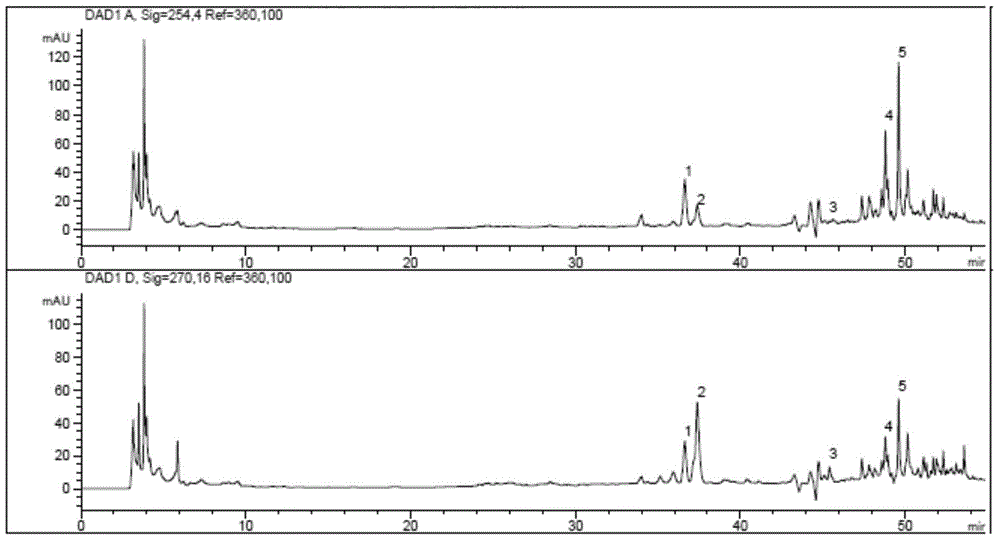
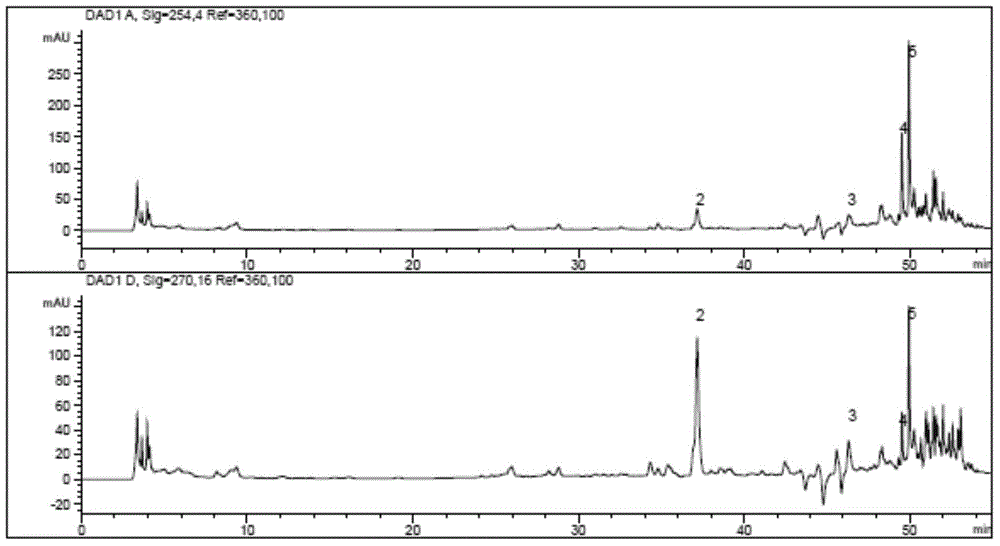
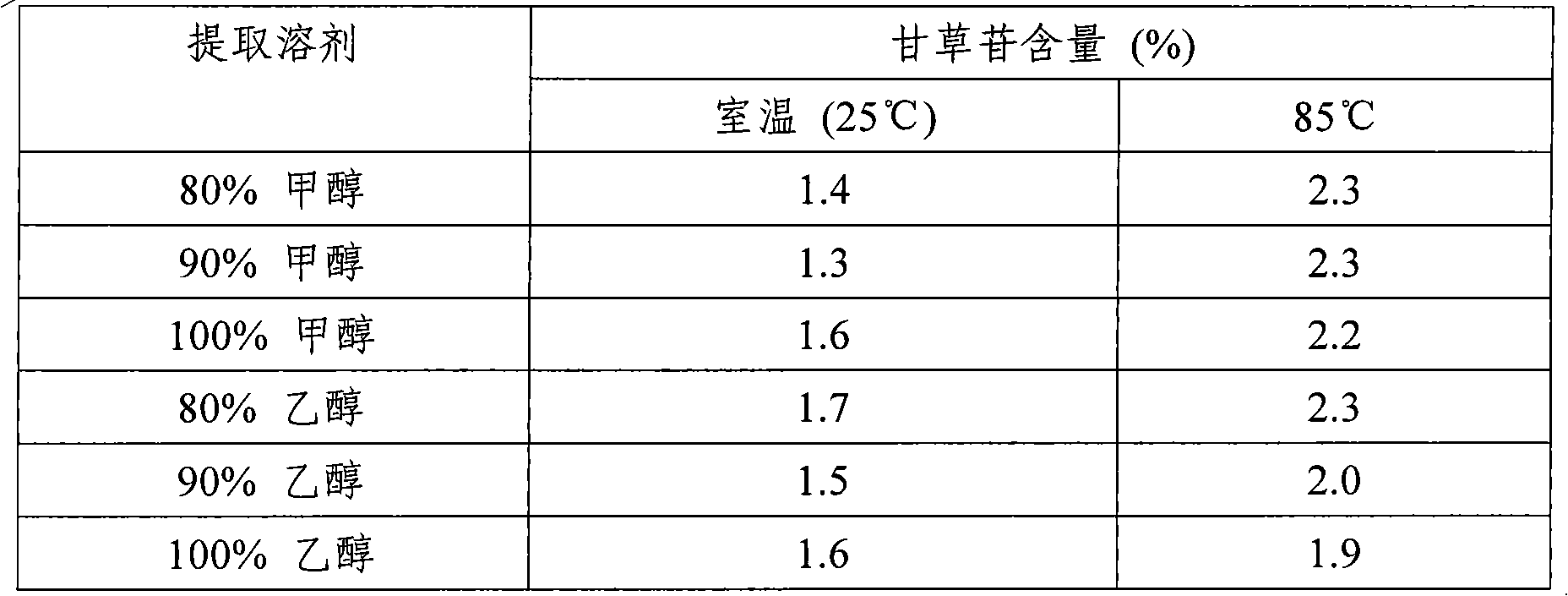
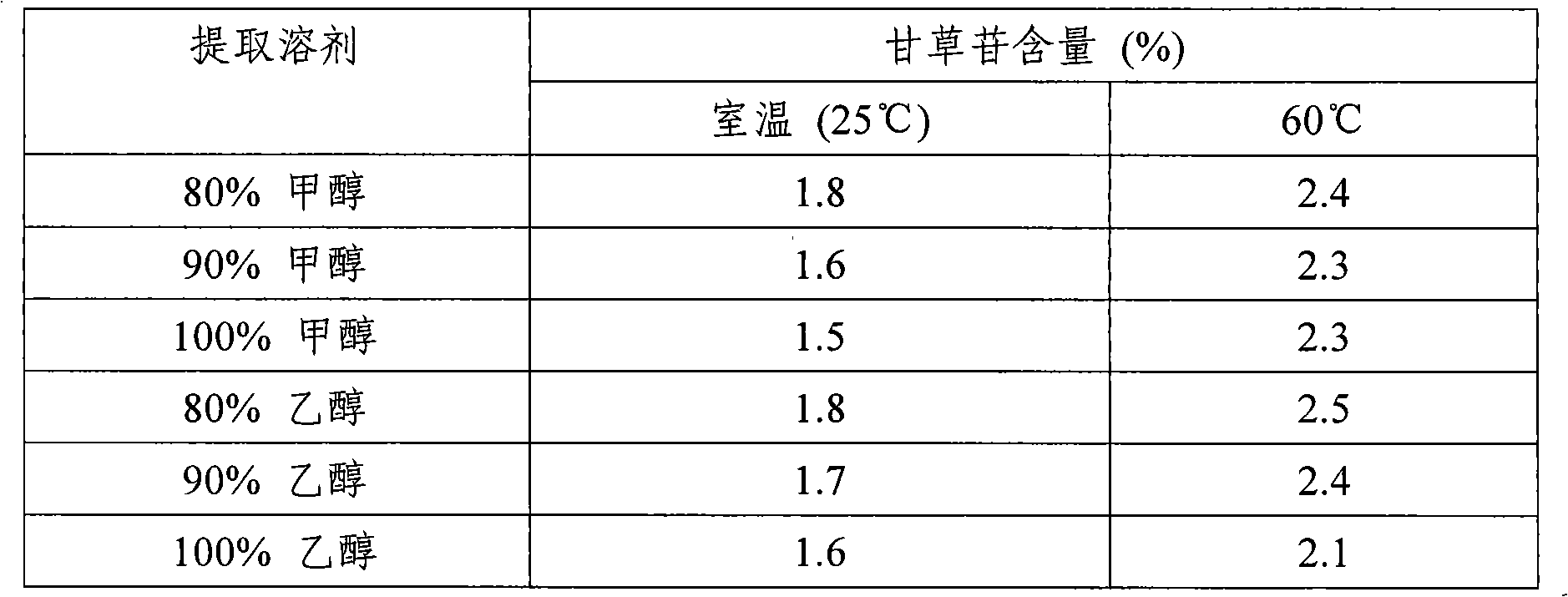
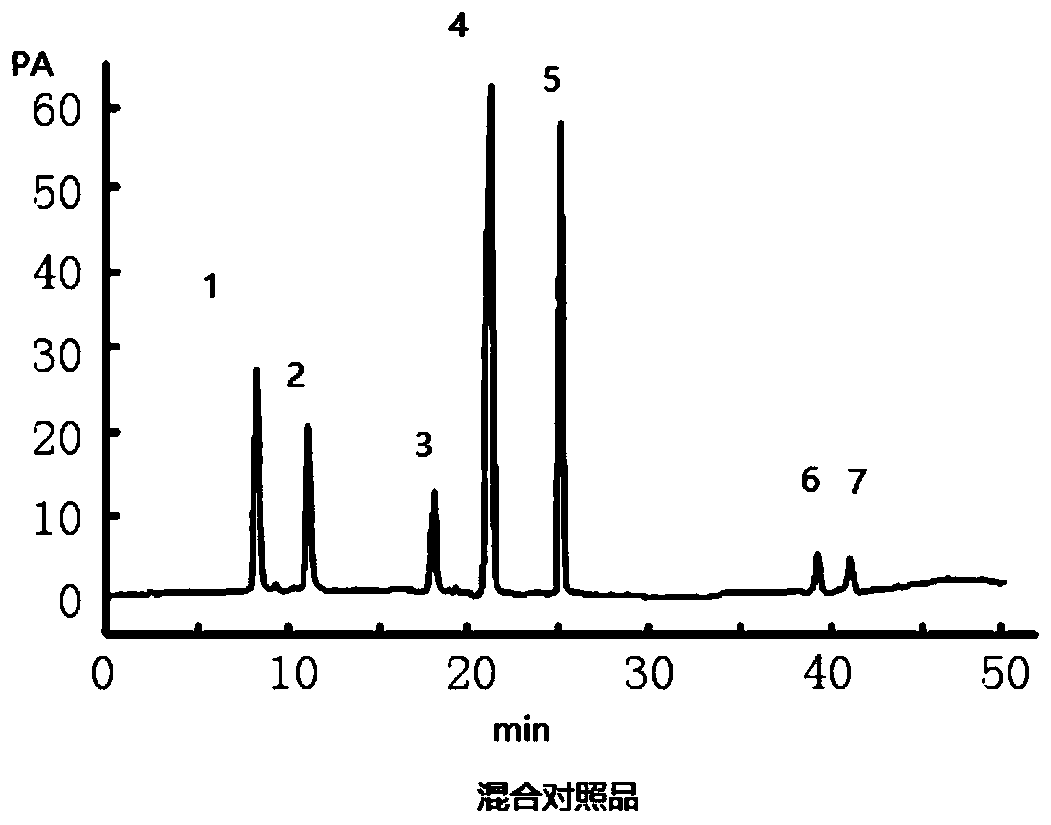
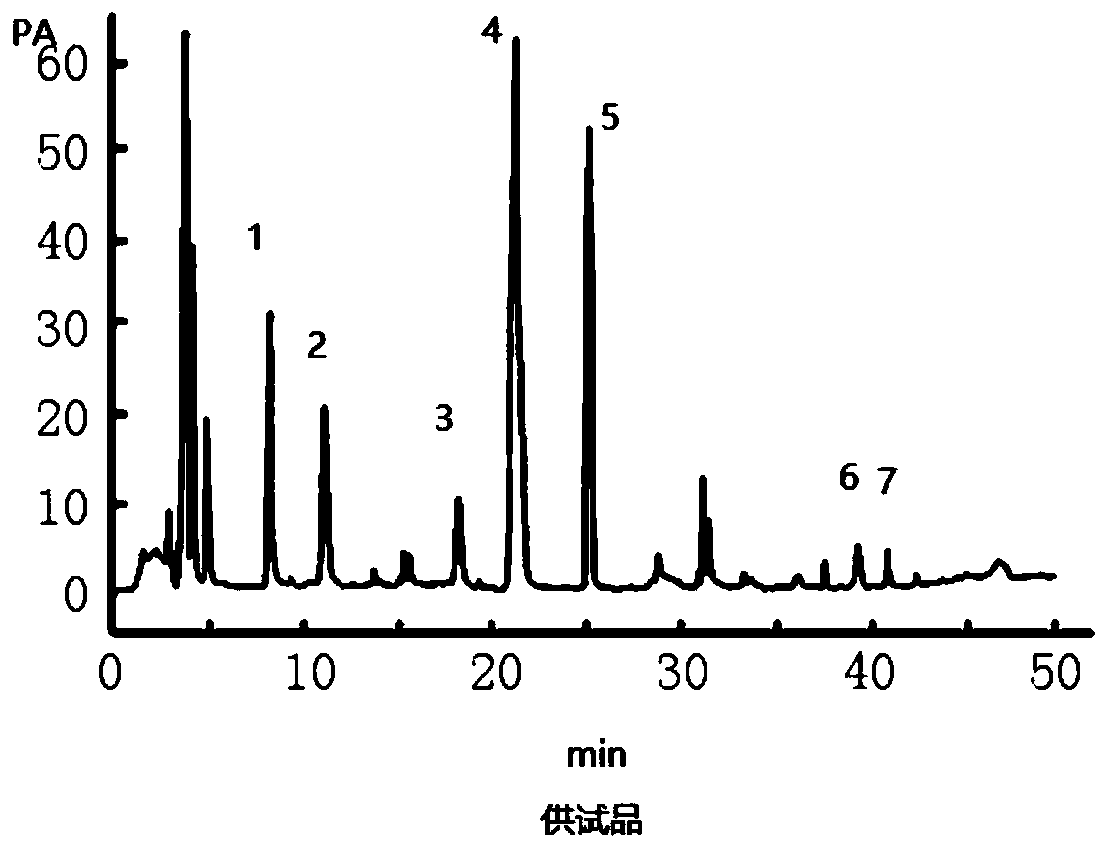
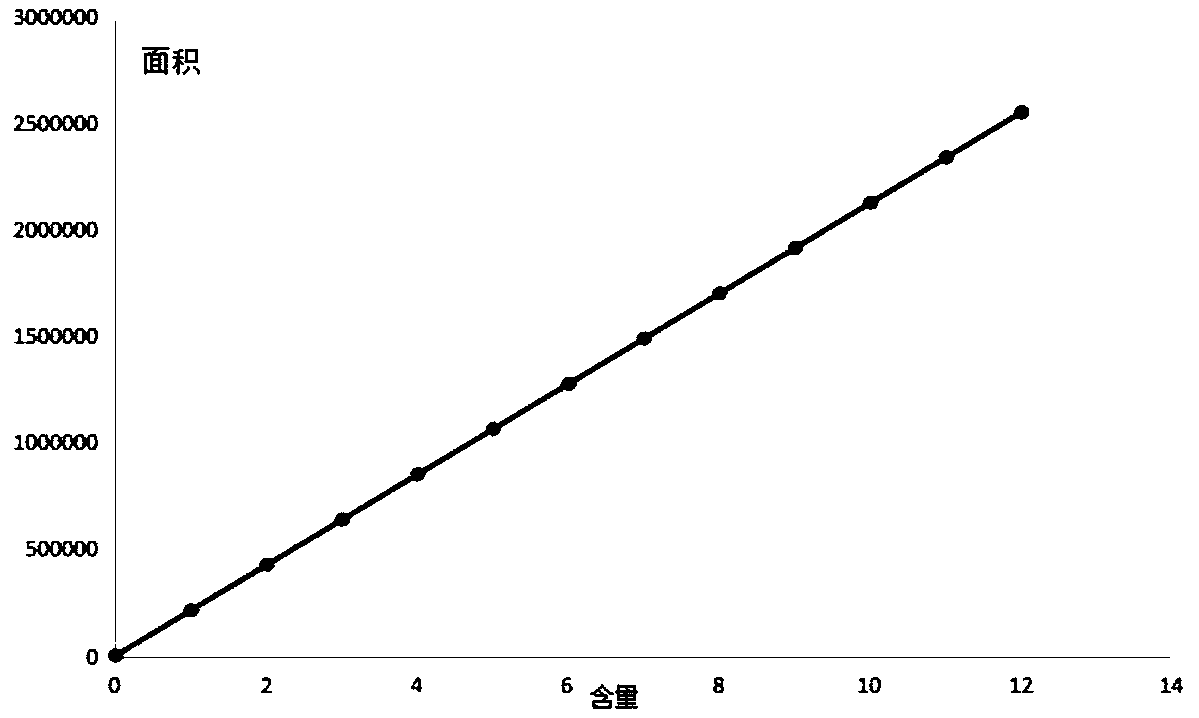
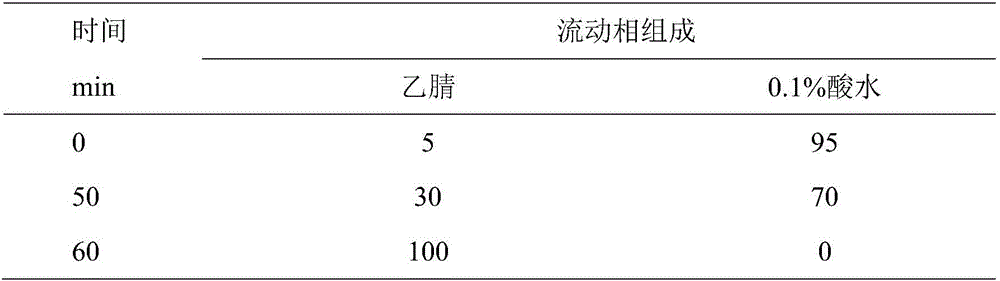
