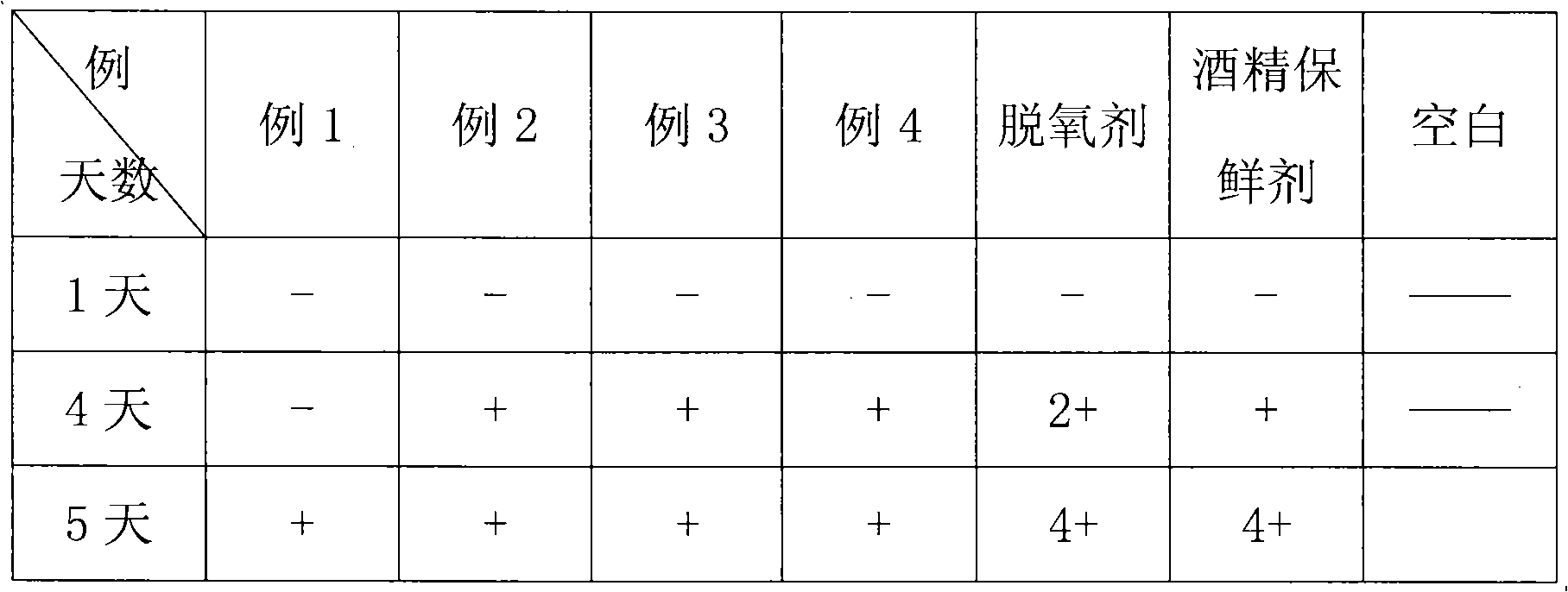
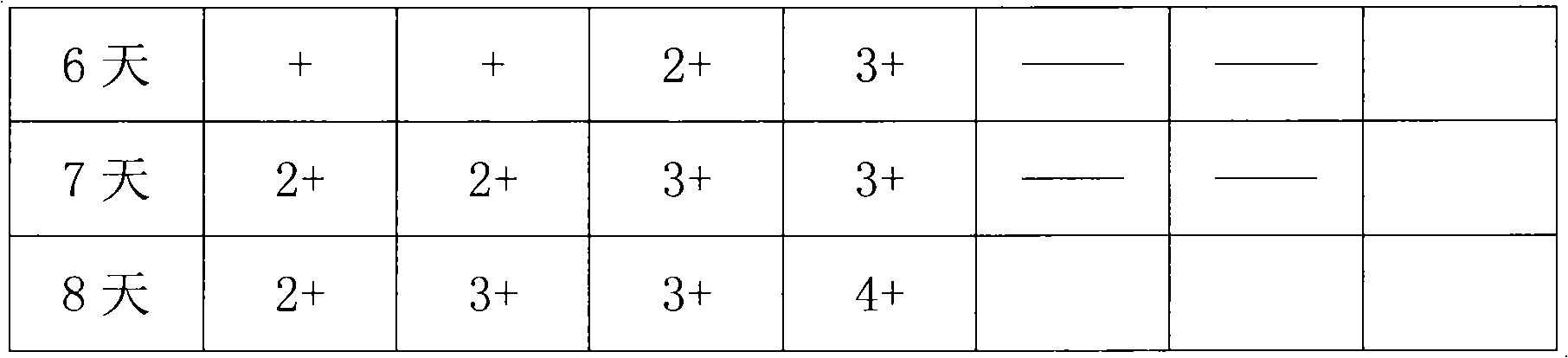
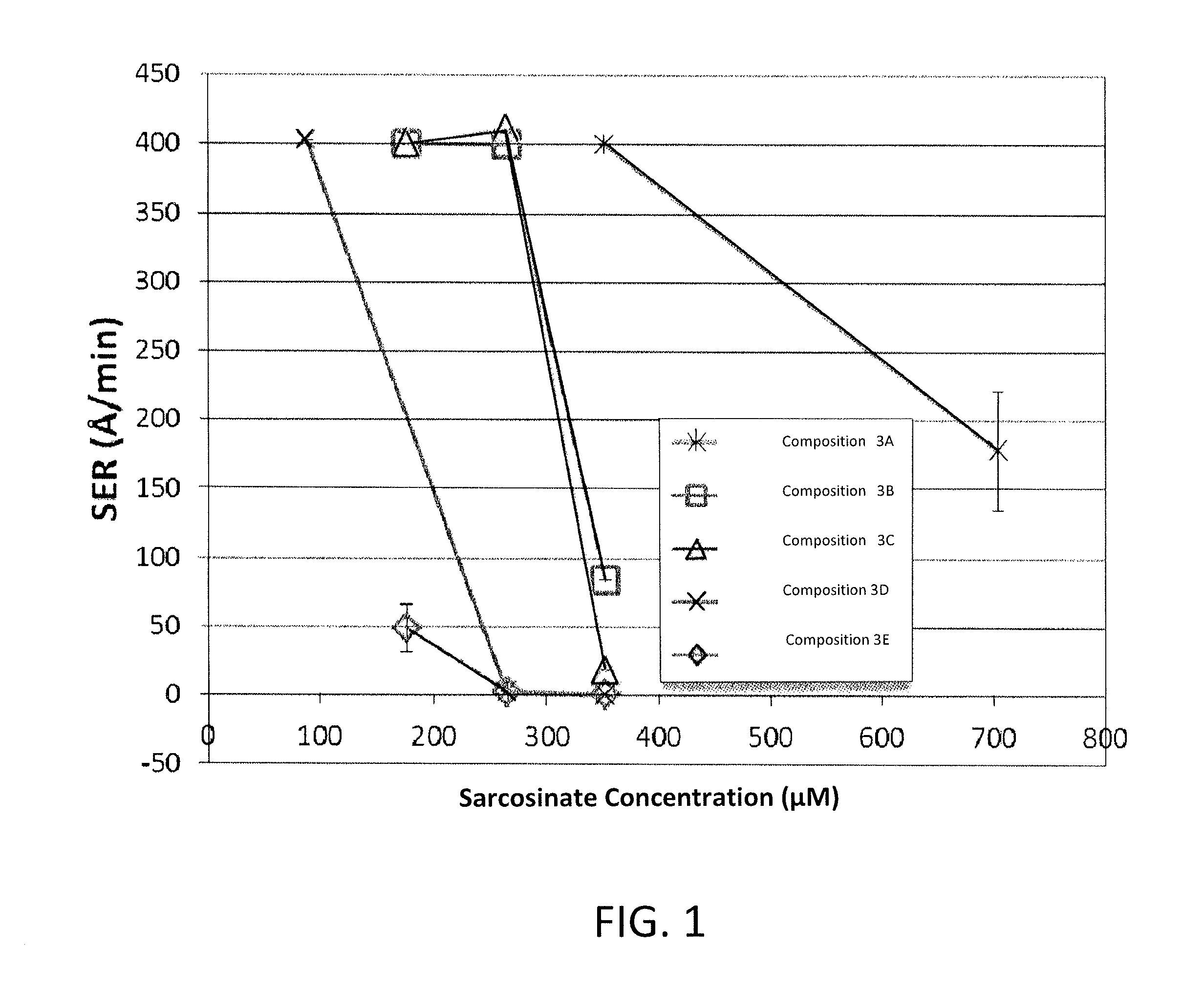
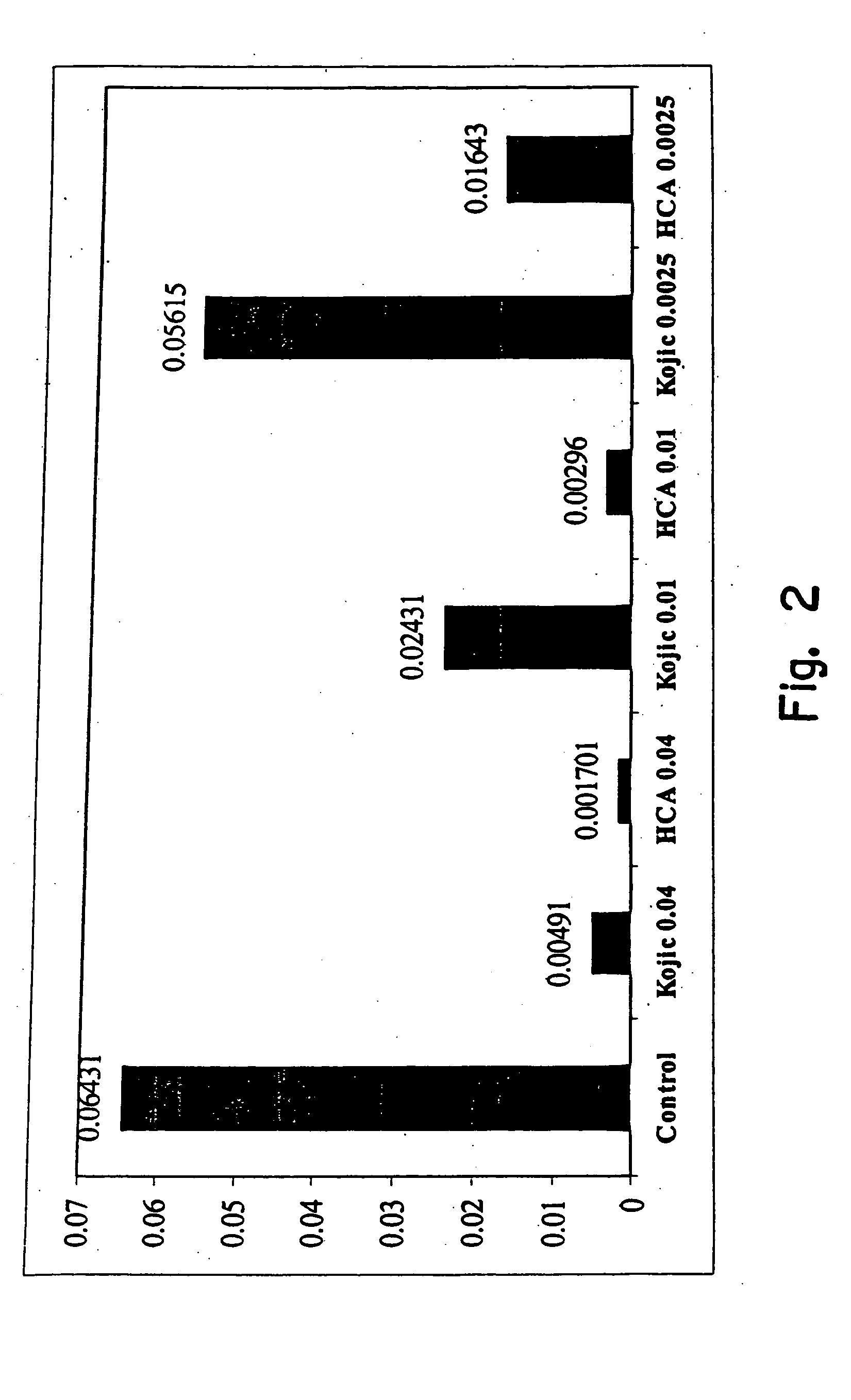
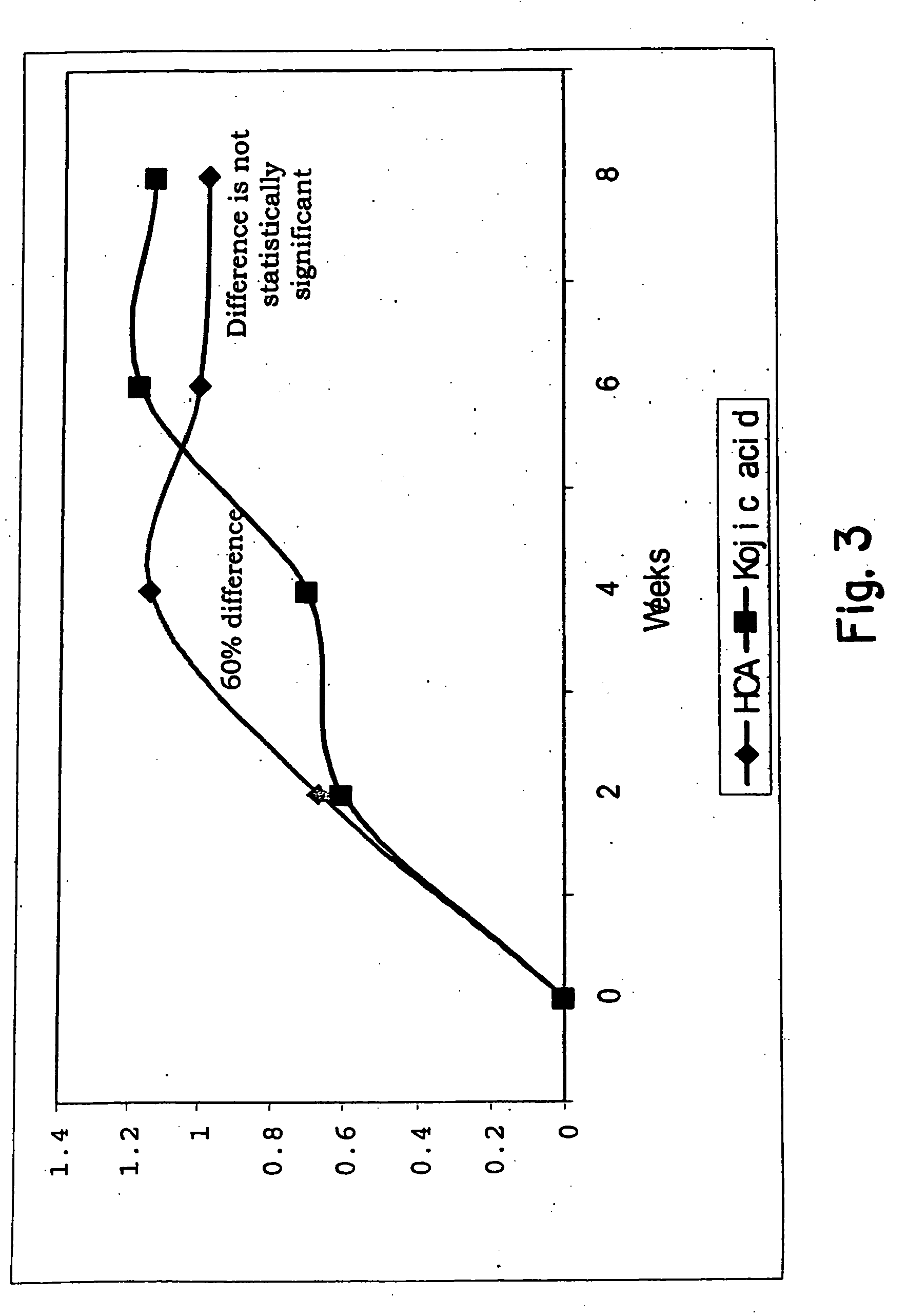
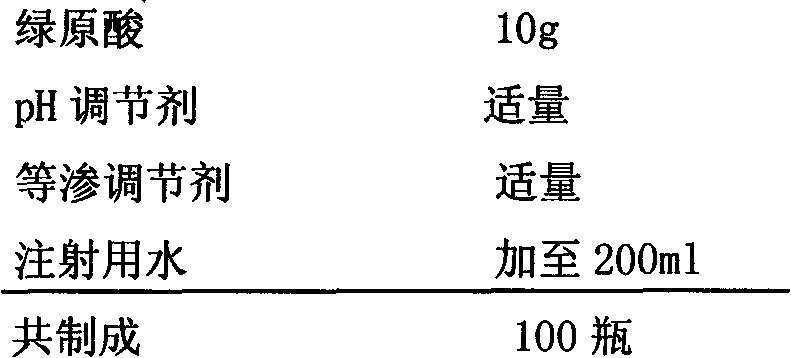
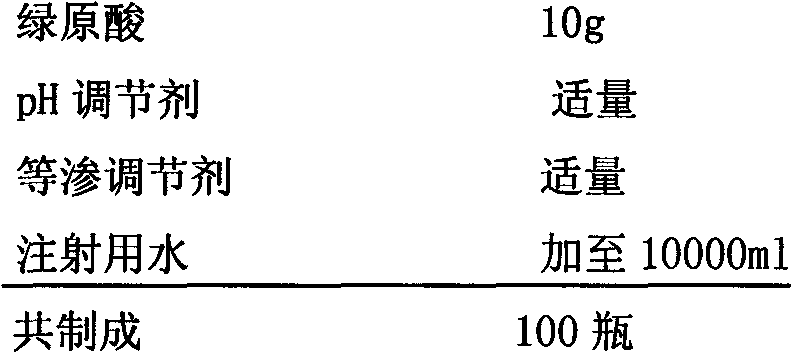
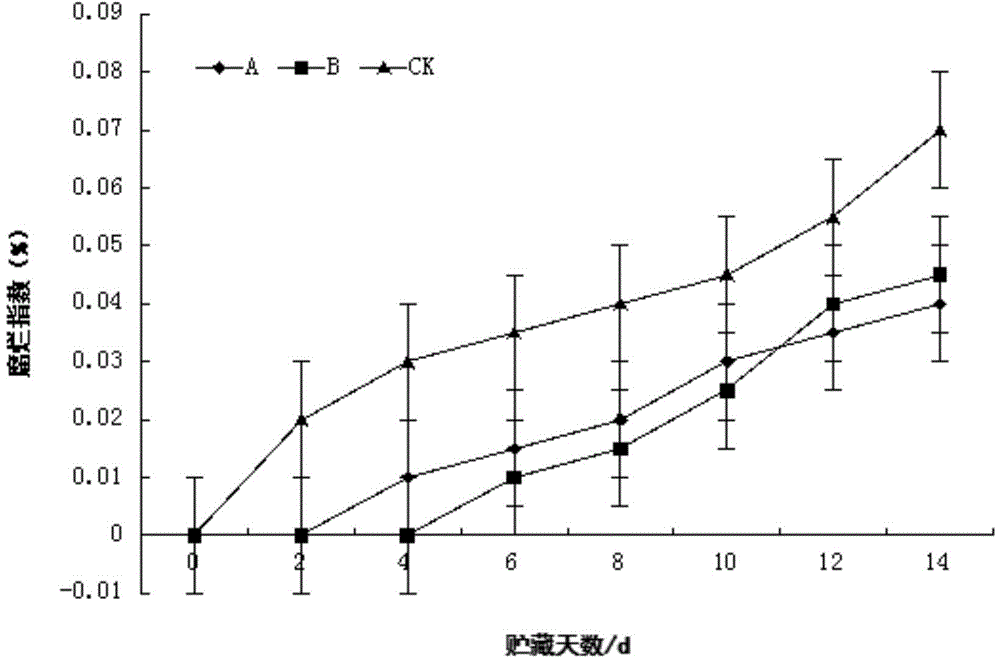
