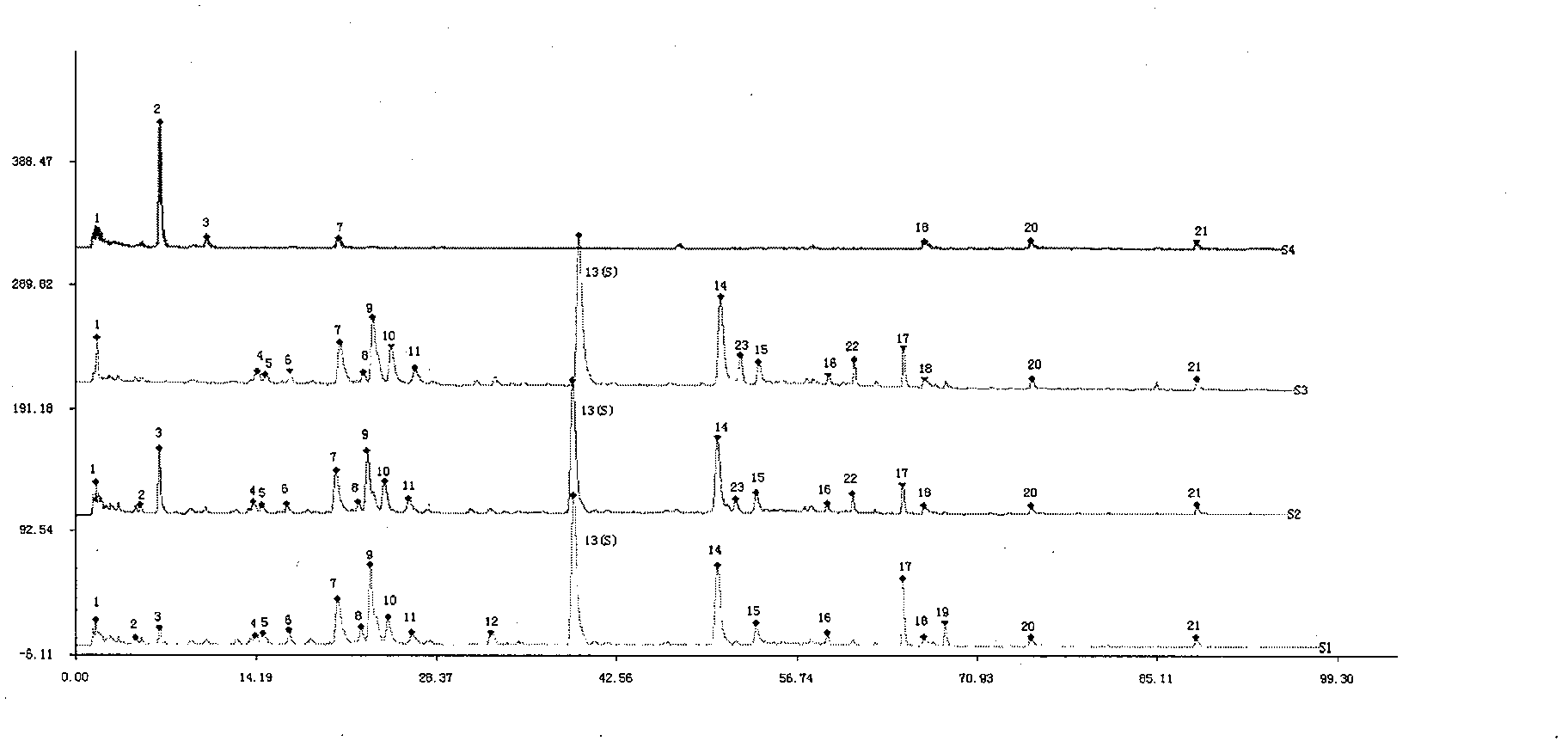
