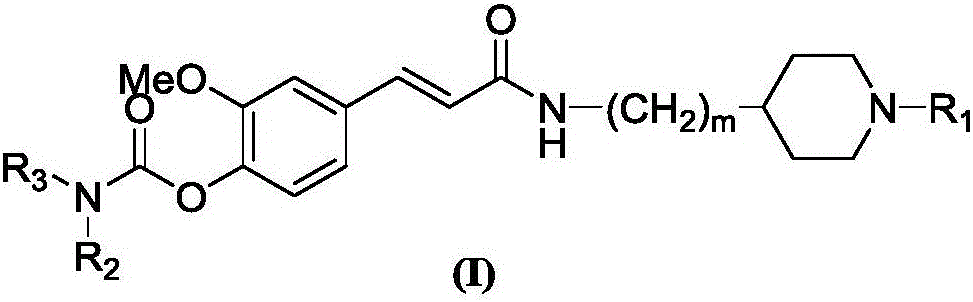
4-carbamate-3-methoxy cinnamic acid cyclamine alkyl amide compound, and preparation method and application thereof
A cinnamic acid cycloamine alkyl amide, carbamate technology, applied in organic chemistry, drug combination, muscle system diseases, etc., can solve the problems of single target, poor long-term efficacy in AD patients, and many toxic and side effects , to achieve the effects of high yield, good blood-brain barrier permeability, and simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-19
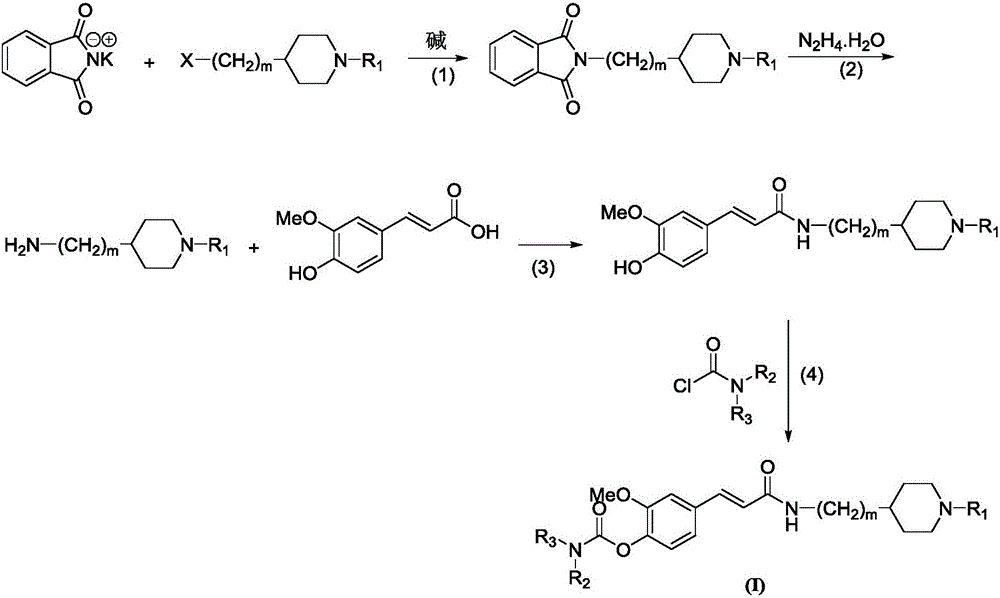
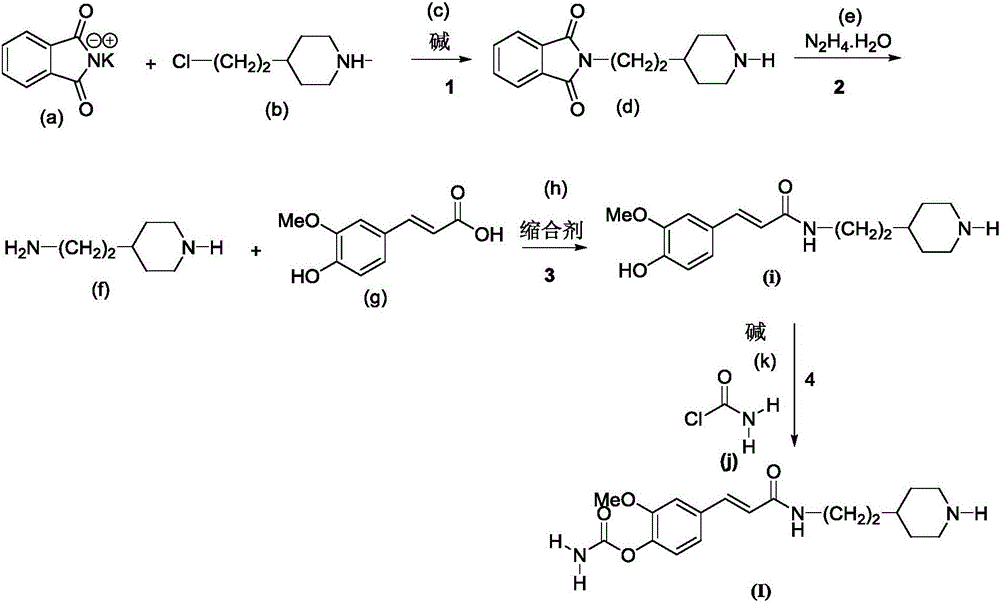
[0038] A preparation method of 4-carbamate-3-methoxycinnamic acid cyclic amine alkyl amides compound, comprises the steps:
[0039] The first step: using phthalimide potassium salt as the starting material, reacting with 1-substituted-4-chloroalkylpiperidine in the first solvent and the first basic condition to obtain phthalimide imidoalkylamine compounds;
[0040] The second step: adding the phthalimide alkylamine compound obtained in the first step into the second solvent, adding N 2 h 4 .H 2 0, warming up to reflux and stirring reaction (reaction progress tracked with TLC); after the reaction was finished, suction filtration while hot, a small amount of ethanol washed the filter cake, the filtrate was evaporated under reduced pressure to remove the solvent, and the residue was purified by column chromatography (eluent: two Chloromethane:methanol=15:1v / v) to obtain alkylamino primary amine compounds;
[0041] The third step: the primary amine compound obtained in the sec...
Embodiment 20
[0067] The specific process conditions are the same as those in Example 1-2, and the difference is the same as in the investigation of different substituents. The specific substituents are shown in Table 5, and the resulting 4-carbamate-3-methoxycinnamic acid cyclic amine alkyl amides compound , and its chemical structure was confirmed by 1H-NMR, 13C-NMR and ESI-MS.
[0068]
[0069]
[0070]
[0071]
[0072]
[0073]
[0074]
Embodiment 21
[0076] A kind of preparation method of the salt of 4-carbamate-3-methoxycinnamic acid cycloaminoalkyl amides compound, comprises the steps as follows, 4-carbamate-3-methoxycinnamic acid cycloaminoalkyl Mix the base amide compound (I) with acetone, stir evenly, add acid, heat and reflux and stir for 15 to 30 minutes, cool to room temperature after the reaction, evaporate the solvent under reduced pressure, recrystallize the residue with acetone, and filter the precipitated solid , to obtain the salt of 4-carbamate-3-methoxycinnamic acid cycloaminoalkylamide compound (I). Among them, its chemical structure was confirmed by 1HNR and ESI-MS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com