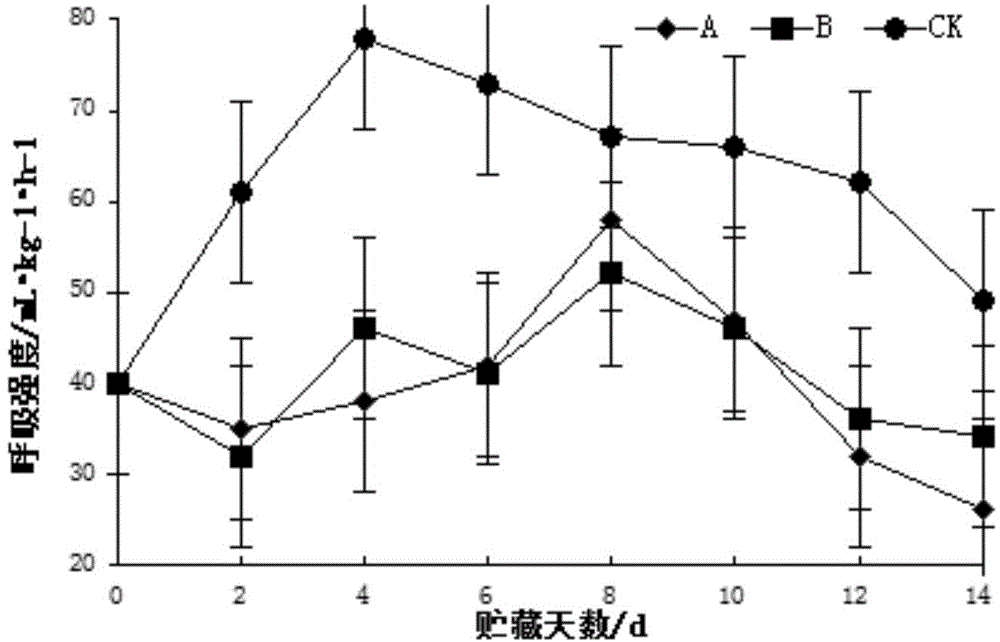
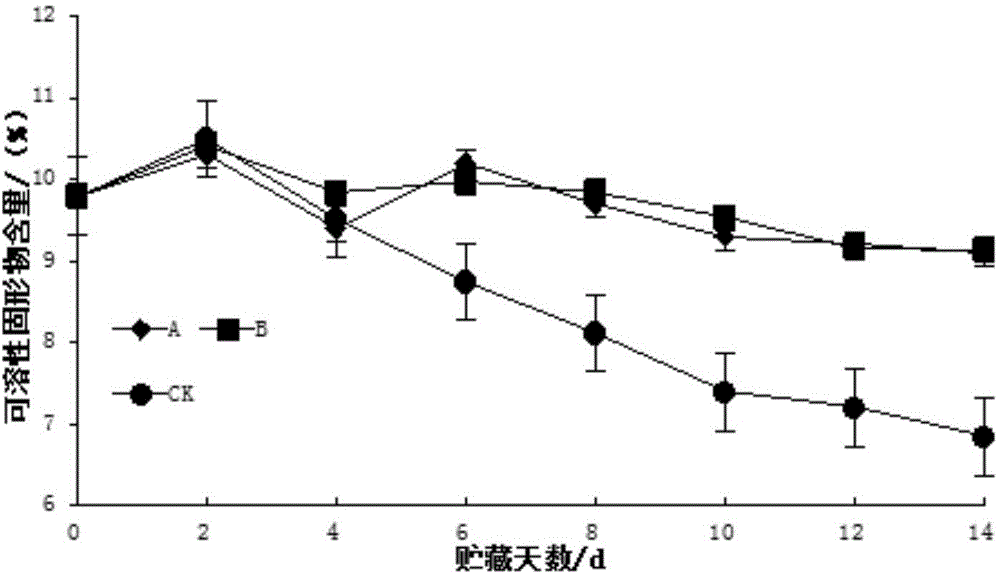
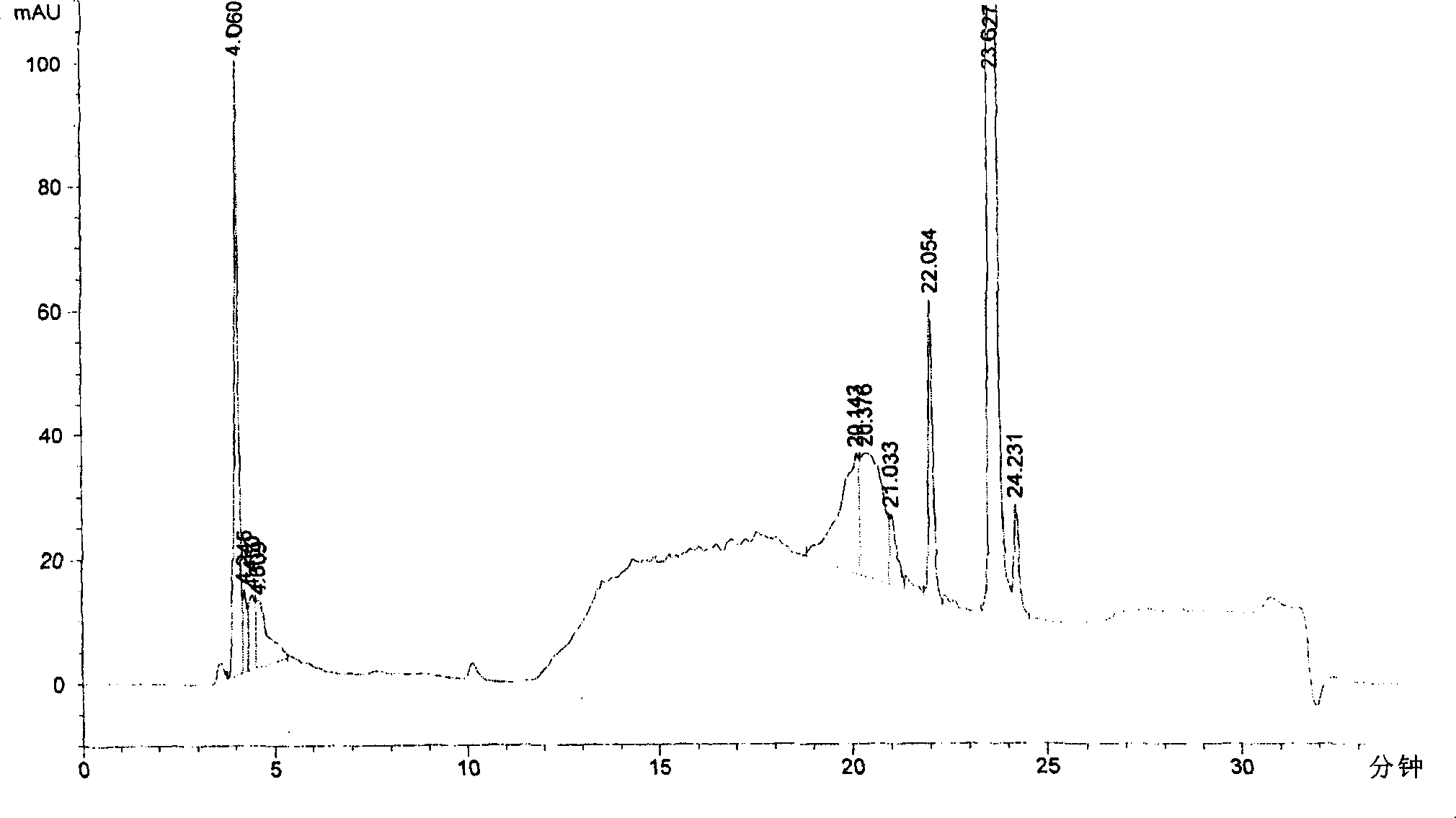
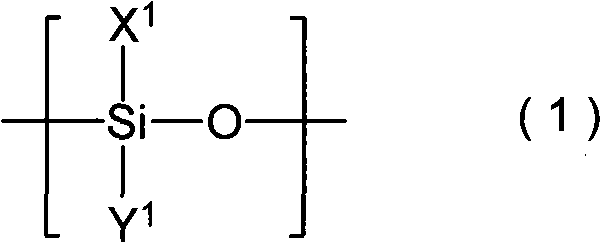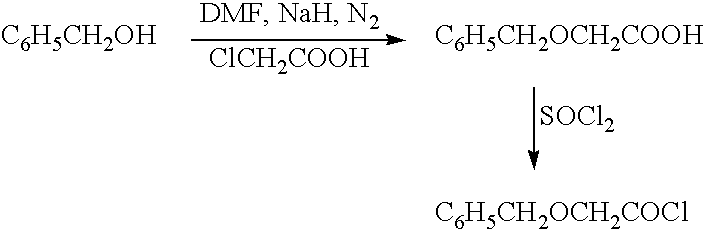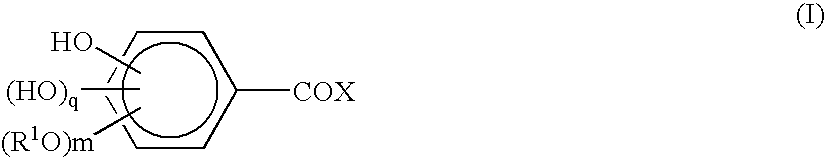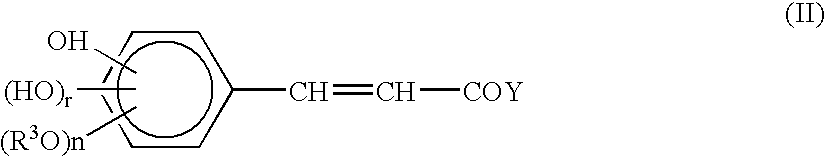Patents
Literature
1271 results about "Cinnamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