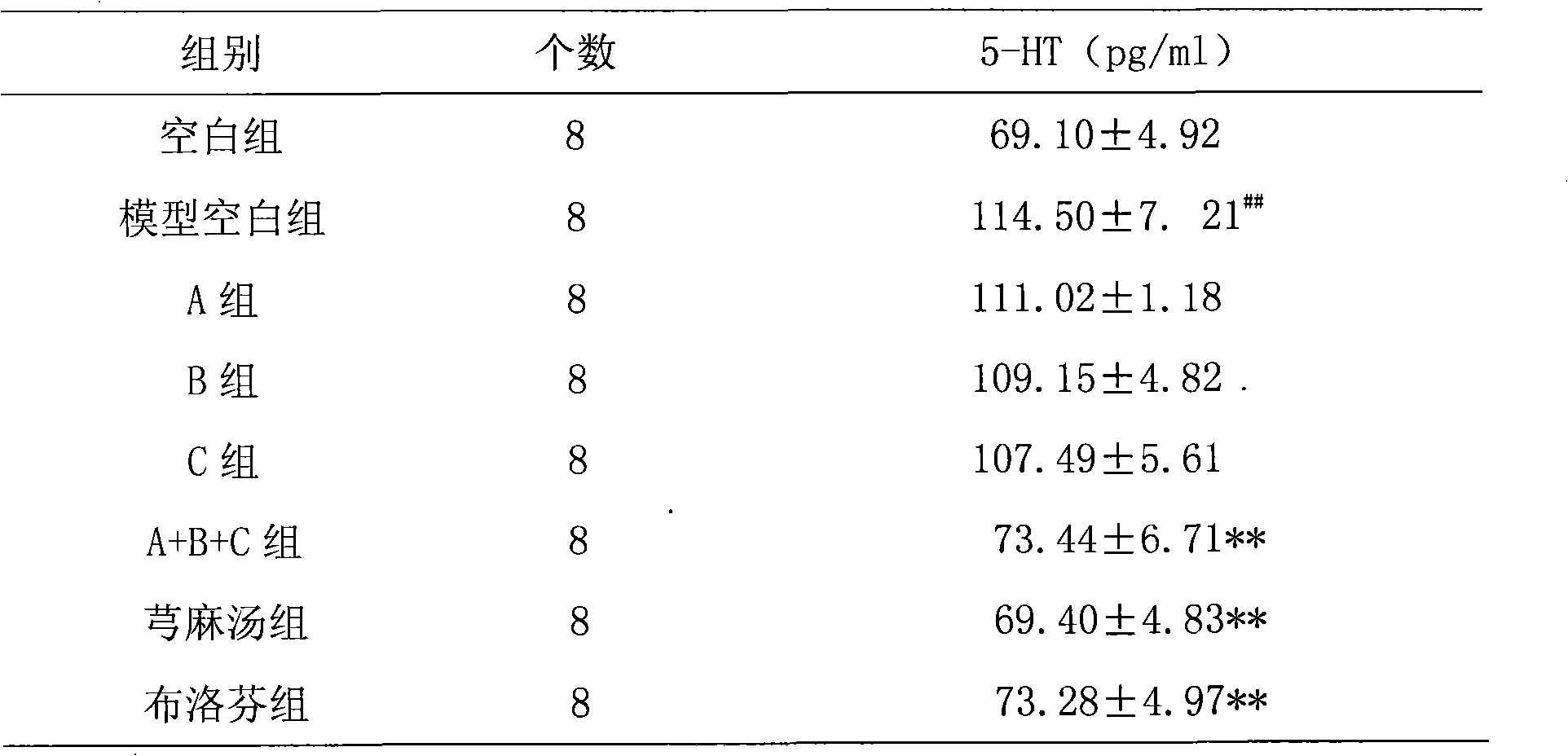
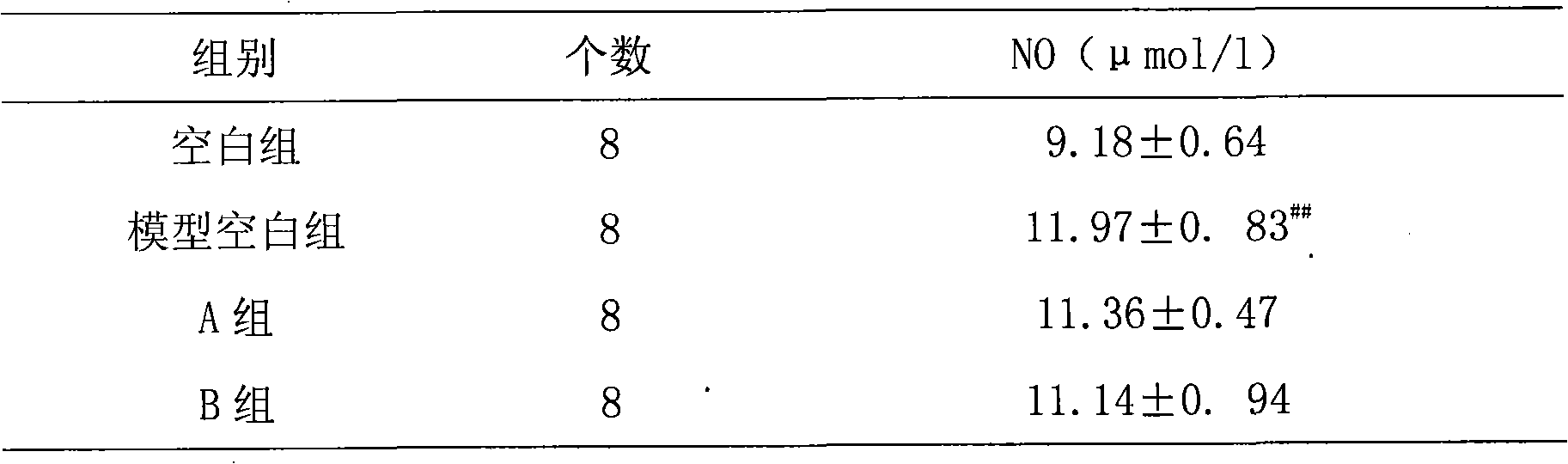
