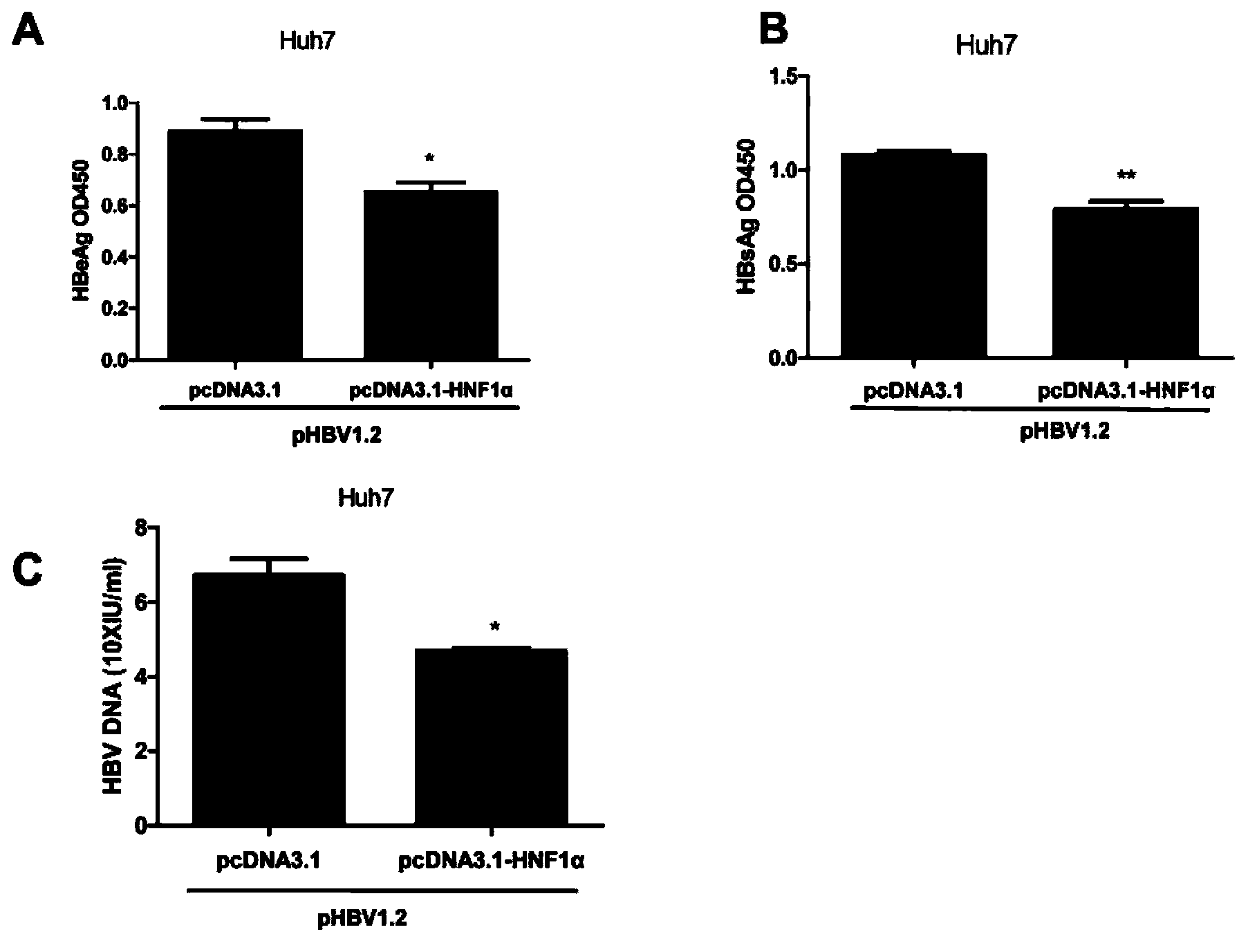
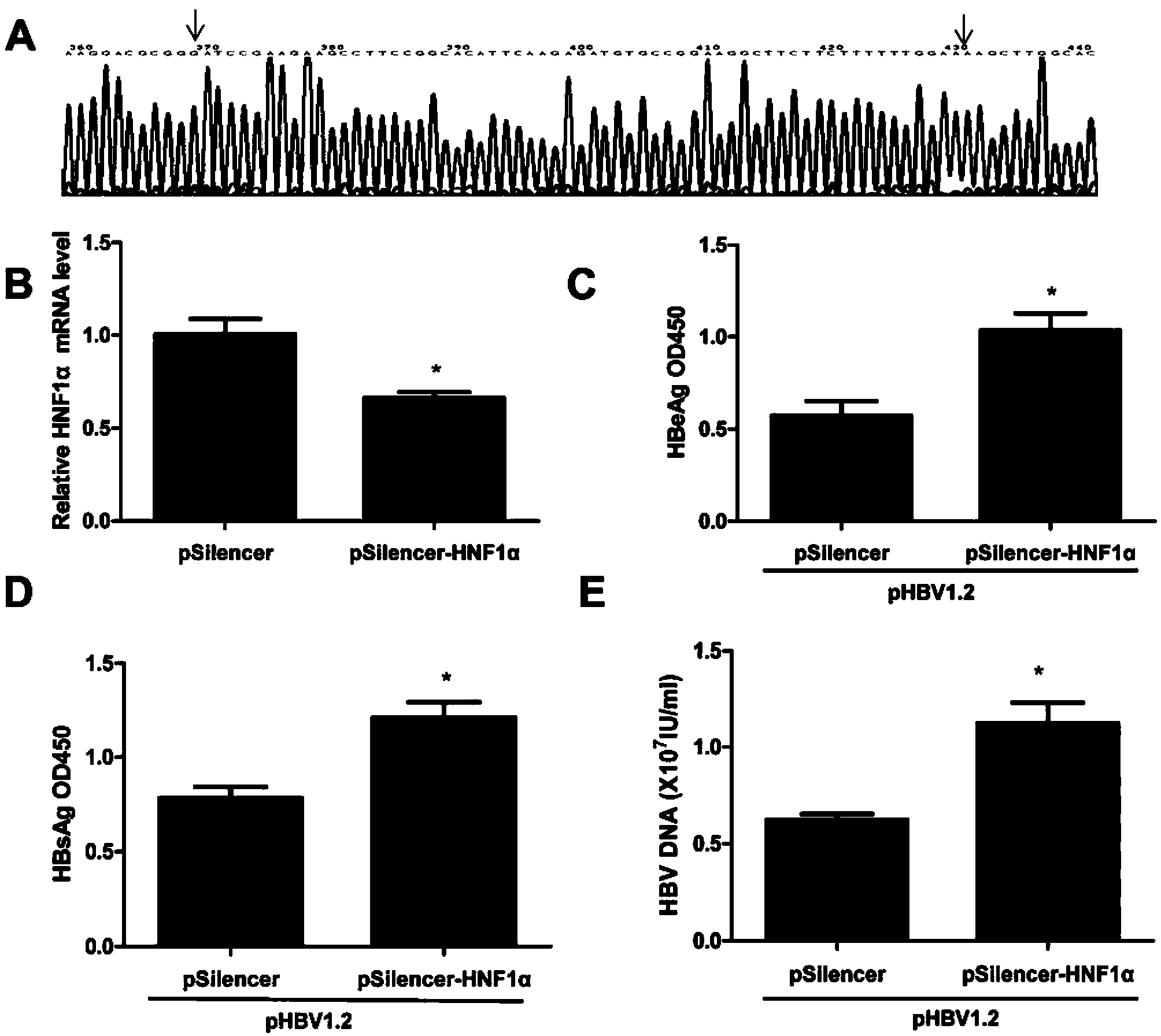
Novel anti-HBV endogenous protein HNF1alpha and application thereof
The invention discloses a novel anti-HBV endogenous protein HNF1alpha and an application thereof. The amino acid sequence of HNF1alpha is represented by SEQ ID No.1. HNF1alpha can inhibit liver cells from secreting and expressing s antigen (HBsAg) and e antigen (HBeAg), and can reduce HBV DNA load. The HNF1alpha can perform the effect of inhibiting HBV expression through combining with a specific locus of an HBV enhancer I. HNF1alpha can be developed into an effective anti-HBV medicine. Also, a basis for researching and developing anti-HBV medicines is established.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989BReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