Patents
Literature
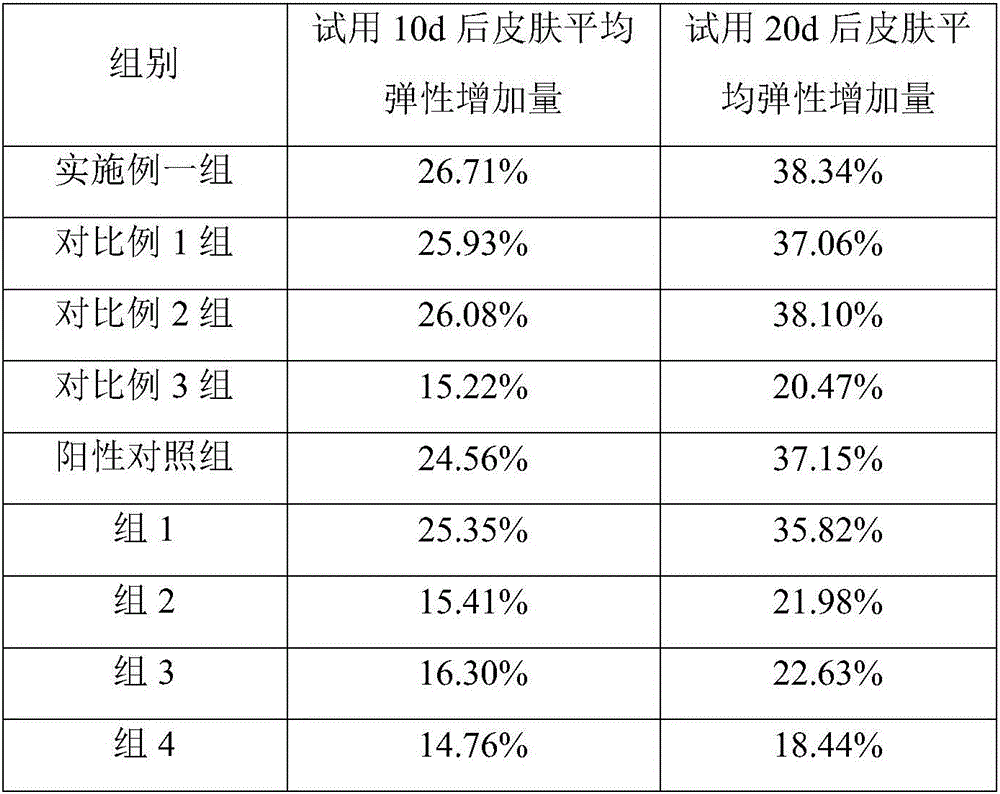
157 results about "Liquiritin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
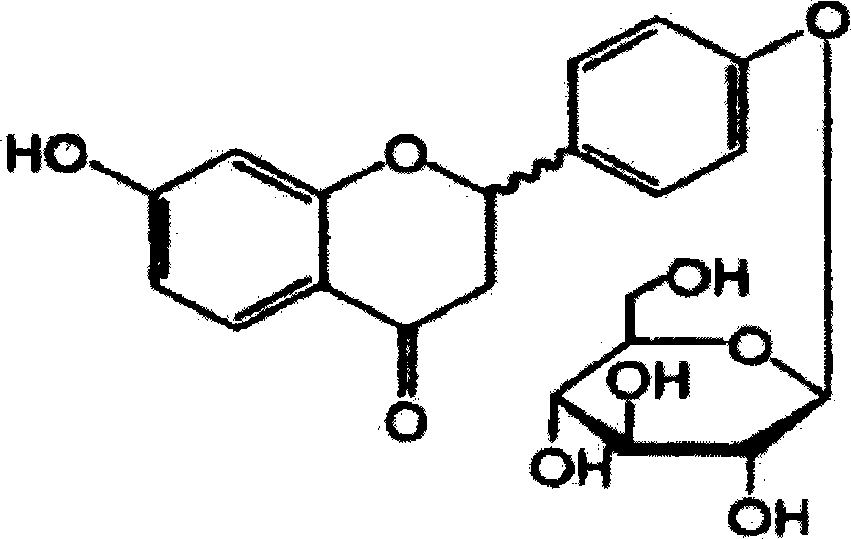
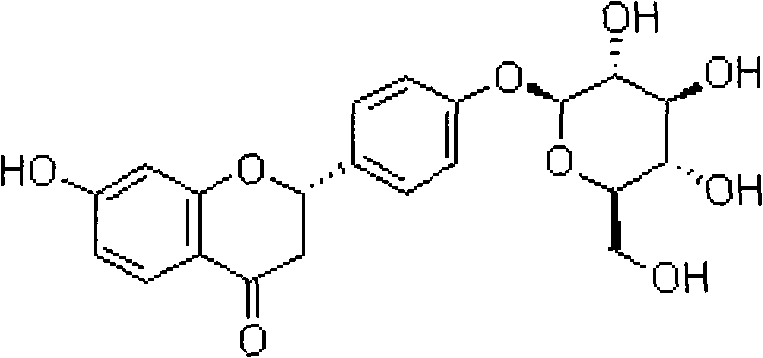
Liquiritin is the 4'-O-glucoside of the flavanone liquiritigenin.
