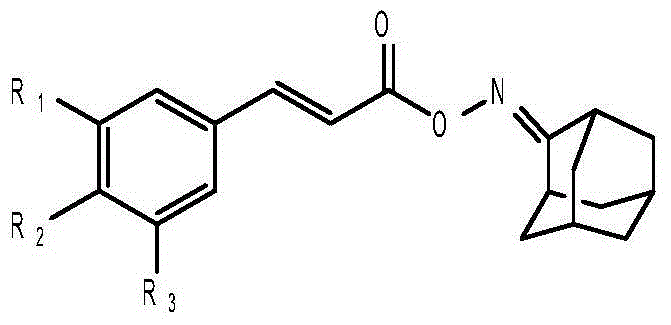
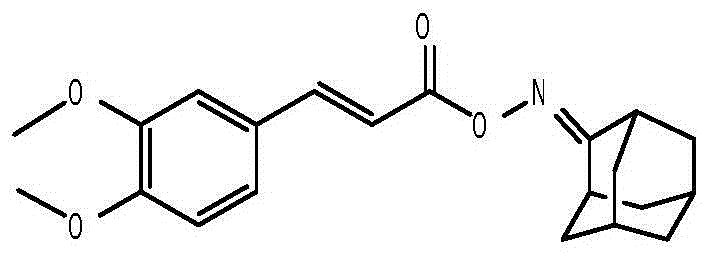
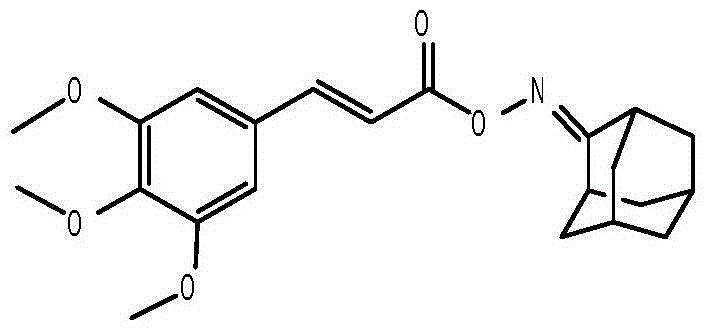
Anti-hepatitis B virus active phenylpropanoids adamantine ketoxime ester compound
A technology of phenylpropanoid adamantanone oxime and ester compounds, which is applied in the fields of antiviral agents, oxime preparation, organic chemistry, etc., and can solve drug withdrawal rebound, nucleoside analog drug resistance, and endanger the effect of hepatitis B virus vaccine And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of the first phenylpropanoid oxime ester compound 3,4-dimethoxycinnamic acid-2'-adamantanone oxime ester, the steps are as follows:
[0050] (1) Add 10 mmol of adamantanone, 12 mmol of hydroxylamine hydrochloride, 12 mmol of sodium acetate trihydrate, and finally add 50 ml of ethanol to dissolve, and stir and react at 60° C. for 2 hours. After stopping the reaction, the solution was concentrated to remove ethanol to obtain a concentrate. The concentrate was dissolved in 50 ml of dichloromethane, washed three times with 70 ml of hydrochloric acid solution with a concentration of 1 mol / L, and the upper layer solution was separated and removed to obtain the lower layer of dichloromethane solution. After acid washing, the dichloromethane solution was washed three times with 70 ml of water, and then the lower dichloromethane solution was separated. The dichloromethane solution washed with water was finally washed three times with 70 ml of saturated aq...
Embodiment 2
[0054] The preparation method of the second phenylpropanoid oxime ester compound 3,4,5-trimethoxycinnamic acid-2'-adamantanone oxime ester, the steps are as follows:
[0055] (1) Same as step (1) of Example 1.
[0056] (2) Dissolve 10mmol of 3,4,5-trimethoxycinnamic acid in 10ml of dichloromethane, add 50mmol of thionyl chloride, stir and reflux at 65°C for 5 hours, stop the reaction and concentrate to obtain 3, 4,5-Trimethoxycinnamoyl chloride.
[0057] (3) Dissolve the 2-adamantanone oxime prepared in step (1) in 15 milliliters of dichloromethane, add 12 mmol of triethylamine and 12 mmol of 3,4,5-trimethoxy Cinnamoyl chloride, after reacting at 0°C for 30 minutes, then continue to react at room temperature for 12 hours, stop the reaction, concentrate under reduced pressure, and separate by silica gel column chromatography. The mobile phase is composed of petroleum ether with a boiling point of 60-90°C: The volume ratio of ethyl acetate was gradually changed from 4:1 to 1.5...
Embodiment 3
[0059] The preparation method of the third phenylpropanoid oxime ester compound 3,4-(methylenedioxy)cinnamic acid-2'-adamantanone oxime ester, the steps are as follows:
[0060] (1) Same as step (1) of Example 1.
[0061] (2) Dissolve 10mmol of 3,4-(methylenedioxy)cinnamic acid in 10ml of dichloromethane, add 50mmol of thionyl chloride, stir and reflux at 65°C for 5 hours, stop the reaction and concentrate to obtain 3,4-(Methylenedioxy)cinnamoyl.
[0062](3) Dissolve the 2-adamantanone oxime prepared in step (1) in 15 milliliters of dichloromethane, add 12 mmol of triethylamine and 12 mmol of 3,4-(methylenedioxy ) cinnamoyl chloride, after reacting at 0°C for 30 minutes, then continue to react at room temperature for 12 hours, stop the reaction, concentrate under reduced pressure, and separate by silica gel column chromatography. The mobile phase is composed of petroleum ether with a boiling point of 60-90°C : The volume ratio of ethyl acetate is gradually changed from 4:1 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com