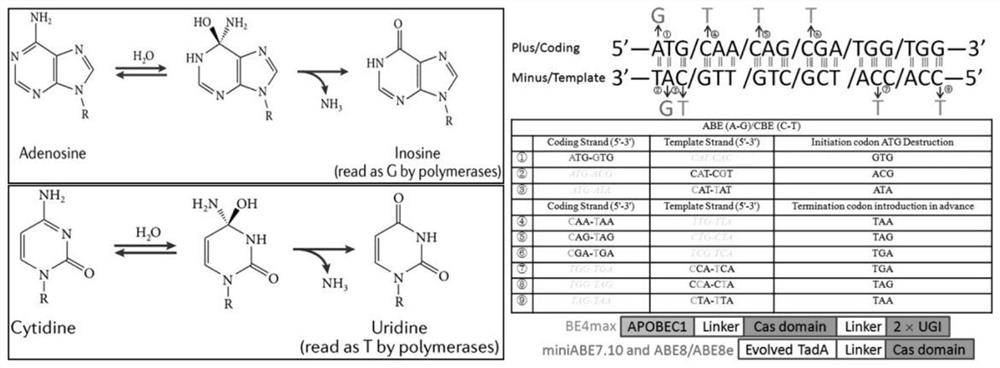
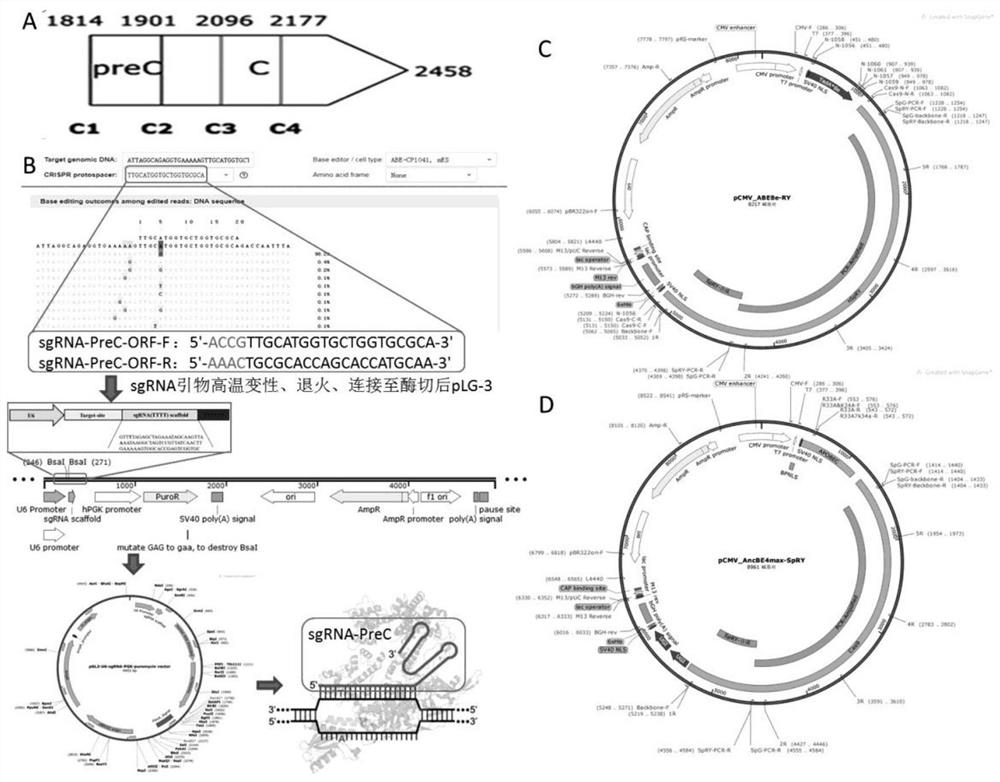
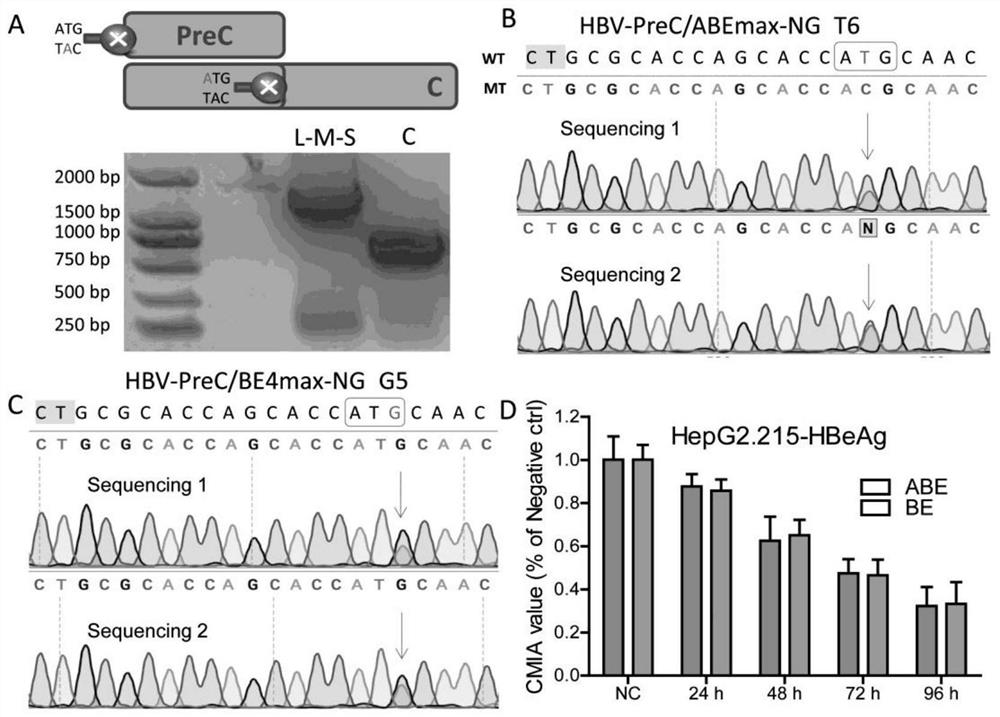
Method for closing target gene and removing HBV e antigen based on base editing technology
PendingCN114561392AReduce dependenceLow miss rateHydrolasesNucleic acid vectorEscherichia coliAntigen
The invention belongs to the technical field of pathogen biological treatment, and particularly relates to a method for closing a target gene and removing an HBV e antigen based on a base editing technology, which comprises the following steps: analyzing a positive chain of HBV genomes contained in HepG2.215 and HepAD38, determining a base editing target spot, designing and synthesizing a universal sgRNA primer of an ATG