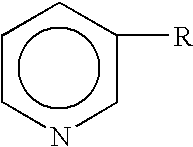
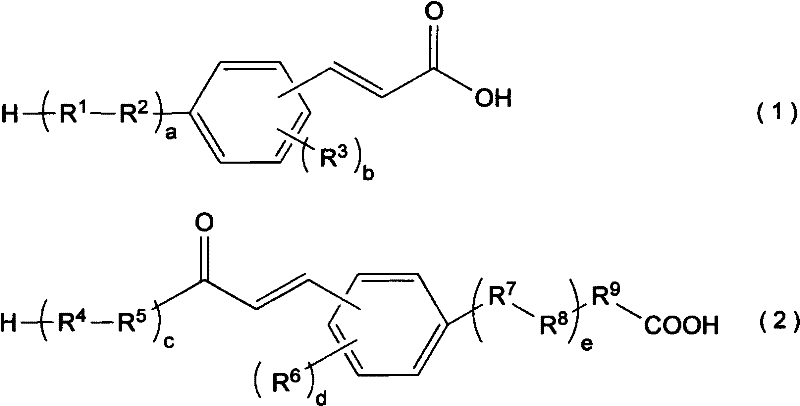
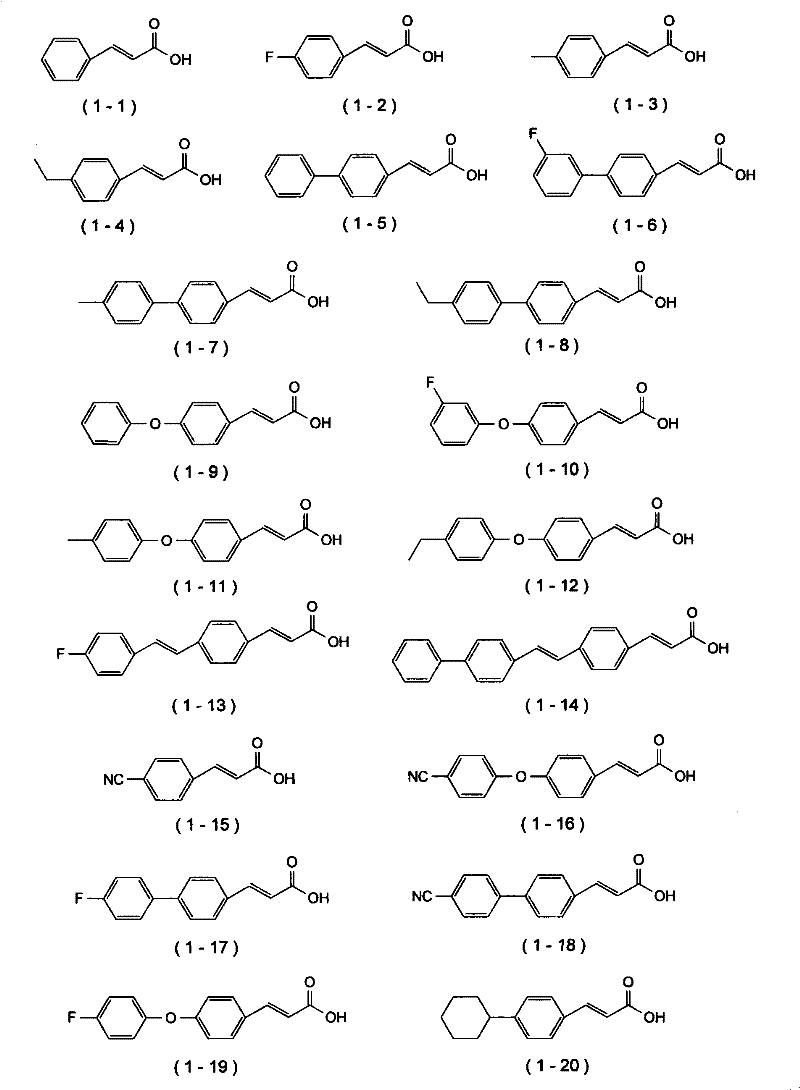
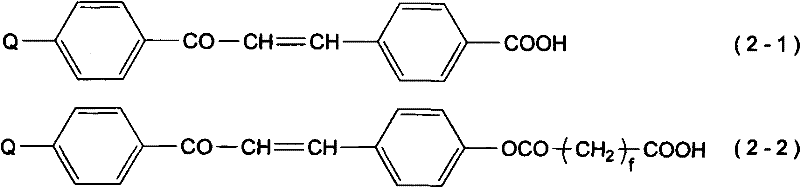
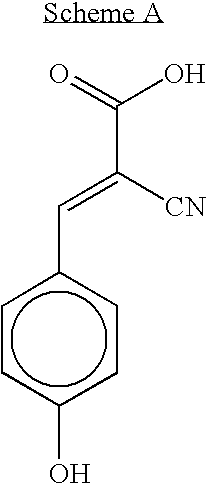
Cinnamic acid is an organic compound with the formula C₆H₅CH=CHCOOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.