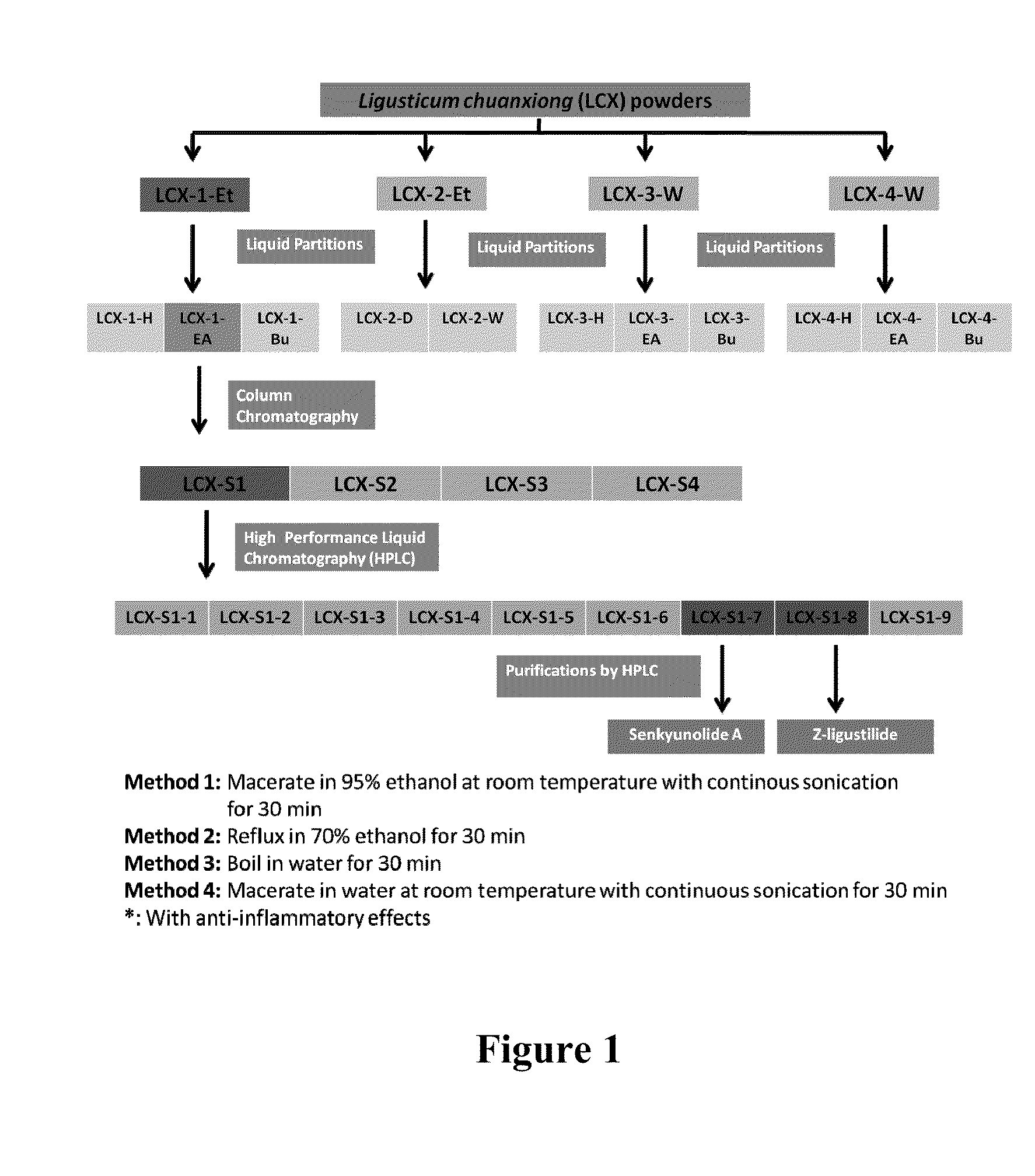