Quality detection method of traditional Chinese medicine preparation
ActiveCN111044624AReasonable quality inspectionEasy to separateComponent separationBiotechnologyGallic acid ester
The invention relates to a quality detection method of a traditional Chinese medicine preparation. The method comprises the following steps: respectively taking gallic acid, albiflorin, paeoniflorin,ferulic acid, liquiritin, beta-ecdysterone, senkyunolide I, glycyrrhizin, cinnamic acid, cinnamyl aldehyde, paeonol, ammonium glycyrrhizinate and ligustilide as reference substances, and establishinga fingerprint spectrum of the traditional Chinese medicine preparation by adopting high performance liquid chromatography; identifying Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba,moutan bark, ginseng, cassia bark, licorice root and the root of bidentate achyranthes of the traditional Chinese medicine preparation by adopting thin-layer chromatography. The traditional Chinese medicine preparation is prepared by using the following raw materials: Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba, cassia bark, moutan bark, zedoray rhizome, ginseng, licorice root and the root of bidentate achyranthes. With the two detection ways, comprehensive quality evaluation can be conducted on the meridian-warming decoction traditional Chinese medicine preparation. Themethod is simple, convenient, high in accuracy and high in reproducibility; a scientific basis can be provided for quality detection and evaluation of the meridian-warming decoction traditional Chinese medicine preparation, and the product quality is effectively controlled.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Prepn process of licoflavone from licorice and licorice slag
InactiveCN1810796ASimple processHigh yieldSugar derivativesSugar derivatives preparationLicorice acidOrganic solvent
The present invention discloses preparation process of licoflavone from licorice and licorice slag, and features licoflavone is extracted from crushed licorice or licorice slag after extracting glycyrrhetic acid through adding organic solvent for soaking, filtering, and concentrating the filtrate; and that the prepared licoflavone contains licochalcone, isoliquiritigenin, glabidin, liquiritin, liquiritigenin and other components. The preparation process is simple, high in yield and suitable for industrial production.
Owner:车庆明
Method for separating and preparing liquiritin
ActiveCN101289480AHigh purityGuaranteed puritySugar derivativesPlant ingredientsMethanol waterAlcohol
The invention relates to the separation of natural medicines, in particular to a separation and preparation method of liquiritin; wherein, extracting solution is obtained by decocting the ground licorice plants with ten times of water, and then extract is obtained after the extracting solution is concentrated, and then ethanol is added for alcohol precipitation twice, and then a 6000Da molecular weight membrane separator is used for separating the extract, and then membrane separation permeation liquid is separated by X-5 macro-porous absorption resin and is eluted with the ethanol water, and the obtained macro-porous resin separation components are separated by an efficient industrial chromatographic column and eluted with gradients with the methanol water and then the liquiritin is obtained by collecting, concentrating, freezing and drying the eluting solution. The method is high in product purity, great in preparation amount and stable and reliable in process and especially suitable for separating and preparing great amount of highly purified liquiritin compounds from the traditional Chinese medicine licorice.
Owner:浙江华谱新创科技有限公司
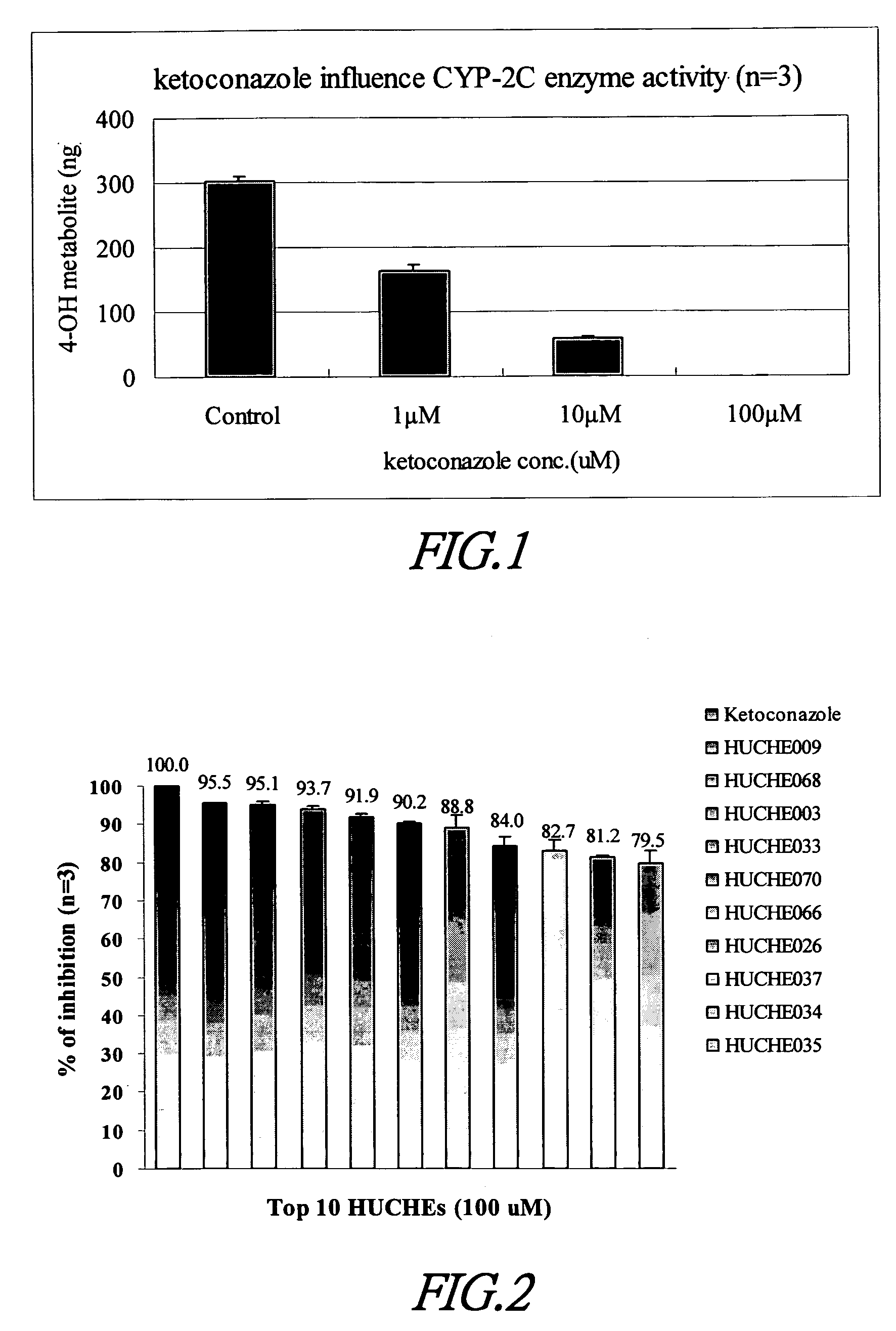
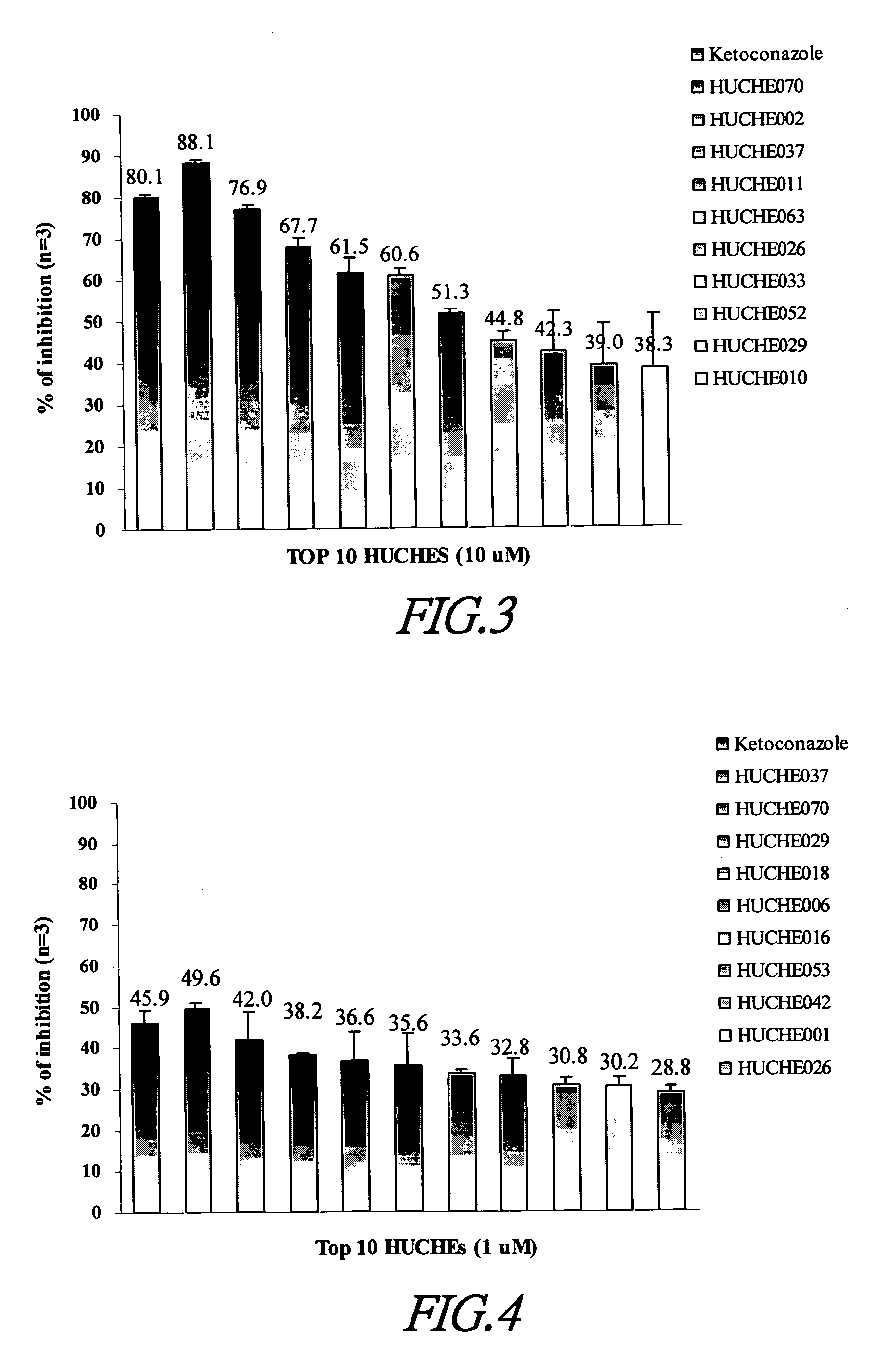
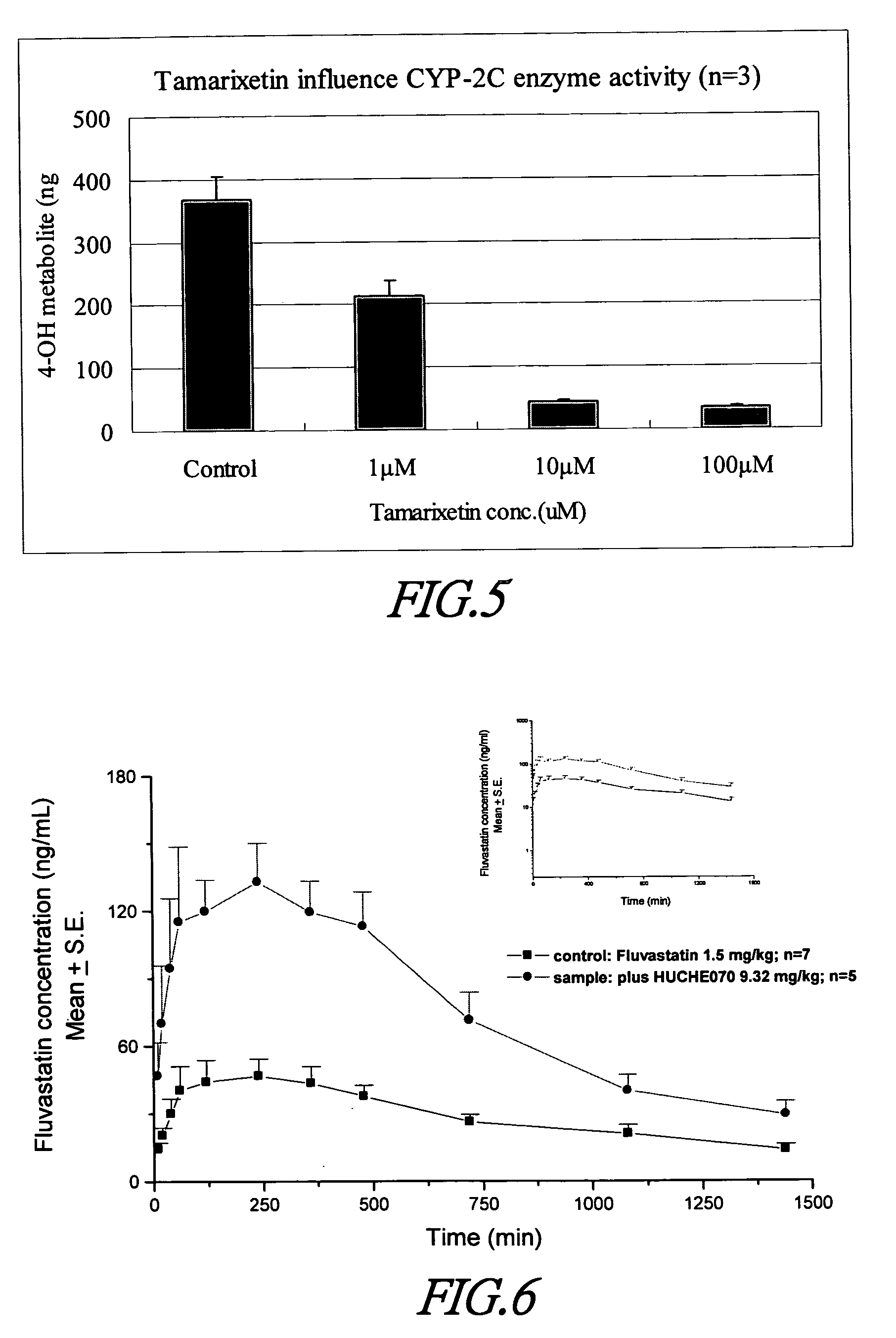
Cytochrome P450 2C9 inhibitors
InactiveUS20060069042A1Improve bioavailabilityBiocideKetone active ingredientsEriodictyolTamarixetin
This invention is to provide multiple specific inhibitors of cytochrome P450 isozyme CYP2C9. These inhibitors can be derived from any combinations with the following compounds including: Tamarixetin, Formononetin, isoliquritigenin, Phloretin, luteolin, Quercitrin, quercetin, myricetin, Wongonin, Puerarin, Genistein, Nordihydroguaiaretic acid, Narigenin, Capillarisin, Chrysin, Fisefin, eriodictyol, 6-Gingerol, Isorhamneti, isoquercitrin, Morin, (+)-Taxifolin, isovitexin, 3-Phenylpropyl Acetate, Oleanolic acid, ursolic acid, β-Myrcene, cinnamic acid, Luteolin-7-Glucoside, Liquiritin, (+)Limonene, Homoorientin, Swertiamarin, Embelin, Daidzein, Poncirin, (−)-Epicatechin, ergosterol. These natural products can be used to enhance the bioavailability of therapeutic agents (drugs).