Regulation of Mammalian Keratinous Tissue Using Skin and/or Hair Care Actives
Personal care compositions containing an active selected from the group consisting of phlorogine, phlorgine BG, deoxyArbutin, sucrose dilaurate, bakuchiol, pyrenoine, millet, arlatone dioic acid, cinnamic acid, ferulic acid, achromaxyl, methyl nicotinamide, oil soluble licorice extract, folic acid, undecylenic acid, zinc undecylenate, L-tryptophan, thiamine HCl, hexylresorcinol, lipidami red vine, dragosine, methyl gentisate, inositol, symdiol 68, laminaine, their salts, their derivatives, their precursors, and / or combinations thereof. Methods for regulating the condition of mammalian keratinous tissue by topically applying the personal care compositions are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
Liquid crystal aligning agent, polyorganosiloxane, liquid crystal aligning film, forming method thereof and liquid crystal display element
ActiveCN101735825AImprove stabilityLiquid crystal compositionsPhotomechanical apparatusEpoxyAcetophenone
The present invention relates to a liquid crystal aligning agent, polyorganosiloxane, a liquid crystal aligning film, a forming method thereof and a liquid crystal display element. The present invention provides the liquid crystal aligning agent which can form the liquid crystal aligning film that can generate a pretilt angle with long time stability through an optical aligning method. The liquid crystal aligning agent comprises radiation-sensitive linear polyorganosiloxane which is prepared through the reaction of the following components: specific polyorgansiloxane with epoxy radical; (A) cinnamic acid derivative; and (B) a specific compound with a radiation-sensitive structure that is preferably selected from: acetophenone structure, benzophenone structure, anthraquinone structure, biphenyl structure, carbazole structure, nitro-aryl structure, fluorenes structure, naphthalene structure, anthracene structure, acridine structure and indole structure.
Owner:JSR CORPORATIOON
Bacterium-restraining deoxidization dual-purpose food antistaling agent and preparation thereof
The invention relates to an antibacterial deoxidizing double-effect food preservative, which is formed by combining a deoxidizer component with a bacteriostat component, wherein the deoxidizer component accounts for 60-90 percent, and the bacteriostat component accounts for 10-40 percent. The bacteriostat component comprises an adsorption carrier and an antibacterial solution. The adsorption carrier comprises salep, silicon dioxide, vermiculite, diatomaceous earth, zeolite, bentonite, fiber, etc. The antibacterial solution comprises one of or the composite mixture of edible alcohol and propylene glycol. Antibacterial synergist comprises allyl isothiocyanate, allicin, cinnamic aldehyde, cinnamic acid, carvacrol, eugenol, thymol, citral, etc. The antistaling agent integrates double protection functions of absorbing oxygen, sterilizing and inhibiting bacteria, which is remarkable in preservation effect and wide in application range.
Owner:GUANGDONG GUANGYI TECH IND
High SPF sunscreen formulations
InactiveUS6048517ALow costHigh SPF valueCosmetic preparationsToilet preparationsSalicylic acidHomosalate
Low cost sunscreening formulations having SPF values greater than 40 contain homosalate, octyl salicylate or mixtures thereof, in combination with oxybenzone, and optionally further contain octyl methoxycinnamate, avobenzone or mixtures thereof.
Owner:MSD CONSUMER CARE INC
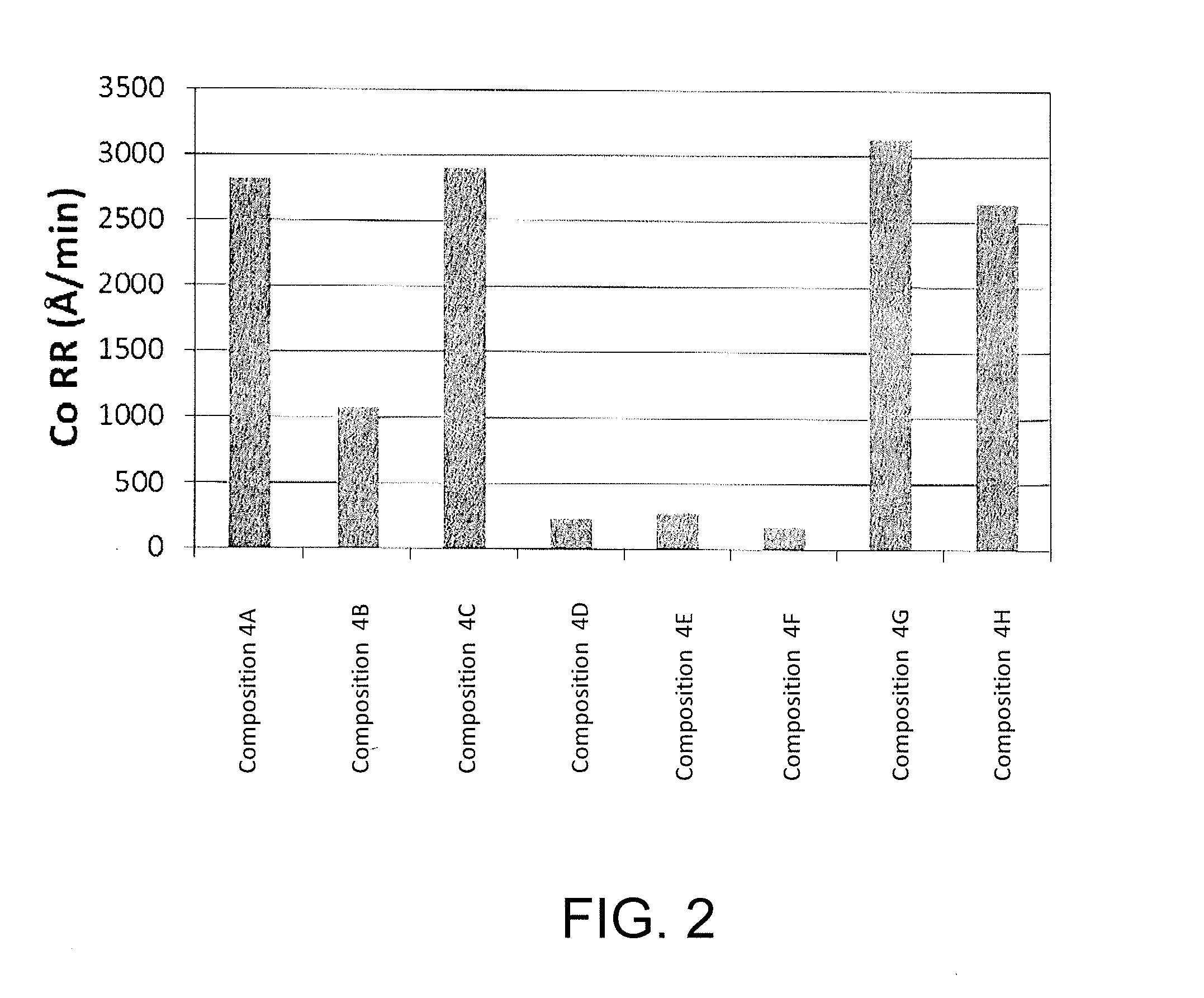
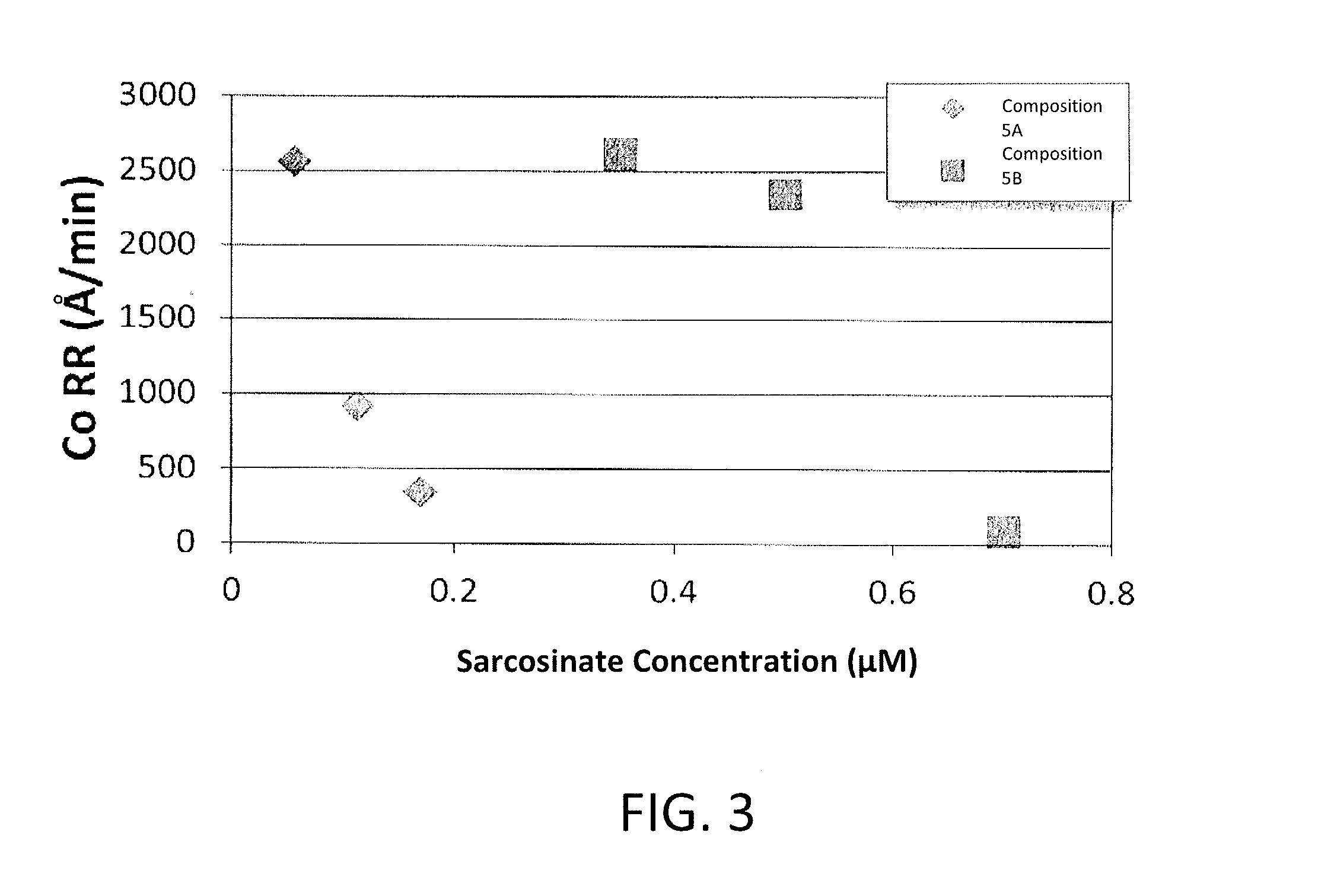
Corrosion inhibitors and related compositions and methods
ActiveUS20160107289A1Decorative surface effectsSemiconductor/solid-state device manufacturingPhosphoric Acid EstersPhosphate
The invention provides methods of inhibiting corrosion of a substrate containing metal. The substrate can be in any suitable form. In some embodiments, the metal is cobalt. The methods can be used with semiconductor wafers in some embodiments. The invention also provides chemical-mechanical polishing compositions and methods of polishing a substrate. A corrosion inhibitor can be used in the methods and compositions disclosed herein. The inhibitor comprises an amphoteric surfactant, a sulfonate, a phosphonate, a carboxylate, an amino acid derivative, a phosphate ester, an isethionate, a sulfate, a sulfosuccinate, a sulfocinnimate, or any combination thereof.