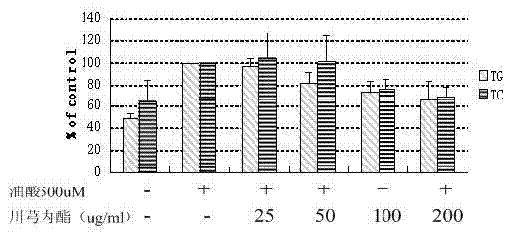
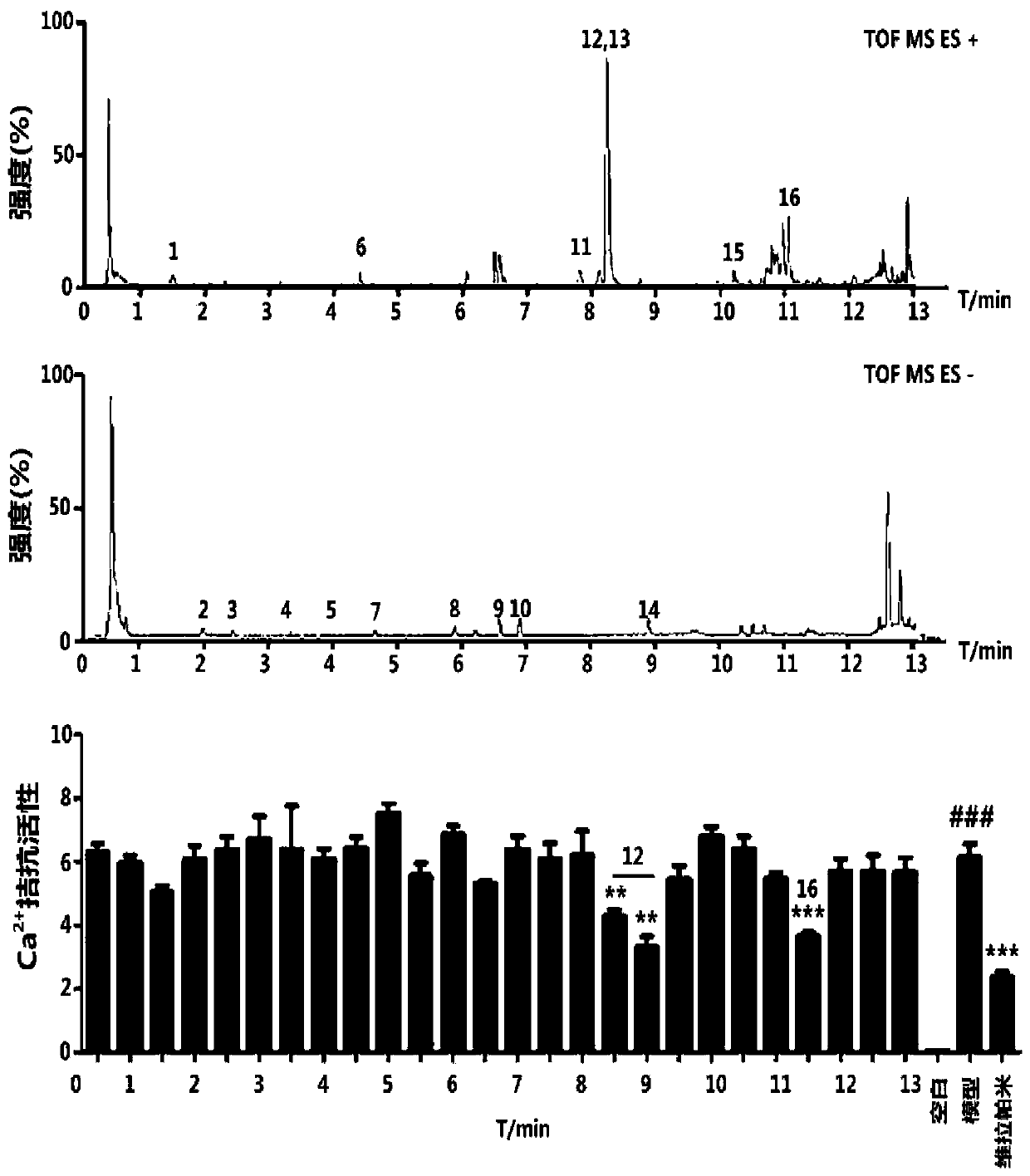
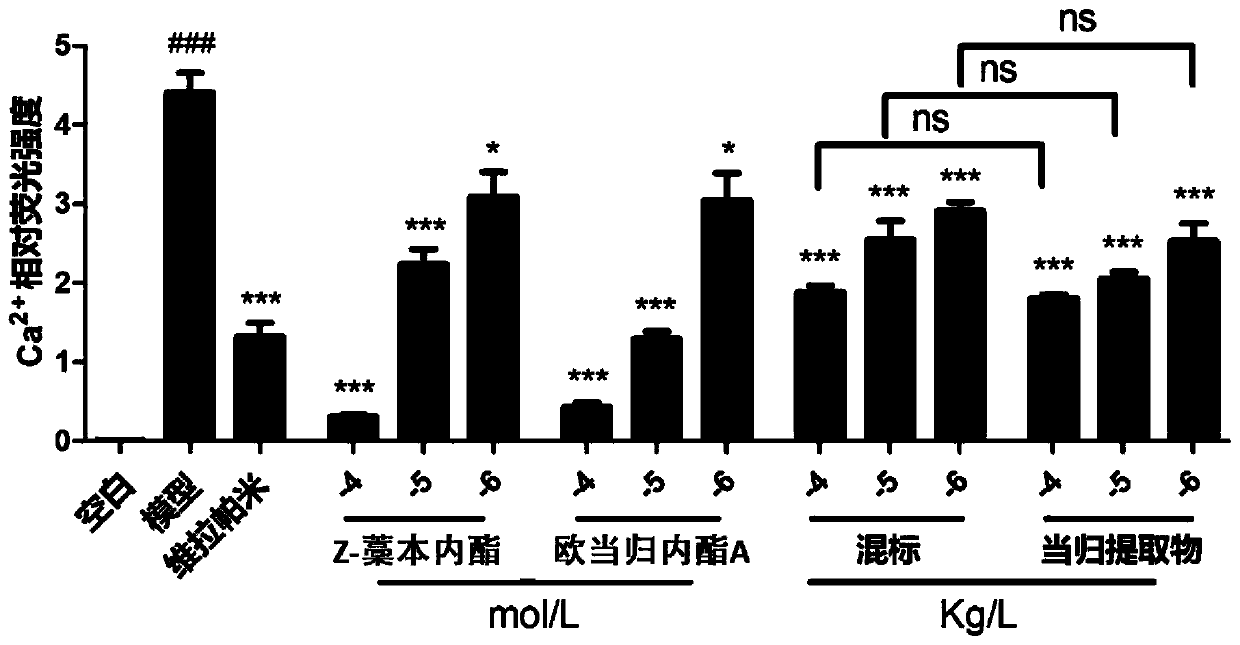
