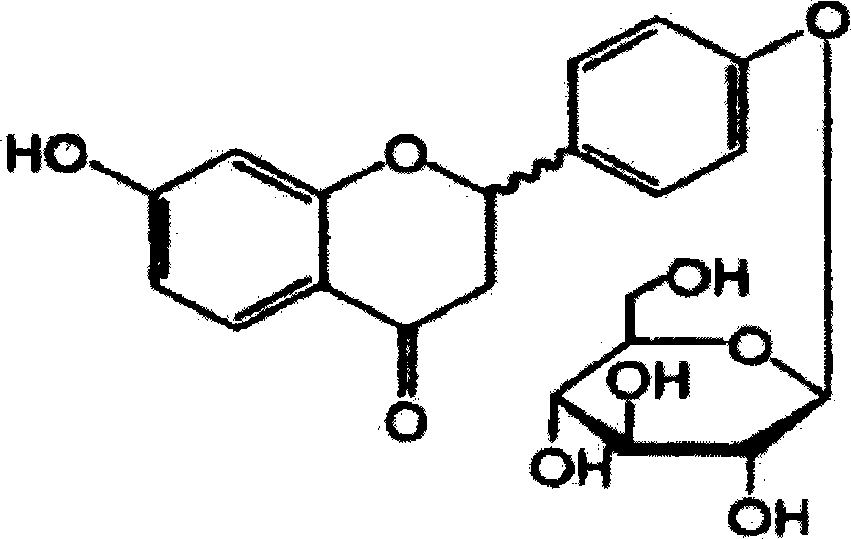
Liquiritin preparation and application thereof
A technology of liquiritin and preparations, applied in the field of liquiritin preparations and applications, can solve problems such as diseases or symptoms that have not yet been seen, and achieve the effect of promoting the excretion of phenol red in the airways of mice, with significant curative effect and strong expectorant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
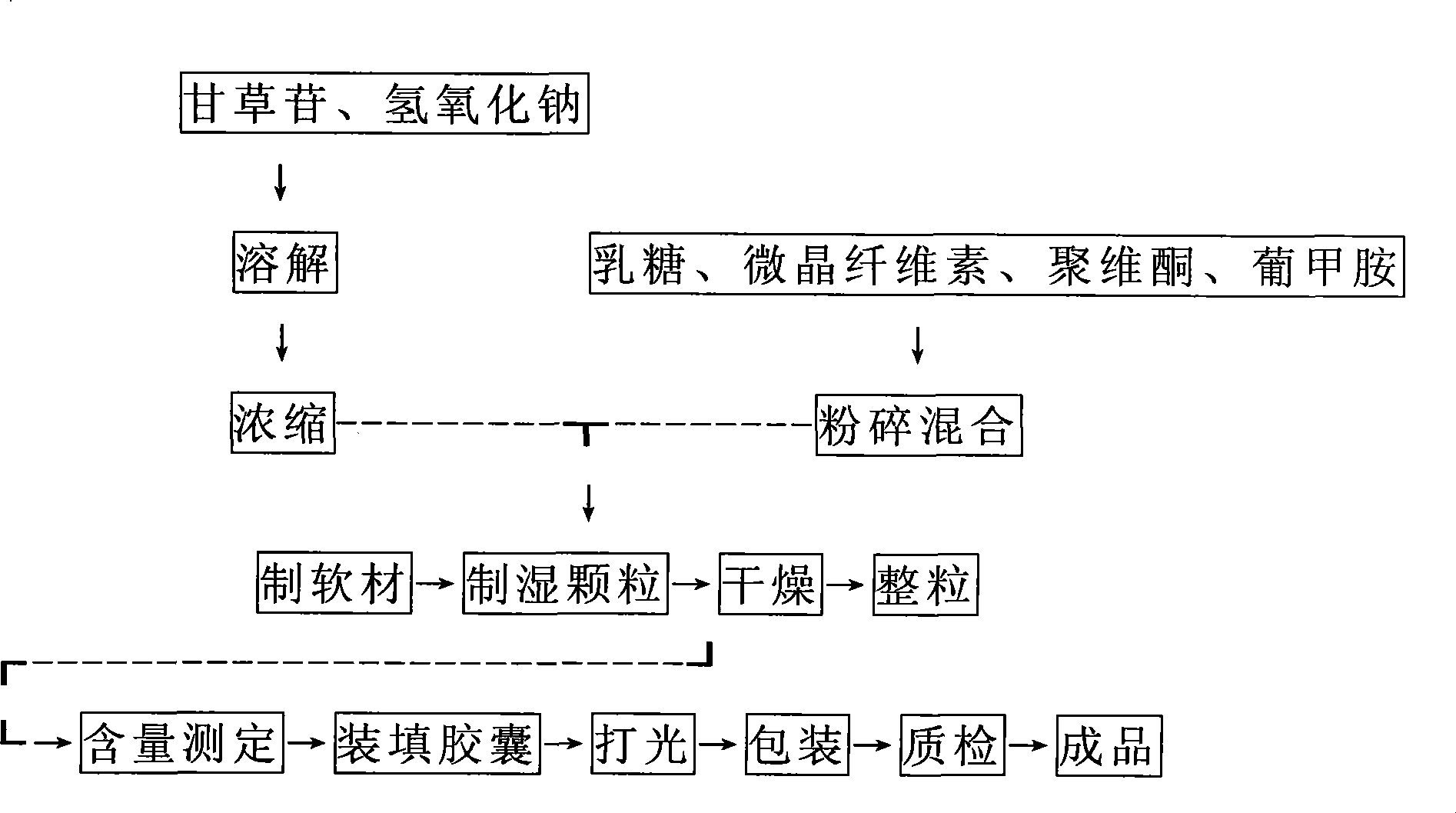
[0015] 1. Composition and preparation process of Liquiritin Capsules
[0016]
[0017]
[0018] 2. Preparation basis and screening process
[0019] 2.1 Preparation Basis
[0020] Formulate Liquiritin Capsules according to pharmacodynamic tests, specifications: Liquiritin content 40mg / capsule.
[0021] The role of excipients in the prescription: sodium hydroxide: solubilizer; povidone: binder; meglumine: solubilizer; microcrystalline cellulose: diluent, disintegrant; lactose: diluent.
[0022] Dissolve liquiritin with ethanol solution of sodium hydroxide. The preliminary test shows that it is not completely dissolved at the theoretical value of complete salification of liquiritin (liquiritin: sodium hydroxide = 40:3.1), and a slight excess of sodium hydroxide is liquiritin. :Sodium hydroxide=40:3.2 can just dissolve completely.
[0023] Then concentrate and dry the solution to a concentrated solution, add different diluents and stir to disperse evenly, make wet granule...
Embodiment 2
[0056] Embodiment 2 pharmacodynamic research
[0057] The experimental protocol was designed with reference to the State Food and Drug Administration's "Compilation of Guiding Principles for Preclinical Research of New Drugs (Western Medicine) (Pharmacology, Pharmacology and Toxicology)". Using 2 kinds of antitussive drug experimental animal models, 2 kinds of expectorant drug experimental animal models and 3 kinds of anti-asthma test methods, the studies on the anti-asthma, antitussive and expectorant aspects of Liquiritin Capsules or Tablets were carried out. The test materials, methods and results are as follows:
[0058] 1. Materials:
[0059] Drugs and Reagents
[0060] 1) Citric acid: Shanghai Chemical Reagent No. 1 Factory, batch number 20070201, prepared with distilled water to a concentration of 15% for later use;
[0061] 2) Codeine Phosphate Tablets: Qinghai Pharmaceutical Factory, batch number 20060615; prepared into 0.5mg / ml suspension with distilled water;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com