Owner:CMC MATERIALS INC
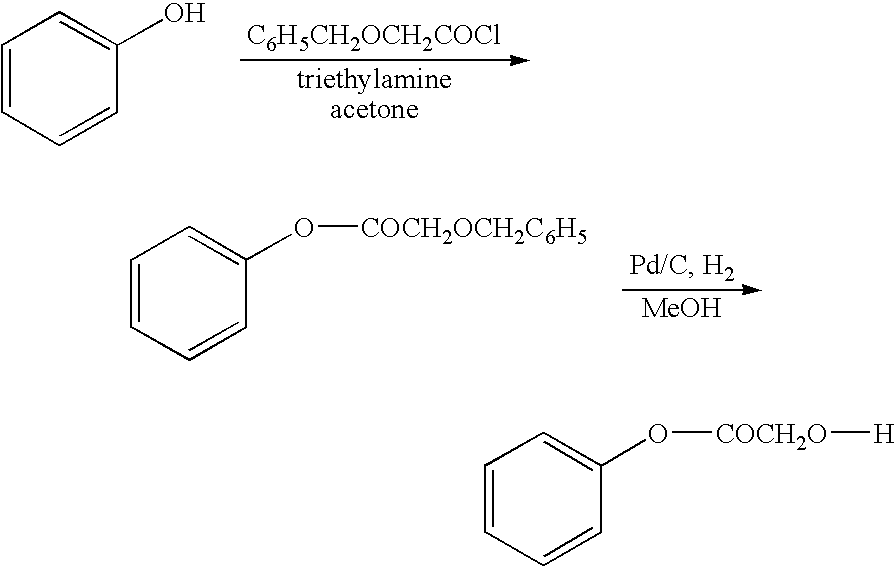
Functionalized phenolic esters and amides and polymers therefrom
InactiveUS20060173065A1Alter efficacyAlter valueBiocideOrganic chemistryBenzoic acidPhenylacetic acid
The present invention relates to a compound of the formula: R-AR—O—Y—R′Wherein R represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid, —NO2, —NH2, —NHCOCH3, and —NH—Y—R′, which is attached directly to AR or attached through an aliphatic chain. The carboxylic acid moiety in R includes but is not limited to the following carboxylic acids: benzoic acids, cinnamic acids, ferulic acid, caffeic acid, syringic acid, salicylic acid, vanillic acid, phenylacetic acids, phenylpropionic acids, and sinapinic acid. -AR—O— is a biologically active phenolic moiety comprising 1 to 6 substituted or unsubstituted aryl rings that are directly bonded to each other, fused together, or joined through a linking group. Y represents a member selected from: —COCH2O— (glycolic ester moiety) —COCH(CH3)O— (lactic ester moiety) —COCH2OCH2CH2O— (dioxanone ester moiety) —COCH2CH2CH2CH2CH2O— (caprolactone ester moiety) —CO(CH2)mO— where m is an integer between 2-4 and 6-24 inclusive —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; and R′ is either hydrogen or a benzyl or an alkyl group, the alkyl group being either straight-chained or branched. The resultant functionalized phenolic compounds, used singly or in combinations, and their polymers have controllable degradation profiles, releasing the active component over a desired time range. The polymers are useful for biomaterials and biomedical devices, wherein said biologically active phenolic moiety is a residue of a phenolic compound.
Owner:BEZWADA BIOMEDICAL LLC
Stabilized ascorbic acid compositions and methods therefor
ActiveUS20050154054A1Improve stabilityImprove solubilityCosmetic preparationsBiocideSolubilityCaffeic acid
The present invention relates to ascorbic acid single-phase solution compositions that provide enhanced stability, enhanced solubility and an enhanced photoprotective effect as compared to prior compositions. The compositions comprise L-ascorbic acid; a cinnamic acid derivative such as p-coumaric acid, ferulic acid, caffeic acid, sinapinic acid, a derivative thereof, and a combination thereof; a solvent comprising a glycol ether and an alkanediol; and water; the composition having a pH of no more than about 3.5. The compositions may also comprise a form of Vitamin E and are useful for treatment of radical-induced damage to a subject, particularly the skin of a subject.
Owner:LOREAL USA CREATIVE INC
Salt and heat sensitive, substantive UV-absorbing polymers
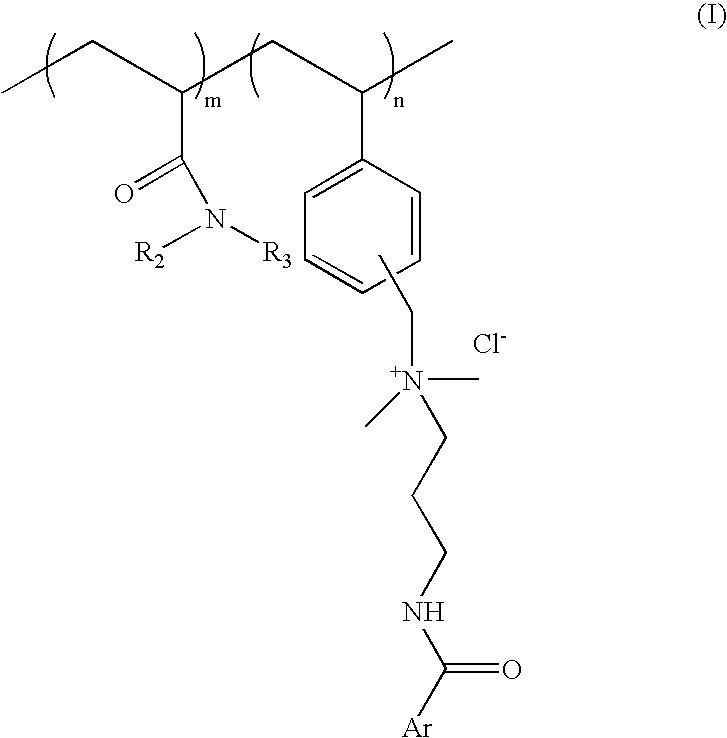
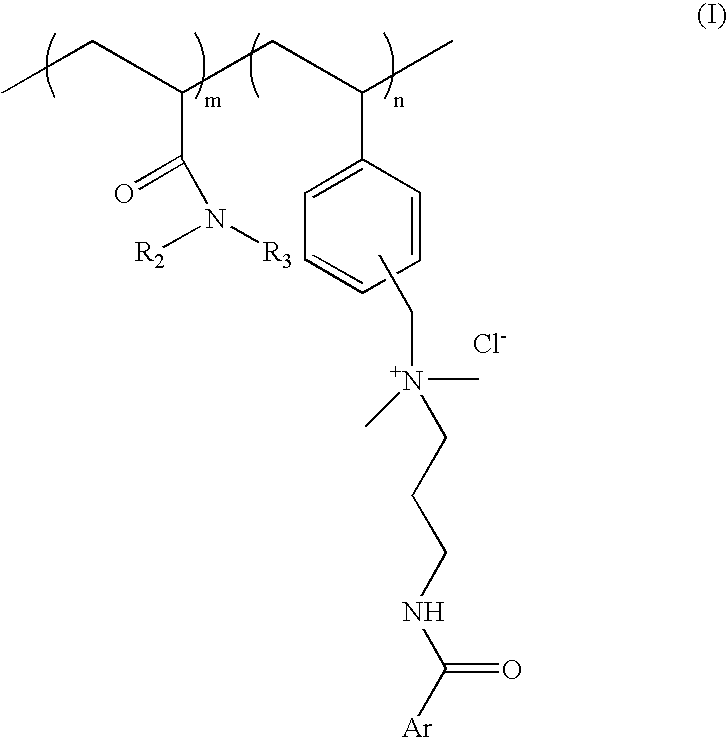
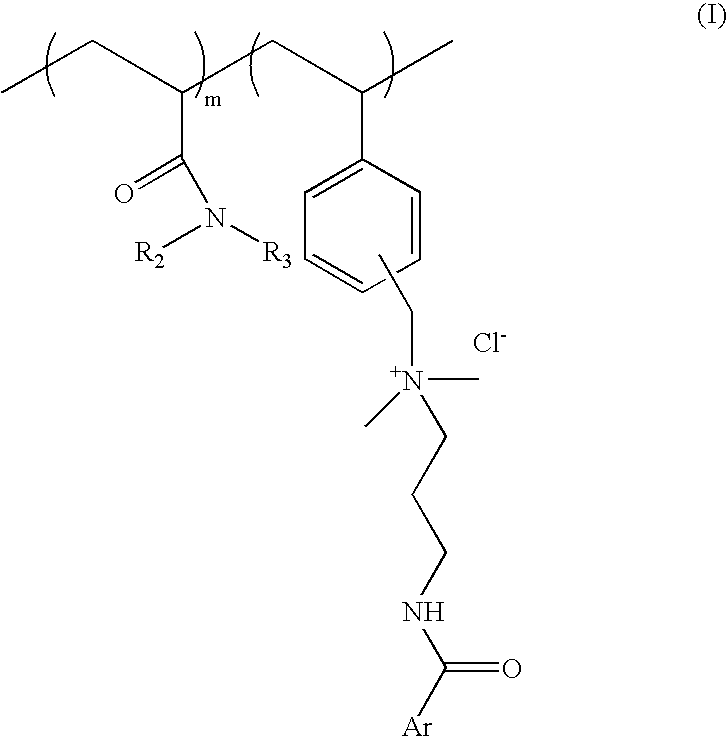
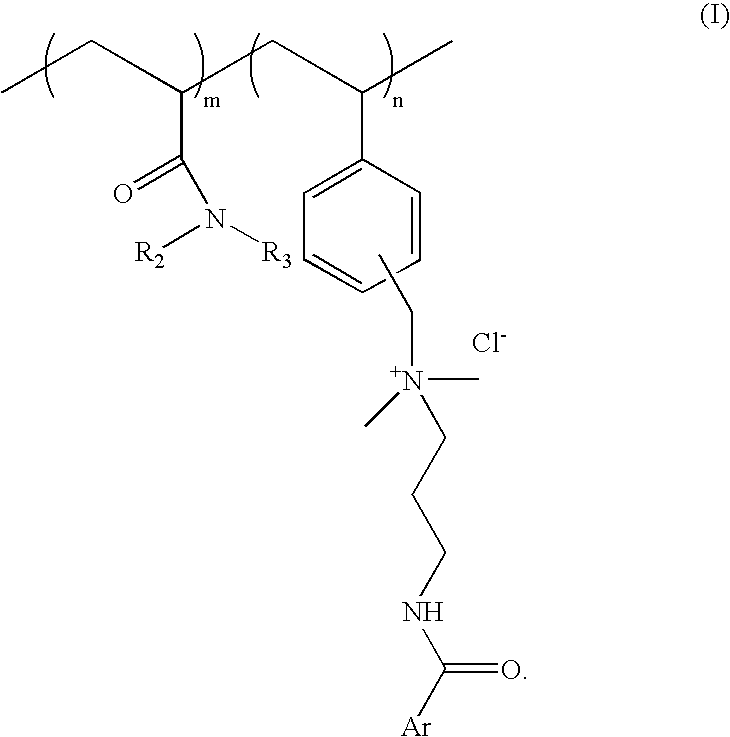
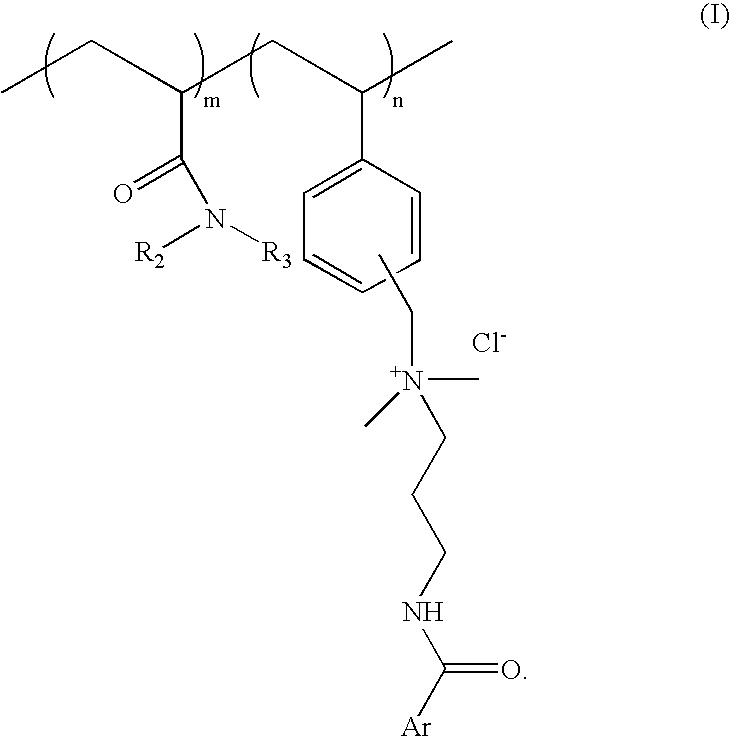
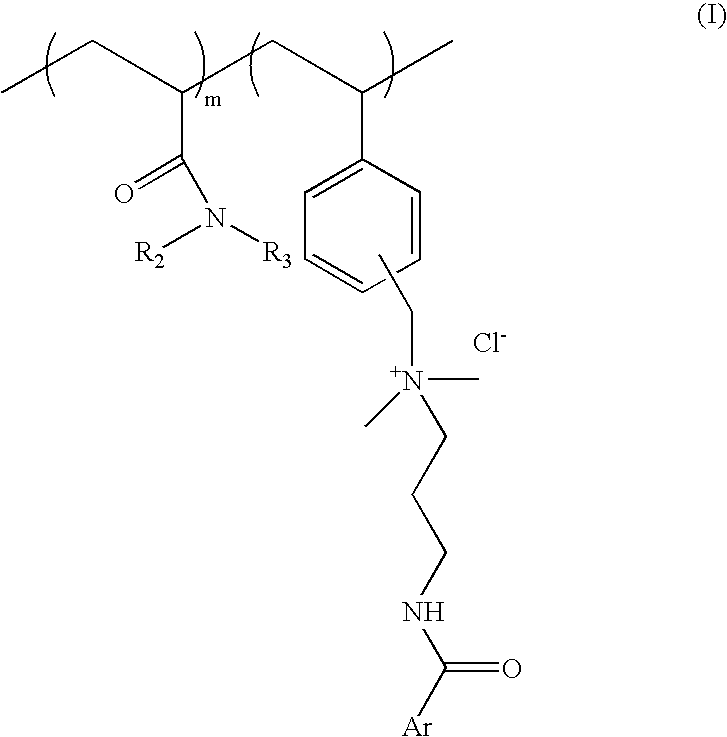
Substantive UV-absorbing, water-soluble, cationic polymers containing cinnamidoalkylamines and benzamidoalkylainines, with 'inverse temperature dependant solubility' are described in the present invention. They are water-soluble at ambient conditions and yet they are water-resistant at the temperature of human body as well as in the presence of electrolytes. These properties make these macromolecules useful for personal care as well as fabric care products. The present invention also describes the hair, skin and fabric care compositions containing the said polymers of Formula I wherein, ArCO is an UV-absorbing moiety of an organic sunscreen acid or mixtures of organic sunscreen acids selected from p-methoxy cinnamic acid and p-dimethyl amino benzoic acid; R2 and R3 are selected from hydrogen, alkyl and cycloalkyl group containing from 1 to 6 carbon atoms; m is an integer from 5 to 9 and n is an integer between 1 to 5 and m+n=10.
Owner:GALAXY SURFACTANTS
Composition and method for treating hyperpigmented skin
InactiveUS20070166251A1Enhanced ability to lighten skin colorImprove breathabilityCosmetic preparationsHair cosmeticsSkin colorGlycol synthesis
Owner:LIPO CHEM
Diet cola beverages