Owner:NAT DEFENSE MEDICAL CENT
Method for rapidly detecting index composition in Chinese herbal medicine compound preparation
The invention relates to the field of detection on traditional Chinese medicine quality, relates to a method for rapidly detecting index composition in Chinese herbal medicine compound preparation, and especially relates to a rapid detection method for index composition in a Chinese herbal medicine compound preparation radix-stephaniae-tetrandrae radix-astragali soup. According to the method, methanol with the concentration of 50% is employed for ultrasonic extraction, and an HPLC-DAD process is applied to simutaneously determine the content of fangchinoline, tetrandrine, calycosin-7-glucoside, liquiritin and glycyrrhizic acid in radix stephaniae tetrandrae, radix astragali and radix glycyrrhizae in the radix-stephaniae-tetrandrae radix-astragali soup at one time. The method solves the problem that quality control is simultaneously performed on stephaniae tetrandrae, radix astragali and radix glycyrrhizae in the radix-stephaniae-tetrandrae radix-astragali soup, and avoids time and reagent waste caused by multiple detection on the five index compositions. The method is simple in operation and high in working efficiency and precision.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Extraction method for increasing liquiritigenin content in glycyrrhizae radix et rhizoma or glycyrrhizae radix extract
InactiveCN102112142ASkip the column chromatography processSimple stepsOrganic active ingredientsNervous disorderOrganic solventGLYCYRRHIZA EXTRACT
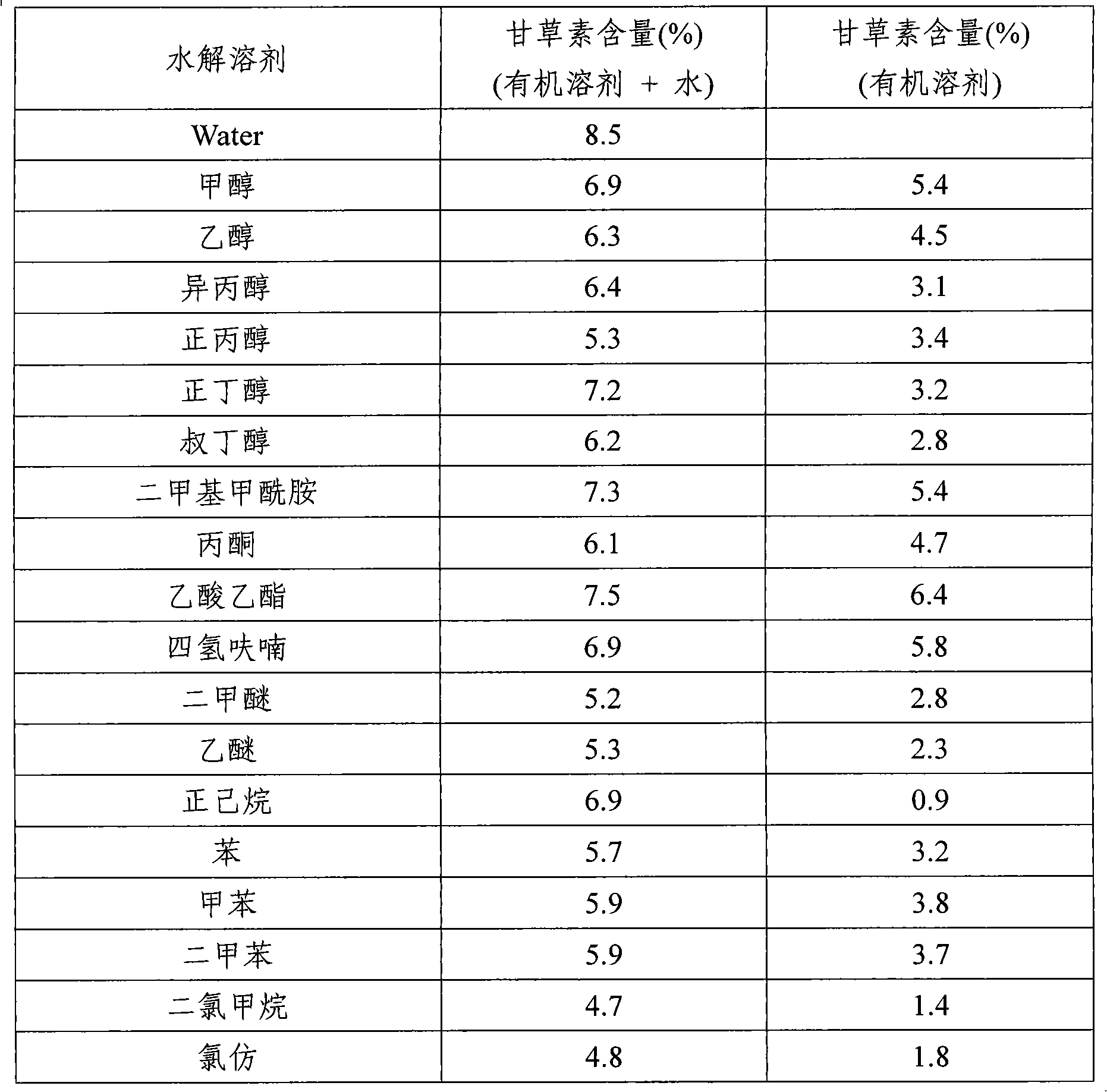
Disclosed herein is an extraction method for increasing liquiritigenin content in Glycyrrhizae Radix et Rhizoma or Glycyrrhizae radix extract, the method comprises: using water, an organic solvent, or a mixed solvent of water and the organic solvent to Glycyrrhizae Radix et Rhizoma to extract a product containing liquiritin (Step 1); and introducing water, an organic solvent, or a mixed solvent of water and the organic solvent into the product containing liquiritin extracted in Step 1, adding acid or base into the solution, and hydrolyzing the mixture to prepare a product containing liquiritigenin (Step 2).
Owner:DAE WON PHARMA
Detection method of cassia twig, peony and anemarrhena decoction
The invention provides a detection method of cassia twig, peony and anemarrhena decoction, where detecting is achieved by means of high-performance liquid chromatography. The detection method includesthe steps of 1) preparing reference solutions, to be specific, collecting controls of gallic acid, mangiferin, paeoniflorin, prim-o-glucosylcimifugin, liquiritin, 5-O-methylvisammioside, cinnamic acid and ammonium glycyrrhetate, and adding methanol solution respectively to obtain the reference solutions; 2) preparing a test solution, to be specific, adding methanol to dissolve lyophilized powderof cassia twig, peony and anemarrhena decoction to obtain the test solution; 3) sucking the reference solutions and the test solution, and injecting them into a liquid chromatograph. The detection method is suitable for detecting gallic acid, mangiferin, paeoniflorin, prim-o-glucosylcimifugin, liquiritin, 5-O-methylvisammioside, cinnamic acid and ammonium glycyrrhetate, allows overall quality of the cassia twig, peony and anemarrhena decoction to be controlled, also helps provide methodological basis for the study on the material basis of the cassia twig, peony and anemarrhena decoction, and is worthy of popularization and application.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE +1
Method for extracting liquiritin from liquorice
The invention discloses a method for extracting liquiritin from liquorice, and the method comprises the following steps: (1) crushing the liquorice, extracting with deionized water and filtering to get liquiritin crude extract solution; and (2) mixing the liquiritin crude extract solution with a resin column, and performing elution, separation, purification and recrystallization. The liquiritin extracted by the method is a natural extract which is high in purity and low in cost, the production efficiency is high and the quality is stable and controllable.