codon of targeted editing inactivated PreC paired with ABE8e and BE4-max, and detecting the HBV e antigen. Connecting to a carrier recovered by BsaI enzyme digestion, transforming Escherichia coli DH5alpha, selecting positive bacterial colonies for cloning, extracting small plasmids, and extracting large plasmids for later use after enzyme digestion and sequencing identification are correct. By changing HBV genetic information, preventing virus protein synthesis and blocking virus replication, a method and a strategy for thoroughly curing and removing the HBV e antigen are explored, and the method and the strategy have great theoretical significance and clinical practice value.
Owner:SHAOXING WOMEN & CHILDRENS HOSPITAL
Synthetic peptides that bind to the hepatitis B virus core and E antigens
InactiveUS20060020110A1Modulate host immune responseMicrobiological testing/measurementVirus peptidesE AntigensCompound (substance)
The present invention relates generally to the field of virology. More particularly, the invention relates to the discovery that peptides, which bind to the Hepatitis B virus (HBV) core and e antigens, can be used to inhibit HBV infection. Embodiments concern “binding partners”, which include peptides, peptidomimetics, and chemicals that resemble these molecules that interact with HBV core and e antigens, biological complexes having HBV core and e antigens joined to said binding partners, methods of identifying such binding partners, pharmaceuticals having binding partners, and methods of treatments and prevention of HBV infection.