InactiveUS20080226800A1Low potencyTotal calories lowFood ingredient as flavour affecting agentLow calorieSuccinic acid
Diet cola beverage and other beverage products and methods for making the same are disclosed. The diet cola beverages comprise water, at least one natural, potent, non-nutritive sweetener, at least one natural, low potency, low calorie sweetener, an acidulant comprising at least one of lactic, citric, tartaric, malic, fumaric, cinnamic, maleic, adipic, glutaric, and succinic acids, a caramel colorant, and cola flavor.
Owner:CONCENTRATE MFG OF IRELAND
Preparation of high purity chlorogenic acid preparation and clinical application thereof
InactiveCN102391119AAntibacterialSuppressor mutationAntibacterial agentsOrganic active ingredientsDiseaseChlorogenic acid
Chlorogenic acid, i.e. 1, 3, 4, 5-tetrahydroxycyclohexanecarboxylic acid-(3, 4-dihydroxycinnamic acid ester), exists in a plurality of plants, and also, high purity chlorogenic acid can be prepared through synthesis. Chlorogenic acid has a lot of biological activity, such as antibacterium, antivirus, antioxidation, antitumor and the like. However, there exists no report and research on oral preparations prepared by high purity chlorogenic acid and application of the preparations in clinics. In the invention, high purity chlorogenic acid is extracted from plants or obtained through synthesis, and is then added with a proper amount of accessories, thus obtaining oral preparations like tablets, capsules, granules, oral solutions, etc. The preparations provided in the invention can be applied in clinics for treating cardio-cerebrovascular diseases, infections, hepatitis B, tumors and other diseases, and also can be used in health care medicines for heat clearing and detoxifying, face nursing and skin moistening, as well as hangover relieving, etc.
Owner:肖文辉 +1
Preparation method of double-sensitivity cyclodextrin supermolecule aggregate
The invention discloses a preparation method of a double-sensitivity cyclodextrin supermolecule aggregate, belonging to the technical field of functional materials. The preparation method comprises the following steps of preparing a host molecule-photosensitive 4-hydroxycinnamic acid-cyclodextrin (4HCA-CD) by using 4-hydroxycinnamic acid (4HCA) to modify beta-cyclodextrin (beta-CD); using trithioester with adamantine (AD) at tail end as a chain transfer agent, and preparing temperature-sensitive object polymer-double-arm adamantine-poly(N-isopropyl acrylamide)-adamantine (AD-PNIPAM-AD) by using a reversible addition-fragmentation chain transfer free radical polymerization (RAFT) method; constructing a double-sensitivity supermolecule inclusion complex 4HCA-CD / AD-PNIPAM-AD by utilizing comprehensive performance of a beta-CD dewatering cavity and AD; and self-assembling the 4HCA-CD / AD-PNIPAM-AD to form the supermolecule aggregate which is capable of realizing reversible conversion in shape and size by changing light and temperature. The supermolecule aggregate prepared by the preparation method disclosed by the invention has good light / temperature double sensitivities and is capable of carrying out smart response onto external stimulus, so that the supermolecule aggregate has a wide application prospect in the fields of drug loading, controlled release, and the like.
Owner:JIANGNAN UNIV
Swamp Rhodopseudomonas of using nitrite nitrogen in high effect, and application
This invention relates to a kind of photosynthetic bacterium that has a high assimilation utility to nitrite nitrogen. More specifically, this invention provides a Rhodopseudomonas palustris strain that can assimilate nitrite nitrogen, and its r ejuvenation, preservation, biological detection and application. The photosynthetic bacterium is a kind of biological water purifier and biological fertilizer, and can be used in controlling the content of nitrite nitrogen in water body for aquaculture, improve environmental pollution, and eliminate cinnamic acid and benzoic acid. The photosynthetic bacterium can be used as nitrogen-fixing biological fertilizer and cucumber growth promoter.