Owner:天津市尖峰天然产物研究开发有限公司
Preparation process and quality control method of poria cocos, cassia twig, bighead atractylodes rhizome and liquorice decoction material reference
ActiveCN112083099AEnsure consistencyGuaranteed transfer rateComponent separationAgainst vector-borne diseasesRhizomeLiquiritin
The invention relates to a preparation process and a quality control method of a poria cocos, cassia twig, bighead atractylodes rhizome and liquorice decoction material reference. According to the invention, through systematic historical research on prescriptions, an ancient method and a modern extraction method are combined, single factor investigation is adopted, and a preparation process and adrying process of the poria cocos, cassia twig, bighead atractylodes rhizome and liquorice decoction and the material reference thereof are optimized. Furthermore, poria cocos, cassia twig, bighead atractylodes rhizome and liquorice in the material reference are identified by adopting thin-layer chromatography, a fingerprint spectrum with good separation degree and reproducibility is established by adopting high performance liquid chromatography, and liquiritin, cinnamic acid and cinnamaldehyde are quantitatively researched by adopting the high performance liquid chromatography. According to the established preparation process and the quality standard research method of the poria cocos, cassia twig, bighead atractylodes rhizome and liquorice decoction material reference, a systematic research basis is provided for reasonable development of the compound preparation.
Owner:JIANGSU KANION PHARMA CO LTD
Method for measuring content of eighteen ingredients of Huangqi Jianzhong Wan (astragalus mongholicus pill for invigorating stomach and regulating middle warmer)
InactiveCN109828062AEasy to handleGood reproducibilityComponent separationAstragalosideAdditive ingredient
The invention provides a method for measuring content of eighteen ingredients of Huangqi Jianzhong Wan (astragalus mongholicus pill for invigorating the stomach and regulating the middle warmer). Themethod includes the steps of subjecting the Huangqi Jianzhong Wan to ultrasonic extraction and centrifugation, extracting the liquid supernate, detecting the liquid supernate by an ultra performance liquid chromatography-tandem mass spectrometry positive and negative ion mode, preparing a mixed standard product solution from standard products of the eighteen ingredients, building a standard curve,and carrying out the methodological study. The method can realize measuring the eighteen ingredients of the Huangqi Jianzhong Wan, namely ononin, formononetin, calycosin, calycosin-7-glucoside, pterocarpan, astraisoflavan-7-O-beta-D-glucoside, isoflavane, isoliquiritin, liquiritigenin, liquiritin, glycyrrhetinic acid, cinnamic acid, isomucronulatol 7-O-beta-glucoside, astragaloside I, astragaloside II, astragaloside III, astragaloside IV and gallic acid. In the method, the sample pre-treatment process is simple and accordingly time for measurement is short; the method has the advantages of high specificity, high flexibility, good reproducibility and short analysis time, and can be applied to monitoring of production quality of the Huangqi Jianzhong Wan.
Owner:SHANXI UNIV
Method for simultaneous determination of six active components in Niuhuang Ninggong tablet
InactiveCN104897787ASimple methodAccurate methodComponent separationPhosphoric acidColumn temperature
The invention discloses a method for simultaneous determination of six active components consisting of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride in a Niuhuang Ninggong tablet through HPLC. Chromatographic conditions employed in the invention are as follows: a chromatographic column is TC-C18 (4.6 mm * 250 mm, 5 [mu]m); detection wavelength is 280 nm; a mobile phase is methanol-0.05% phosphoric acid; gradient elution comprises three parts, i.e., elution with methanol with a concentration varying in a range of 10 to 80% in the time period from 0 min to 35 min, then elution with methanol with a concentration of 80% in the time period from 35 to 50 min, and finally elution with methanol with a concentration varying in a range of 80 to 10% in the time period from 50 to 60 min; flow velocity is 1.0 mL / min; column temperature is 25 DEG C; and sample size is 10 [mu]L. Under the above-mentioned chromatographic conditions, chromatographic peaks are perfectly separated, and concentrations and peak areas of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride show good linear relation. The method is simple, rapid and accurate, has good repeatability and can provide quality bases for comprehensive evaluation and control of the Niuhuang Ninggong tablet.
Owner:JILIN NORMAL UNIV
Detection method for fingerprint spectrum of medicinal preparation treating hepatitis
InactiveCN105181825AImprove securityImprove stabilityComponent separationDigestive systemChlorogenic acidMedicine
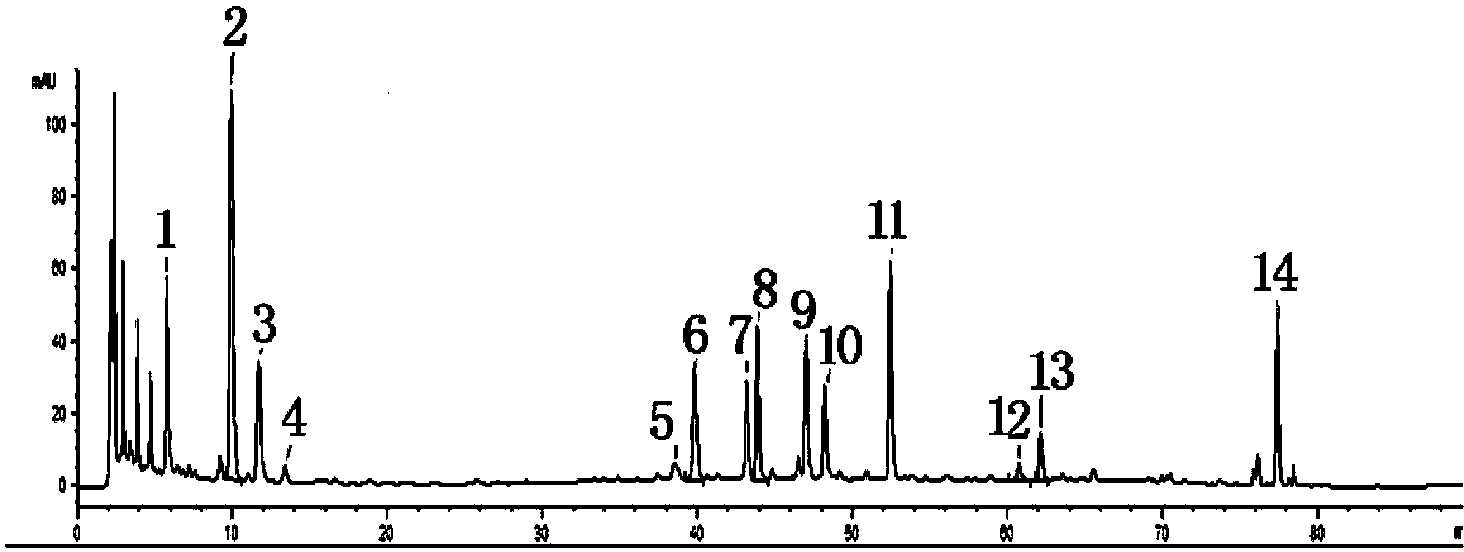
The invention relates to a detection method for a fingerprint spectrum of a medicinal preparation treating hepatitis. In the detection method, through optimization selection of a No.11 peak of baicalin as an internal reference peak of a fingerprint spectrum, retention time of characteristic common peaks: a No.3 peak of chlorogenic acid, a No.4 peak of gentiopicroside, a No.5 peak of paeoniflorin, a No.6 peak of liquiritin, a No.8 peak of ferulic acid and the like of a yindan pinggan capsule is determined, the yindan pinggan capsule can be detected comprehensively and rapidly, which facilitates comprehensive quality detection and whole quality control, and therefore safety and stability of medicine usage is raised.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
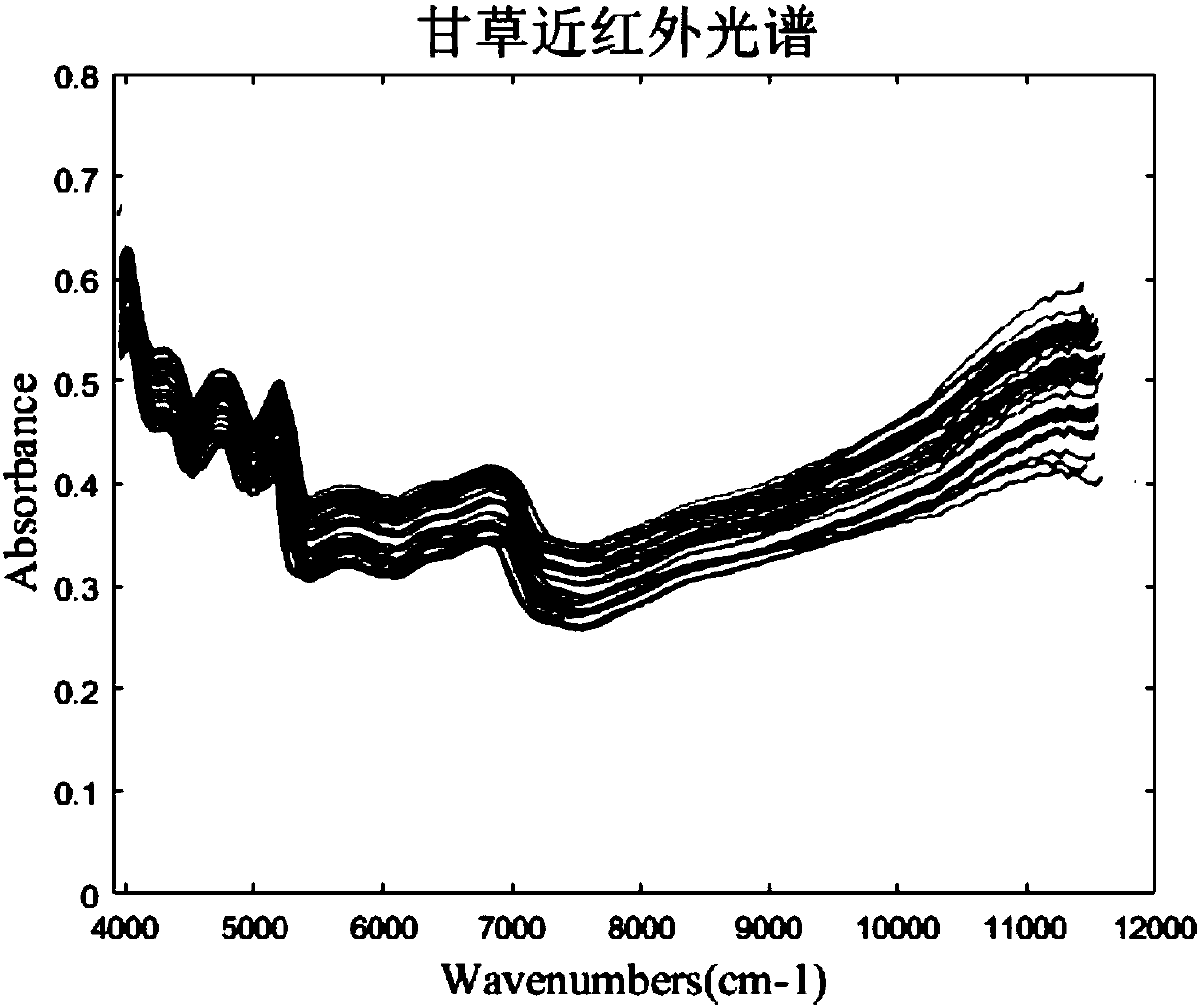
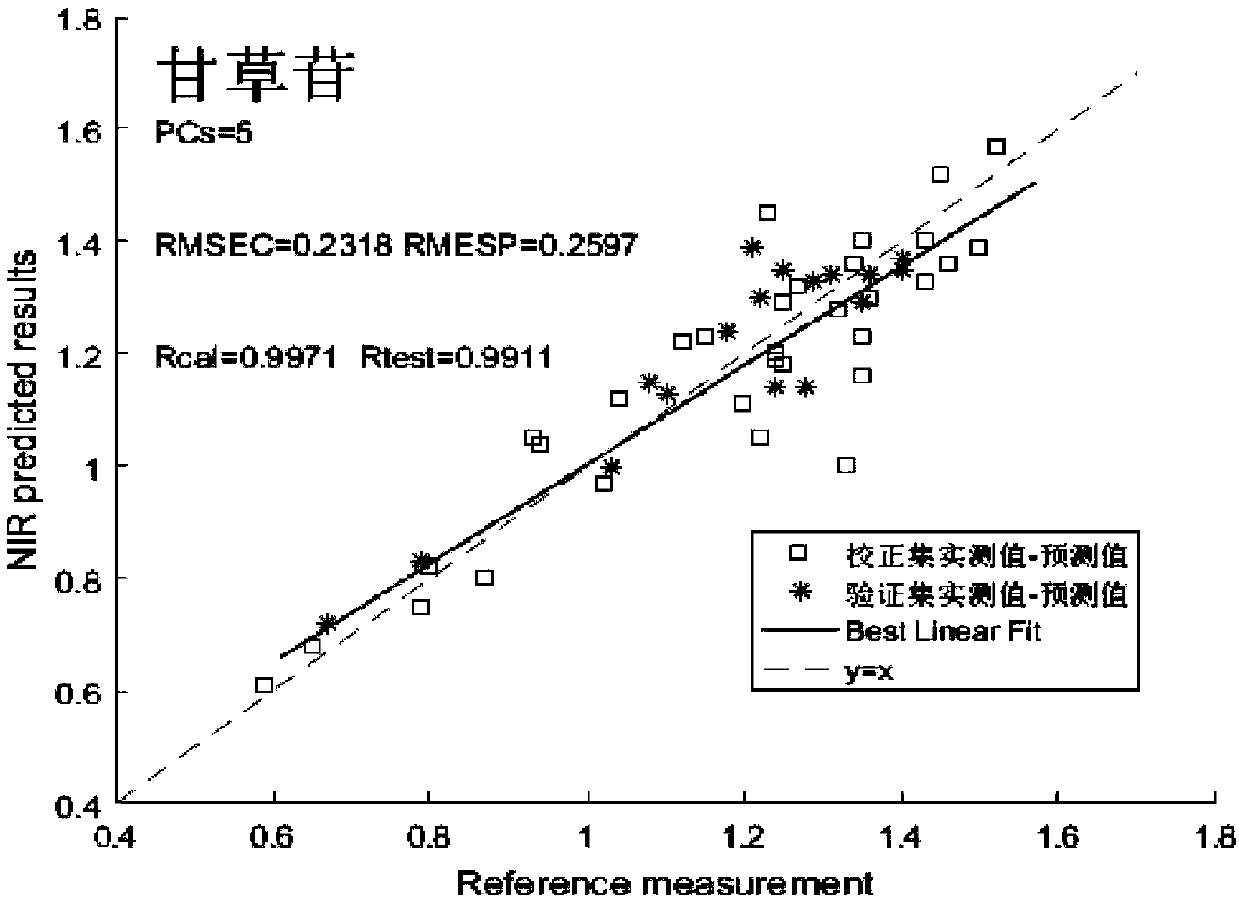
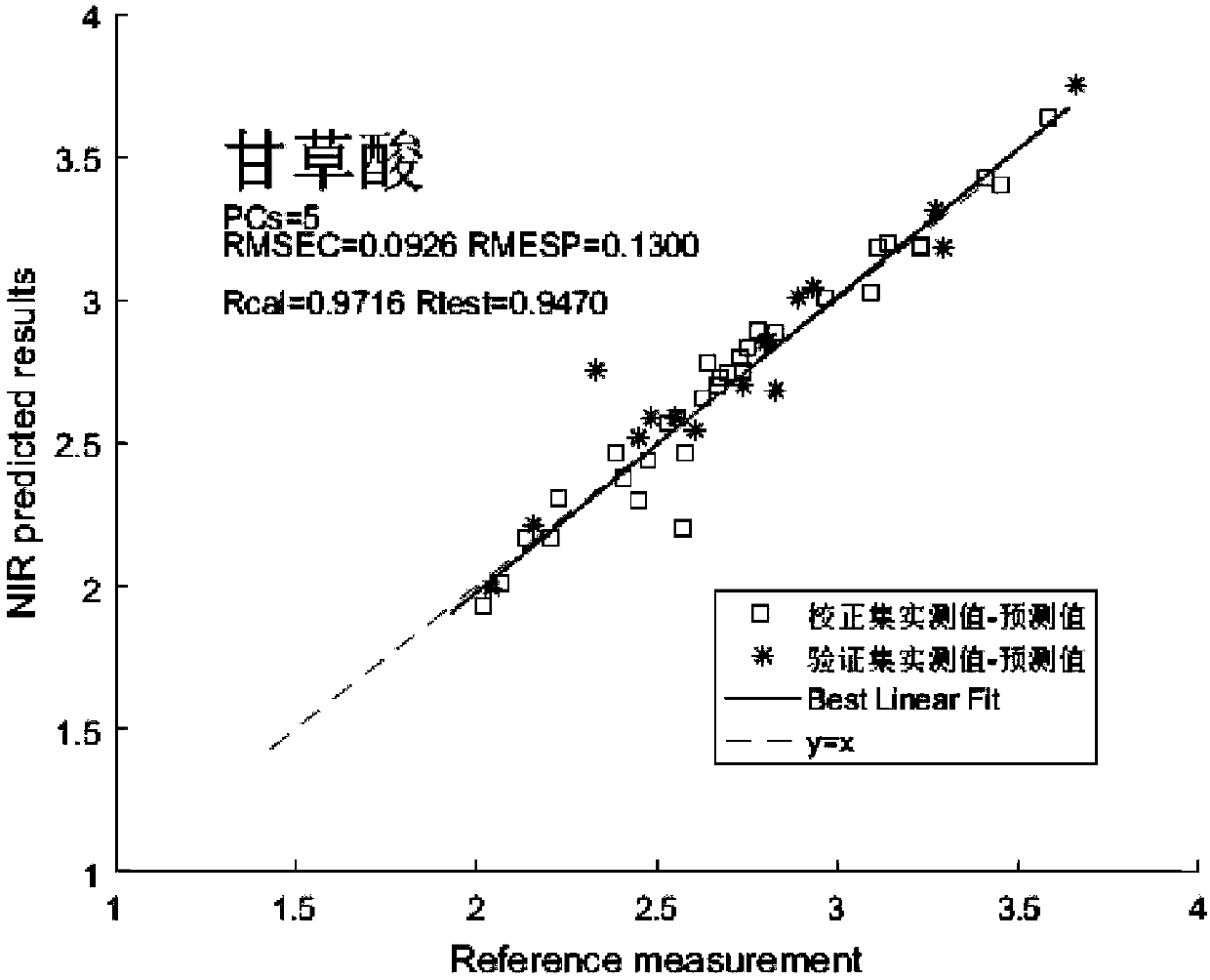
Near infrared spectrum detection method of licorice medicinal material
InactiveCN108562557AEnsure safetyGuaranteed stabilityMaterial analysis by optical meansInfraredMedicine
The invention discloses a near infrared spectrum detection method of a licorice medicinal material. The method includes near infrared detection of licorice moisture / or liquiritin and glycyrrhizic acid, comprising the following steps: (1) preparing a medicinal material sample, crushing and sieving for standby; (2) detecting target components, measuring moisture (oven-drying method) or measuring liquiritin and glycyrrhizic acid (liquid phase method); (3) acquiring near infrared spectral data; (4) establishing a quantitative model, making a correlation between the near infrared data and known component content and water content, and establishing a quantitative calibration model; and (5) carrying out near infrared detection on unknown samples for test. Through the above method, rapid detection of the licorice medicinal material is realized. The method has advantages of simple operation, high accuracy, no loss and the like; and safe, effective, controllable and stable quality of a licoricepreparation is guaranteed.