Owner:SALLBERG MATTI
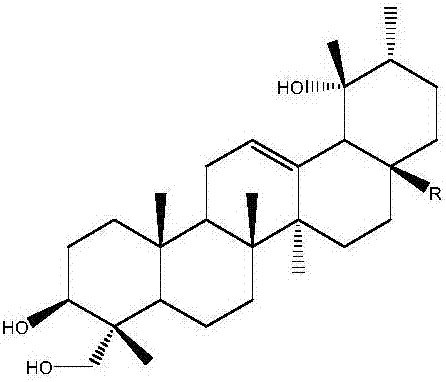
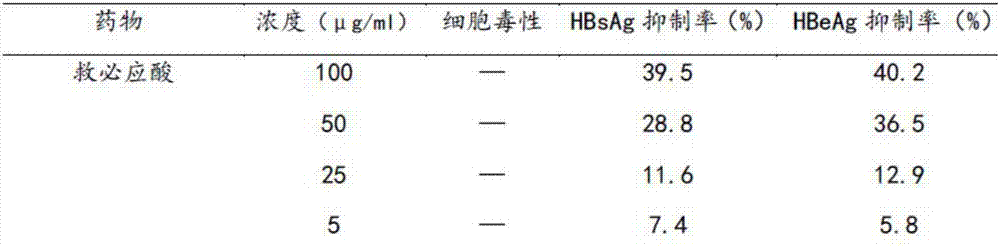
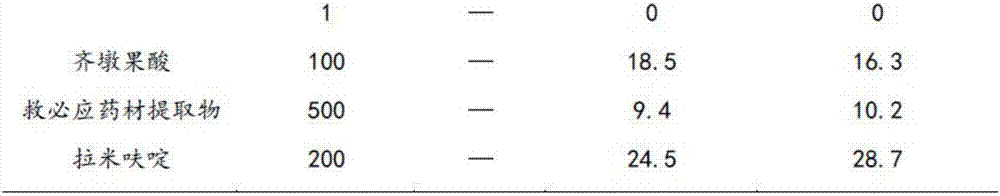
Application of rotundic acid in preparation of anti-hepatitis B virus (HBV) medicine
InactiveCN107281188AGood anti-HBV effectLow effective concentrationOrganic active ingredientsDigestive systemAntigenPositive control
The invention discloses application of rotundic acid in preparation of an anti-hepatitis B virus (HBV) medicine for the first time, and especially discloses application of the rotundic acid in preparation of medicines for inhibiting secretion of HBV surface antigen or HBV E antigen. In-vitro HepG22.2.15 cell pharmacological experiments prove that rotundic acid has excellent anti-HBV effect, and has the advantages of relatively low effective concentration and relatively small cytotoxicity. The rotundic acid has an effect for inhibiting HBsAg and HBeAg, has the inhibition rates for HBsAg and HBeAg respectively being 39 / 5 percent and 40.2 percent at 100mu g / ml, and has the inhibition effect for HBV superior to that of positive control lamivudine, oleanolic acid and ovate leaf holly bark medicinal material extract.
Owner:SUN YAT SEN UNIV +1
PRRSV minor protein-containing recombinant viral vectors and methods of making and use thereof
ActiveUS9981033B2Altered the tropism of the virusAvoid infectionPolypeptide with localisation/targeting motifSsRNA viruses positive-senseE AntigensEpitope
The present invention encompasses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) vaccines or compositions. In particular, the invention encompasses recombinant adenovirus vectors encoding and expressing PRRSV gp2, gp3, gp4, gp5a, gp5 and / or E antigens, proteins, epitopes or immunogens. Such vaccines or compositions can be used to protect animals from PRRSV.
Owner:MERIAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com