Owner:珠海市农科中心有限公司
Liquid crystal aligning agent for retardation film, liquid crystal alignment film for retardation film, and retardation film and process for production thereof
ActiveCN102337140AEfficient manufacturingIncrease production capacityLiquid crystal compositionsPolarising elementsThermal stabilityCinnamic acid
The invention relates to a liquid crystal aligning agent for a retardation film, a liquid crystal alignment film for a retardation film, and a retardation film and a process for production thereof. The invention aims at providing a liquid crystal aligning agent capable of forming a liquid crystal alignment film for a retardation film, a retardation film provided with a liquid crystal alignment film for the retardation film and excellent liquid crystal alignment performance and thermal stability, and a manufacture method for the retardation film, the liquid crystal aligning agent can be in light alignment even few radioactive rays illuminate, and no heating procedure is needed during and after illumination. The invention is a liquid crystal aligning agent for a retardation film comprising [A] polysiloxane provided with a light alignment group. The light alignment group is preferably a group provided with a cinnamic acid structure.
Owner:JSR CORPORATIOON
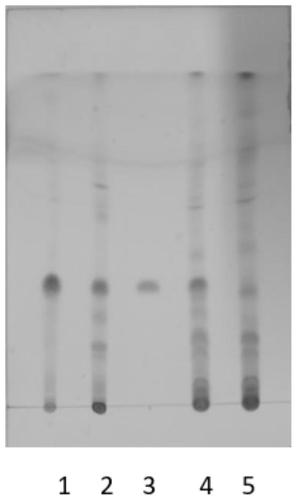
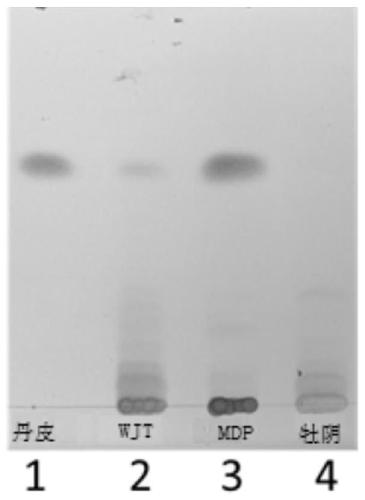
Quality detection method of traditional Chinese medicine preparation
ActiveCN111044624AReasonable quality inspectionEasy to separateComponent separationBiotechnologyGallic acid ester
The invention relates to a quality detection method of a traditional Chinese medicine preparation. The method comprises the following steps: respectively taking gallic acid, albiflorin, paeoniflorin,ferulic acid, liquiritin, beta-ecdysterone, senkyunolide I, glycyrrhizin, cinnamic acid, cinnamyl aldehyde, paeonol, ammonium glycyrrhizinate and ligustilide as reference substances, and establishinga fingerprint spectrum of the traditional Chinese medicine preparation by adopting high performance liquid chromatography; identifying Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba,moutan bark, ginseng, cassia bark, licorice root and the root of bidentate achyranthes of the traditional Chinese medicine preparation by adopting thin-layer chromatography. The traditional Chinese medicine preparation is prepared by using the following raw materials: Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba, cassia bark, moutan bark, zedoray rhizome, ginseng, licorice root and the root of bidentate achyranthes. With the two detection ways, comprehensive quality evaluation can be conducted on the meridian-warming decoction traditional Chinese medicine preparation. Themethod is simple, convenient, high in accuracy and high in reproducibility; a scientific basis can be provided for quality detection and evaluation of the meridian-warming decoction traditional Chinese medicine preparation, and the product quality is effectively controlled.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Photoprotector and/or photoimmunoprotector compositions of the skin and their uses
InactiveUS20070025933A1Prevent and minimise damaging effectPrevent and minimise reactionCosmetic preparationsBiocideBenzoic acidPhototherapy unit
The composition comprises of a component A selected from a hydroxylated derivative of benzoic acid or of cinamic acid, their esters, amides or salts, a glycoside of a hexose, and their mixtures; and a component B selected from quinic acid, shikimic acid, their alkaline metal or alkaline earth salts, their methyl esters, and mixtures of the same. This composition is suitable for protecting the skin against ultraviolet radiation coming from the sun or artificial sources, such as those used in phototherapy units and in sun tanning rooms. For application in the field of dermatology and nutrition, and, in particular, in the photoprotection of the skin and mucosa, photo-ageing and photocarcinogenesis, including protection of the immune system associated with the skin.
Owner:IND FARM CANTABRIA
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Anti-reflective coating composition with improved spin bowl compatibility
InactiveUS6962769B2Extended shelf lifeRadiation applicationsLayered productsAnti-reflective coatingAdditive ingredient
Anti-reflective compositions and methods of using those compositions to form circuits are provided. The compositions comprise a polymer dissolved or dispersed in a solvent system. In one embodiment, the compositions comprise less than about 0.3% by weight of a strong acid. In another embodiment, the weight ratio of strong acid to weak acid in the composition is from about 0:100 to about 25:75. Examples of preferred weak acid compounds include phenolic compounds (e.g., Bisphenol S, Bisphenol A, α-cyano-4-hydroxycinnamic acid), carboxylic acids (e.g., acetic acid), phosphoric acid, and cyano compounds. The polymer and other ingredients are preferably physically mixed in a solvent system. The resulting compositions are spin bowl compatible (i.e., they do not crosslink prior to the bake stages of the microlithographic processes or during storage at room temperature).
Owner:BREWER SCI
Special washing salt for pesticide residues in fruits and vegetables
ActiveCN103436391AHas a bactericidal effectPollutedSurface-active non-soap compounds and soap mixture detergentsInorganic non-surface-active detergent compositionsPesticide residuePollution
The invention discloses a special washing salt for pesticide residues in fruits and vegetables. The special washing salt comprises the following raw materials by weight: 65 to 95 parts of refined salt, 2 to 5 parts of sodium dodecyl benzene sulfonate, 3 to 4 parts of cinnamic acid, 1 to 2 parts of green tea powder, 2 to 3 parts of chitosan, 2 to 4 parts of sodium carbonate, 2 to 3 parts of sodium alpha-allylsulfonate and 0.005 to 0.01 part of essence. The special washing salt provided by the invention has the characteristics of a simple production process, no generation of waste gas, waste water and industrial residues, environment friendliness and the like and can improve performance of products of a same kind on the washing market at present and realize green washing. Compared with other washing agents, the washing salt uses salt as a basic raw material, so production cost for the washing salt is reduced and the washing salt performs a synergistic effect with a surfactant to reinforce a removal effect on pesticide residues; moreover, the washing salt uses salt has certain sterilization and disinfection effects, the advantages of no pollution, no toxicity, high-efficiency removal of pesticide residues, etc. and wide development prospects in fields like washing products.
Owner:中盐东兴盐化股份有限公司
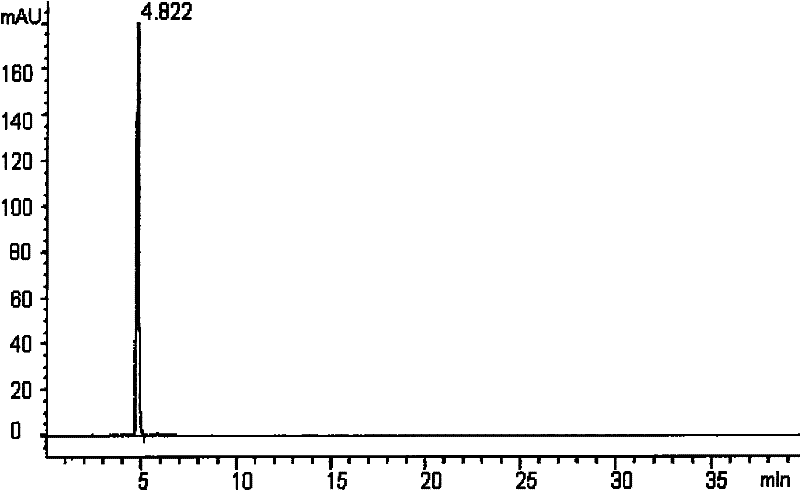
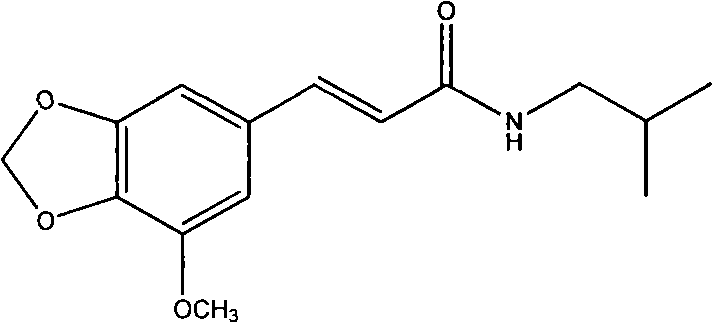
Application of 5'-methoxy-3',4'-methylenedioxy cinnamic acid isobutyl amide in preparing antidepressant medicaments
ActiveCN102240281ASignificantly antagonizes body temperature dropSignificantly antagonizes drooping eyelidsNervous disorderPill deliveryMethylenedioxyCinnamic acid
The invention belongs to the field of traditional Chinese medicine preparation, and relates to application of an amide compound 5'-methoxy-3',4'-methylenedioxy cinnamic acid isobutyl amide extracted from plants in preparing antidepressant medicaments.
Owner:TIANJIN TASLY PHARMA CO LTD
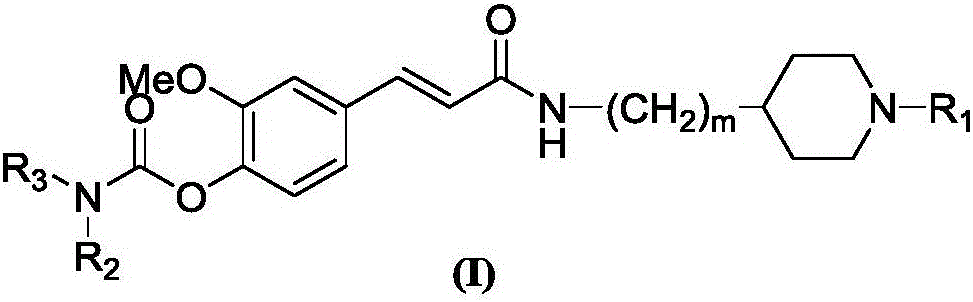
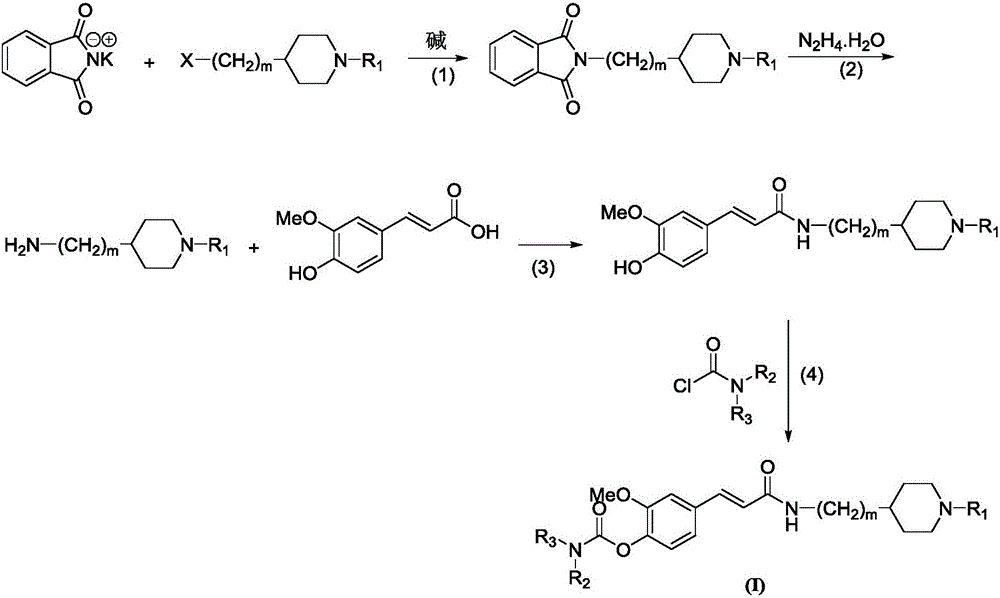
4-carbamate-3-methoxy cinnamic acid cyclamine alkyl amide compound, and preparation method and application thereof
InactiveCN105837497AEasy to prepareGood repeatabilitySenses disorderNervous disorderHuntingtons choreaSide effect
The invention discloses a 4-carbamate-3-methoxy cinnamic acid cyclamine alkyl amide compound, and a preparation method and application thereof. The compound prepared in the invention has low toxic and side effect, and can treat neurodegenerative diseases including, but not limited to, vascular dementia, the Alzheimer's disease, the Parkinson's disease, the Huntington's disease, HIV-associated dementia, multiple sclerosis, amyotrophic lateral sclerosis, neuropathic pain, glaucoma and the like.