Owner:WUXI JIYU SHANHE PHARM CO LTD +1
Mask solution containing growth factors and dry mask
InactiveCN106667788AReduce dosageImprove long-term stabilityCosmetic preparationsToilet preparationsTryptophanBiology
The invention provides a mask solution containing growth factors. The mask solution is prepared from components in parts by weight as follows: 0.00001-0.0005 parts of growth factors, 0.1-2 parts of short peptide, 0.5-5 parts of chitosan, 0.5-5 parts of beta-glucan, 0.1-0.3 parts of allantoin, 0.5-5 parts of liquiritin, 1-5 parts of polyglycerine, 0.1-1 part of xanthan gum, 1-5 parts of glycerin, 2-6 parts of sorbitol, 2-6 parts of propylene glycol, 0.1-1 part of antibacterial peptides and 65-85 parts of water. The growth factors consist of epidermal growth factors and alkaline fibroblast growth factors in a weight ratio being (1-1.2): (1-1.2); the short peptide is histidine-seryl-tryptophan. The mask solution belongs to the technical field of cosmetics, has good skin moistening and conditioning effects and can have good moisturizing, moistening, anti-aging effects and the like.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Method for measuring seven functional components of liquorice in cosmetics
InactiveCN103822977ASimple processing methodQualitatively accurateComponent separationEvaporationLicochalcone A
The invention relates to a method for measuring seven functional components of liquorice in cosmetics. The method comprises the following steps: adding 60 to 80 percent of methyl alcohol into a sample to be tested, performing repeated ultrasonic extraction and centrifugation, combining centrifugal supernate, adding the supernate into a rotary evaporating flask, performing rotary evaporation till constant volume is not exceeded, transferring the methyl alcohol and the sample into a graduated tube, metering the volume through methyl alcohol, filtering through a filter membrane, performing liquid chromatography measurement on filtrates, adopting a diode array detector, using octadecylsilane chemically bonded silica as a filler column, detecting at 190 to 400 nm, using an acetonitrile-phosphoric acid solution as a flowing phase, performing gradient elution, and collecting seven functional components which are liquiritin, isoliquiritin, glycyrrhizin, dipotassium glycyrrhizinate (glycyrrhizic acid), licochalcone A, glabridin and glycyrrhetinic acid. The method is high in resolution ratio and sensitivity, good in selectivity, short in time, simple in sample treatment method, and suitable for the micro analysis of seven functional components of liquorice in the cosmetics, and fills a gap of the measurement of seven functional components of liquorice in the cosmetics.
Owner:LIAONING PROVINCE INST FOR FOOD & DRUG CONTROL
Extract for treating pharyngolaryngitis and preparation method thereof
ActiveCN102188483AEasy additionEasy processingAlcoholic beverage preparationRespiratory disorderMenthoneDrying time
The invention discloses an extract for treating pharyngolaryngitis. Medicinal materials such as 1 part of fine-leaf schizonepeta herb, 4 parts of platycodon root and 2 parts of raw liquorice are prepared by weight. The extract is prepared by the following steps of: 1) drying the medicinal materials, wherein the drying temperature is 40 to 60 DEG C, and the drying time is 3 to 4 hours; 2) crushing the medicinal materials into coarse powder; 3) filling the medicinal materials into a supercritical extraction tank, adding ethanol or methanol serving as an entrainer, and performing extraction for 3 to 4 hours under the conditions that the pressure is 5 to 10MPa, the temperature is 40 to 60 DEG C and the flow is 15 to 20L / h in the primary reduced pressure separation, and the pressure is 5 to 10MPa and the temperature is 35 to 50 DEG C in the secondary reduced pressure separation; and 4) dehydrating and drying the extract. By the supercritical preparation process, flavone, saponin and terpene components can be effectively enriched. The extract is characterized by comprising the following components in percentage by weight: more than or equal to 2.8 percent of glycyrrhizinic acid, more than or equal to 0.7 percent of liquiritin, more than or equal to 0.3 percent of platycodon D and 0.04 percent of menthone. The extract has anti-inflammatory and analgesic effects, and has more remarkable curative effect; and the preparation process is simple, the extraction rate is high, and the cost is low.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Method of establishing fingerprint spectrum of infant diarrhea stopping drug preparation
ActiveCN106802327AImprove effectivenessImprove securityComponent separationDrugs preparationsPharmaceutical drug
The invention belongs to the field of drug analysis and particularly relates to a method of establishing a fingerprint spectrum of an infant diarrhea stopping drug preparation. According to the method, by taking the infant diarrhea stopping drug preparation as a detection object, the method of establishing the fingerprint spectrum of the infant diarrhea stopping drug preparation is established, common characteristic peaks including peak 1, peak 2 liquiritin, peak 3 ammonium glycyrrhizinate, peak 4, peak 5, peak 6, peak 7 and peak 8 are affirmed, the peak 2 liquiritin is selected as an internal reference peak in the fingerprint spectrum, and a relative retention time of each common characteristic peak is determined; and in addition, with combination of information of a plurality of chromatographic peaks in the fingerprint spectrum, the quality of the infant diarrhea stopping drug preparation can be comprehensively and rapidly detected, so that comprehensive quality detection and whole quality control of the infant diarrhea stopping drug preparation are facilitated, and improvement on the use safety and stability of the drug is facilitated. Simultaneously, the method of establishing the fingerprint spectrum of the infant diarrhea stopping drug preparation has the advantages of high precision, good repeatability, high stability and the like.