Owner:NANYANG NORMAL UNIV
Ambient stable beverage
InactiveUS20020034568A1Weak tasteSuppression of unwanted preservative notesTea extractionPreservativePasteurization
An ambient stable tea based beverage that contains a tea extract and a preservative system. The preservative system contains cinnamic acid, one or more essential oils and one or more pasteurization adjuncts that become fungicidal when activated by heat.
Owner:THOMAS J LIPTON DIV OF CONOPCO
Biofuel and chemical production by recombinant microorganisms via fermentation of proteinacious biomass
Provided herein are metabolically modified microorganisms characterized by having an increased keto-acid flux when compared with the wild-type organism and comprising at least one polynucleotide encoding an enzyme, and causing the production of a greater quantity of a chemical product when compared with the wild-type organism. The recombinant microorganisms are useful for producing a large number of chemical compositions from various nitrogen containing biomass compositions and other carbon sources. More specifically, provided herein are methods of producing alcohols, acetaldehyde, acetate, isobutyraldehyde, isobutyric acid, n- butyraldehyde, n-butyric acid, 2-methyl-l-butyraldehyde, 2-methyl-l -butyric acid, 3- methyl-l-butyraldehyde, 3 -methyl- 1 -butyric acid, ammonia, ammonium, amino acids, 2,3-butanediol, 1,4-butanediol, 2-methyl-l, 4-butanediol, 2-methyl- 1,4-butanediamine, isobutene, itaconate, acetoin, acetone, isobutene, 1,5-diaminopentane, L-lactic acid, D- lactic acid, shikimic acid, mevalonate, polyhydroxybutyrate (PHB), isoprenoids, fatty acids, homoalanine, 4-aminobutyric acid (GABA), succinic acid, malic acid, citric acid, adipic acid, p-hydroxy-cinnamic acid, tetrahydrofuran, 3-methyl-tetrahydrofuran, gamma-butyrolactone, pyrrolidinone, n-methylpyrrolidone, aspartic acid, lysine, cadeverine, 2-ketoadipic acid, and / or S-adenosyl-methionine (SAM), from a suitable nitrogen rich biomass.
Owner:RGT UNIV OF CALIFORNIA
Anti-freezing cooling liquid and preparation thereof
ActiveCN101376802AGood storage stabilityImprove stabilityHeat-exchange elementsAnti freezingSodium silicate
The invention provides an antifreezing cooling liquid which comprises glycol, water, decanedioic acid, sodium silicate and a foam killer; wherein, the cooling liquid also contains cinnamic acid and mannite. The invention also provides a preparation method of the antifreezing cooling liquid; wherein, the method includes the step of uniformly mixing the glycol, the water, the decanedioic acid, the sodium silicate and the foam killer. The storage stability of the antifreezing cooling liquid provided by the invention is better; besides, the antifreezing cooling liquid provided by the invention extensively improves the restraining effect to aluminum corrosion.
Owner:BYD CO LTD
Inhibitor for preventing browning of cut vegetable
InactiveCN101664055APrevent browningPlay the role of anti-corrosion and fresh-keepingFruit and vegetables preservationSODIUM METAPHOSPHATECarrageenan
The invention relates to an inhibitor for preventing browning of a cut vegetable, comprising the following components: one of solvent water and ethanol or mixture thereof; one of sodium alginate, xanthan gum, carrageenan and chitosan, one or more of sodium tripolyphosphate, sodium pyrophosphate and 6-sodium metaphosphate, one or more of phytic acid, ascorbic acid, cinnamic acid, citric acid, kojicacid and edible acetic acid, and one or more of potassium sorbate, sodium dehydroacetate, sodium chloride and 4-hexylresorcinol. The inhibitor is used by soaking, and the soaking time varies with thetemperature and the air pressure of the processing environment. All the components used in the inhibitor are permitted in the GB2760-2007 (hygienic standards of food additives), and the inhibitor issulfite-free, safe and non-toxic, and can be used for fresh cut vegetables and can play the multiple roles of pH regulation, enzyme activity inhibition, oxygen removal and isolation and water retention to effectively prevent browning and play a role of anticorrosion and refreshment.
Owner:SHANGHAI ACAD OF AGRI SCI
Multi-effect mixed biological preservative paper and using method thereof for juicy peaches
ActiveCN104831585AImprove food safetyImprove securityNon-fibrous pulp additionFruit and vegetables preservationSodium phosphatesEvaporation
The invention belongs to the technical field of preservation of botanical fruits and particularly relates to multi-effect mixed biological preservative paper and a using method thereof. The multi-effect mixed biological preservative paper is made by combining biological preservative materials with common packaging paper and can preserve juicy peaches in the long run. The biological preservative materials are lysozyme, Vc derivatives, chitosan, galangal and a few of auxiliary elements comprising beta-cyclodextrin, sodium phosphates and cinnamic acid. Compared with the conventional methods, the multi-effect mixed biological preservative paper has remarkable advantages in food safety, environmental protection and simplicity degrees of operation, can be recycled and reproduced and has the advantages of sterilization, preservation, cooling, nutrition less water evaporation and the like. In addition, the multi-effect mixed biological preservative paper is applicable to industrial batch production and used for many orchard workers for preserving and storing juicy peaches in the market.
Owner:NANJING UNIVERSTIY SUZHOU HIGH TECH INST
Method for preparing cinnamon extraction, cinnamon extraction and its composition and use
ActiveCN101167802AIncreased sensitivityImprove the immunityMetabolism disorderPlant ingredientsLiver functionsPharmacy
The invention relates to a preparation method of cinnamon extract, the cinnamon extract prepared thereby, a composition containing the extract and the use of the extract in pharmacy or preparation of health products. The preparation method comprises the following steps: a) uniformly mixing the cinnamon raw material with an aqueous solvent; b) extracting the mixture obtained in step a); c) separating the residue to obtain the cinnamon extract. The obtained cinnamon extract contains cinnamyl alcohol, cinnamic acid, cinnamaldehyde, eugenol, etc., and the appearance is a yellow clear liquid with a pH of about 4.6. The invention also provides a composition containing the extract. The cinnamon extract of the invention can be used to prepare medicines or health care products that have the dual effects of lowering blood sugar and blood fat and protecting liver function.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Salt and heat sensitive, substantive UV-absorbing polymers
Owner:GALAXY SURFACTANTS LTD
Beautifying and whitening sun protection pressed powder and preparation method thereof
ActiveCN101780025AWide and strong absorptionImprove securityCosmetic preparationsBody powdersCinoxateMagnesium stearate
The invention relates to a beautifying and whitening sun protection pressed powder and a preparation method thereof. An ultra micro pearl powder, lysine, vitamin E, magnesium stearate, cinoxate and titanium dioxide, and other raw materials are selected as functional compositions used for beautifying, whitening and sun protection. The beautifying and whitening sun protection pressed powder has good effect, has wet and dry two purposes and has the functions of shielding, whitening, nourishing skin, anti-aging, blocking sun, and the like. The beautifying and whitening sun protection pressed powder can play a role in shielding; by applying the matching between traditional Chinese medicine and modern scientific principles, the functional compositions thereof have functional actions; and chemical harmful substances are not present in the raw material compositions, and side effects are not generated. The beautifying and whitening sun protection pressed powder and the preparation method thereof have the advantages of reasonable formula, simple process and extremely obvious use effect.
Owner:TIANJIN TIANSHI BIOLOGICAL DEV
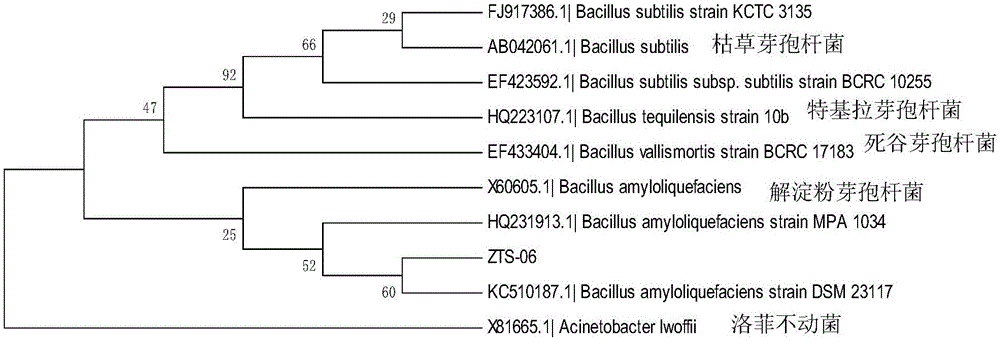
Bacillus amyloliquefaciens strain and application thereof
ActiveCN105062937APromote degradationAlleviate continuous cropping obstaclesBiocidePlant growth regulatorsPropanoic acidToxic material
The invention discloses a Bacillus amyloliquefaciens strain and application thereof. The strain is Bacillus amyloliquefaciens ZTS-06, and the collection number is CGMCC No.11165. The invention also discloses a live strain preparation containing the strain and a preparation method thereof. The live strain preparation is prepared by the following steps: carrying out amplification culture on the Bacillus amyloliquefaciens ZTS-06, inoculating in a fermentation culture medium, culturing to obtain a fermentation liquid, adding propyl gallate, beta-cyclodextrin and soluble starch, and carrying out spray drying to obtain live strain raw powder; and adding abundant soluble starch, and uniformly mixing to obtain the live strain preparation. The strain has favorable degradation effects on phenylallyl propionic acid, para-hydroxybenzoic acid, cinnamic acid and the like, and can relieve succession cropping obstacles caused by phenolic acid and other toxic substances. The living strain preparation has the advantages of short fermentation period, high fermentation liquid bacterium concentration, low live strain loss in the spray drying process, and high stability in the storage process.
Owner:SHANDONG ZOETICLAND BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com