Owner:合肥华润神鹿药业有限公司
Liquiritin preparation and application thereof
InactiveCN101518542ASignificant antitussive effectReduce efficacyOrganic active ingredientsRespiratory disorderSide effectActive component
The invention provides a liquiritin preparation with the molecular formula of C26H30O13 and the molecular weight of 550. The liquiritin is singly prepared or by matching with other active components or auxiliary materials, and the preparation composition is counted in such a way that 1000 particles contain 40g of liquiritin. The liquiritin preparation has stronger effect for relieving cough and eliminating phlegm, therefore, the preparation can be singly prepared by liquiritin or by the liquiritin matching with other active components or auxiliary materials, can be used for improving symptoms of cough and profuse sputum, and can be applied to the preparation of medicine for curing acute cough, chronic cough and profuse sputum. The liquiritin preparation originates from natural plants, can be made into a health-care product, and provides the natural cough-relieving medicine with less toxic side effect and obvious healing effect for clinical treatment.
Owner:ZHEJIANG UNIV
Quality control reference substance for antelope's horn tablets for common cold and application of quality control reference substance
InactiveCN104374841AAdaptableGreat application potentialComponent separationChlorogenic acidChinese patent
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Quality control method of Fuzi Lizhong Wan
InactiveCN108508118AFilling the Gap in Dissolution Quality Control ProgramsImprove uniformityComponent separationQuality controlSpecific time
The invention discloses a quality control method, which uses a high-performance liquid chromatography to determine the dissolving-out amount of liquiritin and glycyrrhizic acid in Fuzi Lizhong Wan ata specific time point and draws a dissolution curve based on the determination result so as to evaluate the product quality consistency, of the Fuzi Lizhong Wan. The method disclosed by the inventionfills a gap in the dissolution quality control item of the Fuzi Lizhong Wan, and enhances the product quality control of the Fuzi Lizhong Wan, thus providing a basis for clinical medication.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
A method for measuring contents of 14 chemical components in a traditional Chinese medicine composition
ActiveCN107703244AEffective quality controlGuaranteed clinical efficacyComponent separationGallic acid esterClinical efficacy
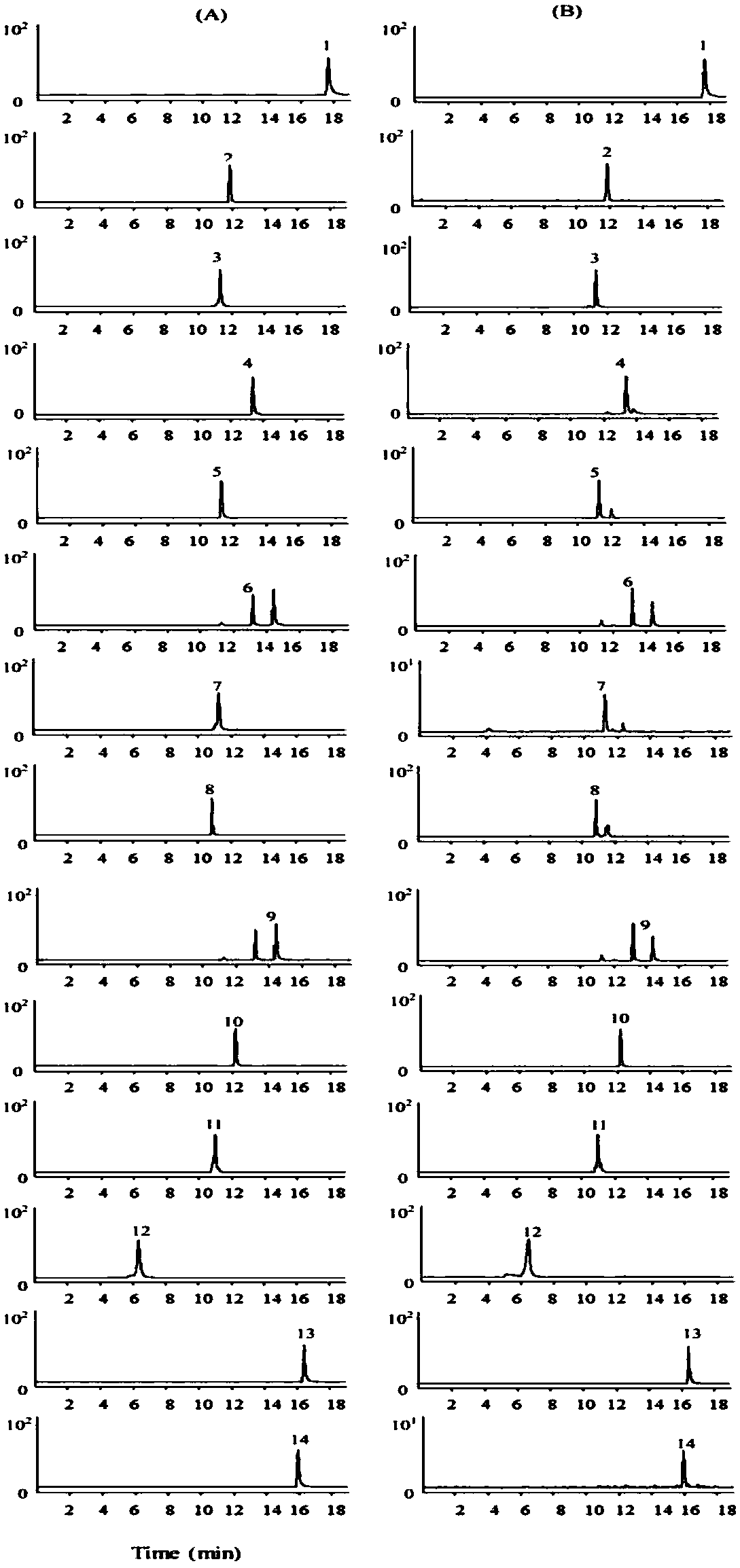
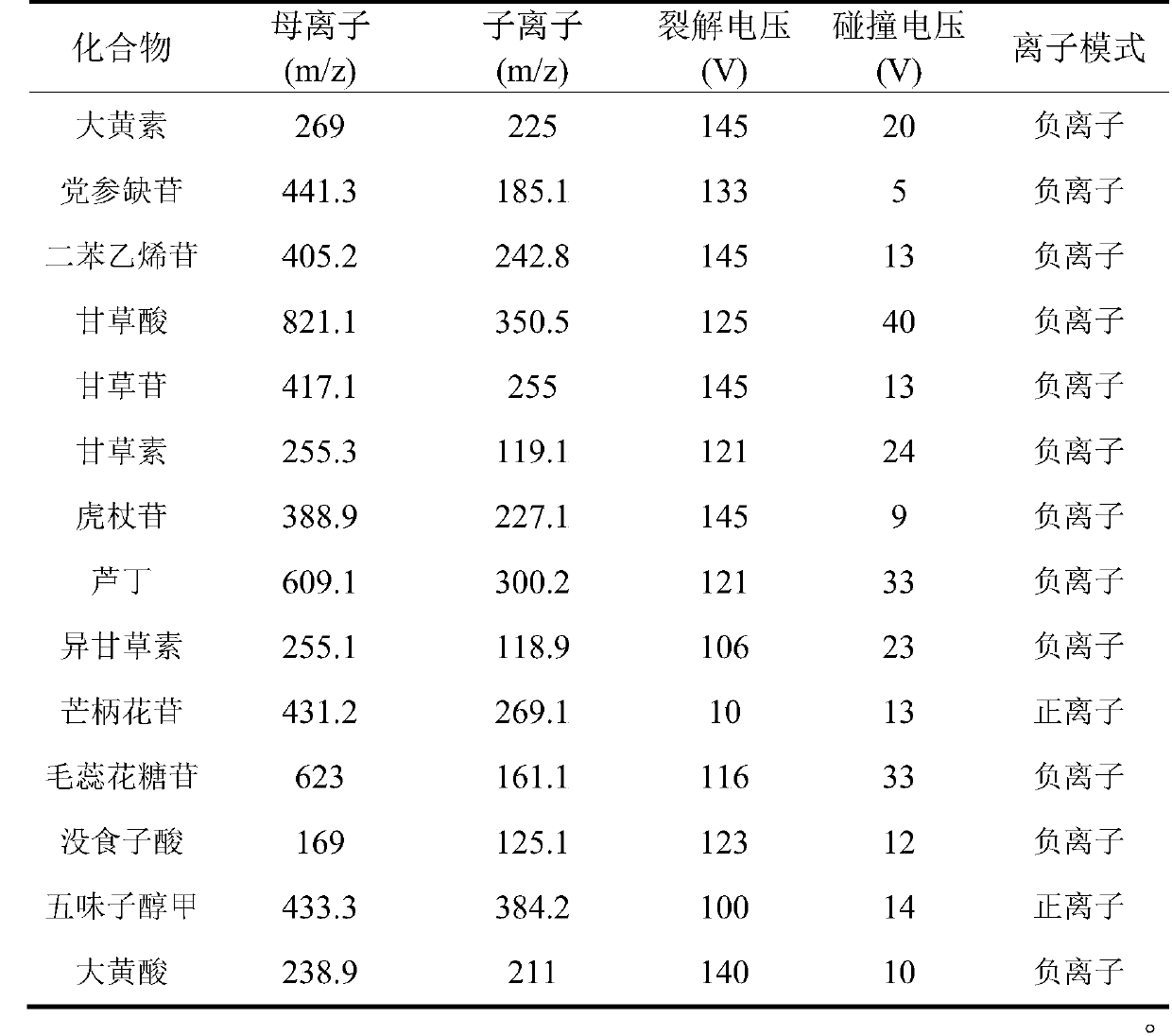
A method for measuring contents of 14 chemical components in a traditional Chinese medicine composition is disclosed. HPLC-MS / MS is adopted to simultaneously measure the contents of the 14 chemical components in the composition. The composition is a pulse-invigorating heart-tonifying pill. The 14 chemical components include emodin, lobetyolin, 2,3,5,4'-tetrahydroxy stilbene-2-O-beta-D-glucoside, glycyrrhizic acid, liquiritin, liquiritigenin, polydatin, rutoside, isoliquiritigenin, ononin, verbascoside, gallic acid, schizandrin, and rhein. The method includes building standard curves of the 14chemical components; acquiring multi-reaction ion chromatograms of the 14 chemical components in the pill; and determining the contents of the 14 chemical components in the pill. The method has characteristics of high sensitivity, high reliability, a high efficiency and capability of being rapid. Through measuring the contents of the 14 chemical components in the pill, quality control on the pillcan be more effectively performed, thus ensuring clinical treatment effects of the pill.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Method for simultaneously measuring seven index components in formula granules of Huagai powder for treatment of wind-cold-caused common cold and asthma by using high performance liquid chromatography
InactiveCN109856270ASimple processing methodFully extractedComponent separationPretreatment methodPseudoephedrine
The invention discloses a method for simultaneously measuring seven index components in formula granules of Huagai powder for treatment of wind-cold-caused common cold and asthma by using high performance liquid chromatography. The seven index components include ephedrine hydrochloride, pseudoephedrine, amygdalin, glycyrrhizic acid, liquiritin, sanggenon C, and sanggenon D. The method comprises the following steps: step one, preparing a standard solution; step two, preparing a test sample solution; step three, carrying out liquid chromatogram separation; step four, performing a content calculation method. According to the invention, the pretreatment method of sample extraction is simple and the index components can be extracted fully. The method is performed accurately and rapidly with high sensitivity and low cost; and various methodological indexes can satisfy the actual detection demands. Seven measured compounds have high linearity in a standard curve linear range, wherein R2 is larger than 0.9990; the within-day precision relative standard offsets of all components are less than 1.0%; and the day-to-day precision relative standard offsets are less than 3.0%. The recovery ratesof the seven index components are in a range of 90.0% to 110.9%.
Owner:ZHEJIANG PHARMA COLLEGE +1
Preparation method of traditional Chinese medicine chemical component
InactiveCN103833806AHigh purityGuaranteed reproducibilitySugar derivativesSugar derivatives preparationChemical compositionDissolution
The invention relates to a separation of a natural chemical component, and in particular to a method for extracting high-purity liquiritin from licorice medicinal material. The method mainly comprises the following steps: adding a proper amount of dilute ethanol into a licorice powder for extraction; concentrating the extract or percolating liquid; adding a proper amount of water for dissolution; filtering; separating by macroporous adsorption resin to obtain a liquiritin crude product; adding a proper amount of a solvent; heating for dissolving; filtering; standing for crystallization; filtering; and drying to obtain high-purity liquiritin. The product is determined by high performance liquid chromatography to have purity higher than 95%. The liquiritin produced by the technical scheme of the invention has high purity and large preparation volume; and the method has low cost, and stable and reliable process, and is especially suitable for separating and preparing of a large amount of high-purity liquiritin compound from the traditional Chinese medicine licorice.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Compound preparation for treating cecal granuloma and preparation method thereof
InactiveCN105920081AEffective expansionLittle side effectsPeptide/protein ingredientsDigestive systemIntestinal wallsChlorogenic acid
The invention discloses a compound preparation for treating cecal granuloma and a preparation method thereof. The compound preparation comprises chloroquine phosphate, diiodohydroxyquinoline, chlorogenic acid, liquiritin, cistanoside, ursolic acid, polysavone, levamisole, albendazole, okra powder, lactein, vitamin B1, lactalbumin and arginine. The compound preparation can effectively expand intestinal tubes, prevent intestinal obstruction, prevent mesentery and intestinal wall inflammatory infiltration and edema, prevent intestinal wall fibrosis, adjust body metabolism, improve immunity, reduce drug side effects and treat cecal granuloma and has a wide application prospect.
Owner:于爱华
Establishment method for HPLC (high performance liquid chromatography) fingerprint of cold treatment medicament
The invention discloses an establishment method for an HPLC (high performance liquid chromatography) fingerprint of a cold treatment medicament. The method comprises the following steps: (1) preparing reference solvents: preparing glycyrrhizic acid, liquiritin, chlorogenic acid and cynaroside reference solvents respectively; (2) preparing a test solvent: weighing bitter sweet granules, performing extraction, and filtering an extracting solvent with a microfiltration membrane to obtain the test solvent; (3) performing measurement to obtain the fingerprint by adopting an HPLC method, wherein a chromatographic condition is gradient elution, a flowing phase A of gradient elution is acetonitrile, B is a 0.05 to 5 percent formic acid aqueous solvent, and the detection wavelength is 254nm; (4) evaluating the similarity: evaluating the fingerprint of a test sample by adopting the Similarity Evaluation System for Chromatographic Fingerprint of TCM (Version 2004 A). the method is high in precision, repeatability and stability, and the quality of the bitter sweet granules can be effectively controlled by adopting an HPLC fingerprint technology.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Quality detection method for traditional Chinese medicine pediatric cold-relieving granules
ActiveCN110736799ASimple and fast operationAnalytical data is accurateComponent separationBaicaleinFluid phase
The invention relates to a quality detection method for traditional Chinese medicine pediatric cold-relieving granules. According to the invention, the contents of baicalin, wogonoside, baicalein, wogonin, liquiritin and ammonium glycyrrhizinate in the traditional Chinese medicine pediatric cold-relieving granules are determined by high performance liquid chromatography. According to the invention, the separation degree between each to-be-detected component in the chromatogram of a test sample and the adjacent peak is greater than 1.5, and negative control has no interference, so that the quality safety of the granules is further ensured, evaluation is comprehensive, and the detection method has the advantages of high practicability, high operability, cost saving and the like in operation.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Method for controlling quality of liquorice and liquorice preparation
The invention relates to a method for controlling quality of a liquorice medicinal material and a liquorice preparation. The method concretely relates to the step of simultaneously determining the content of the liquorice medicinal material and the content of six main active ingredients, namely liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, glycyrrhizic acid and glycyrrhetinic acid in the liquorice preparation by utilizing a high performance liquid chromatography. A new method for controlling the quality of the liquorice and the liquorice preparation is established and the six main active ingredients, namely liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, glycyrrhizic acid and glycyrrhetinic acid are quantitatively determined simultaneously by utilizing the high performance liquid chromatography. Confirmed by methodological investigation and test, the method is simple and convenient, correct in result and good in reproducibility, the defect of the existing quality control is overcome, and the quality control on the liquorice and the liquorice preparation is perfect and scientific.
Owner:惠州市九惠制药股份有限公司
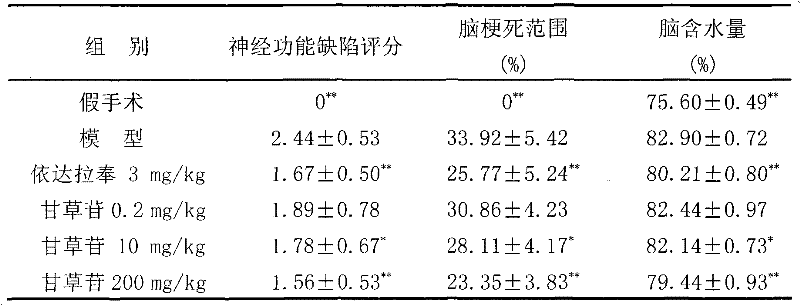
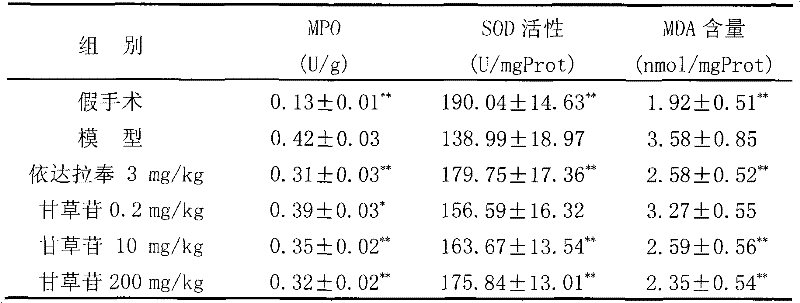
Application of liquiritin in preparing medicine for treating cardiovascular and cerebrovascular diseases
InactiveCN102125576APrevent or improve damageNo adverse reactionOrganic active ingredientsCardiovascular disorderDiseaseMedication dose
The invention belongs to the technical field of medicament, particularly relates to an application of liquiritin in preparing medicament for treating cardiovascular and cerebrovascular diseases, more specifically relates to an application of liquiritin in preparing medicine for treating cerebrovascular diseases. Pharmacological experiments reveal that liquiritin has distinct effects in resisting thrombosis, resisting cerebral ischemia, resisting cerebral anoxia and improving memory. The medicine with liquiritin as active ingredient has low medication dose and high safety, and has wide application prospect.
Owner:北京联合大学应用文理学院
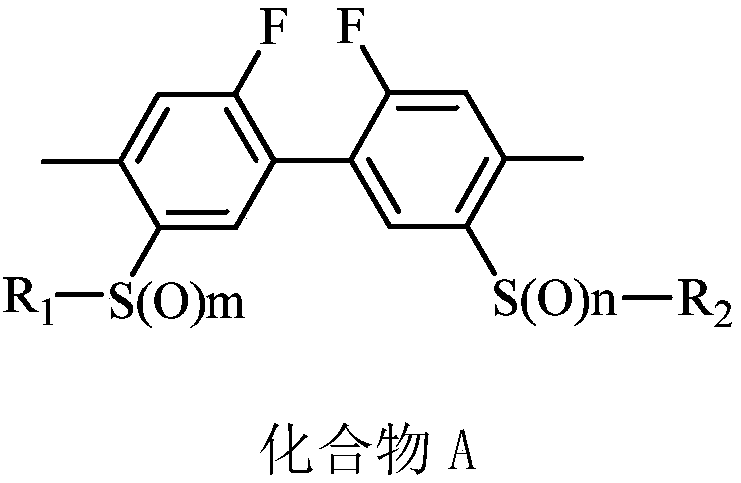
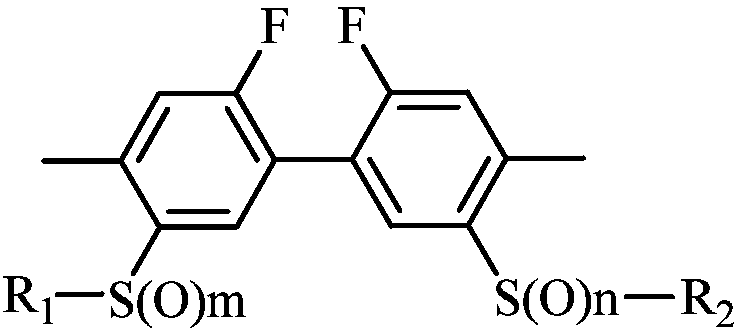
Biphenyl compound-containing solid preparation and applications thereof
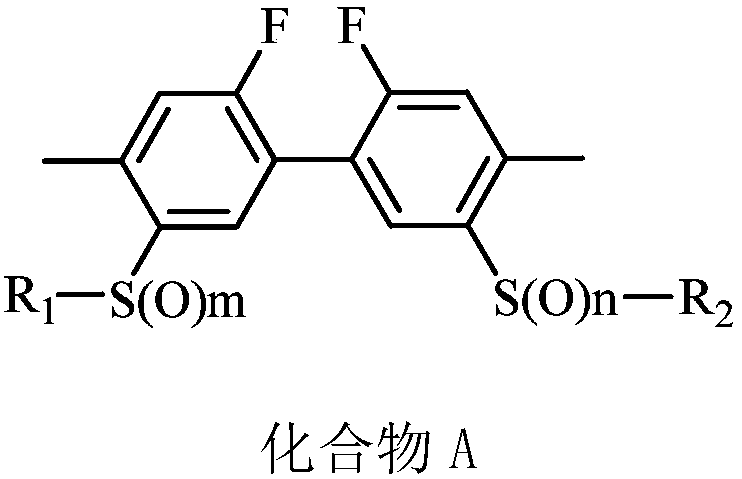
ActiveCN110870478AGood suspensionSuspension qualifiedBiocideAnimal repellantsCompound aChemical compound
The invention belongs to the field of acaricides, and relates to a biphenyl compound-containing solid preparation and applications thereof, wherein a compound A is used as an active component and is matched with at least a carrier and at least an auxiliary agent, the weight percentage content of the active component compound A of the solid preparation is 0.5-90%, the auxiliary agent contains a suspending aid, and the weight percentage content of the suspending aid is 0.01-5%. According to the invention, a proper auxiliary agent system condition is selected and matched, a suspending aid substance liquiritin is added into the solid preparation according to a specific ratio to effectively improve the suspension rate of the solid preparation, and the liquiritin and the structure of the compound A interact, so that the defects of softening and dissolving of solid particles are inhibited, the problems that low-melting-point raw medicine solid preparation particles are prone to melting and softening and low in suspension rate in the prior art are solved, the suspension rate of the solid preparation is qualified, the product performance is guaranteed, and the unexpected effect is achieved.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com