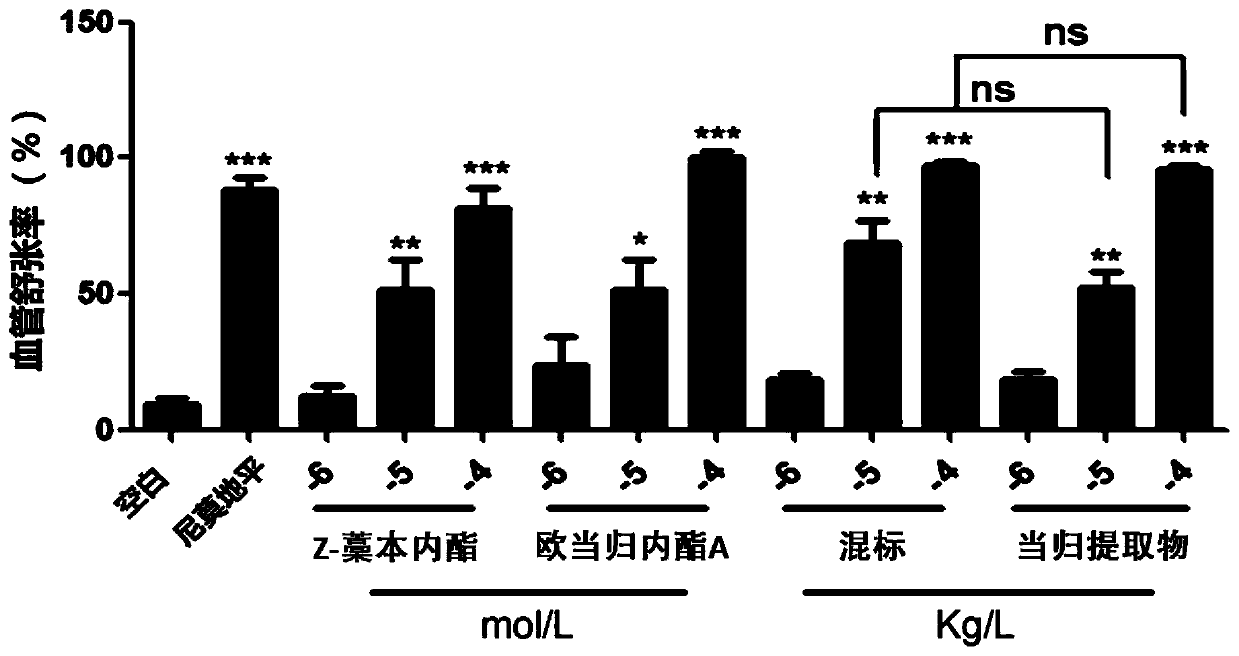
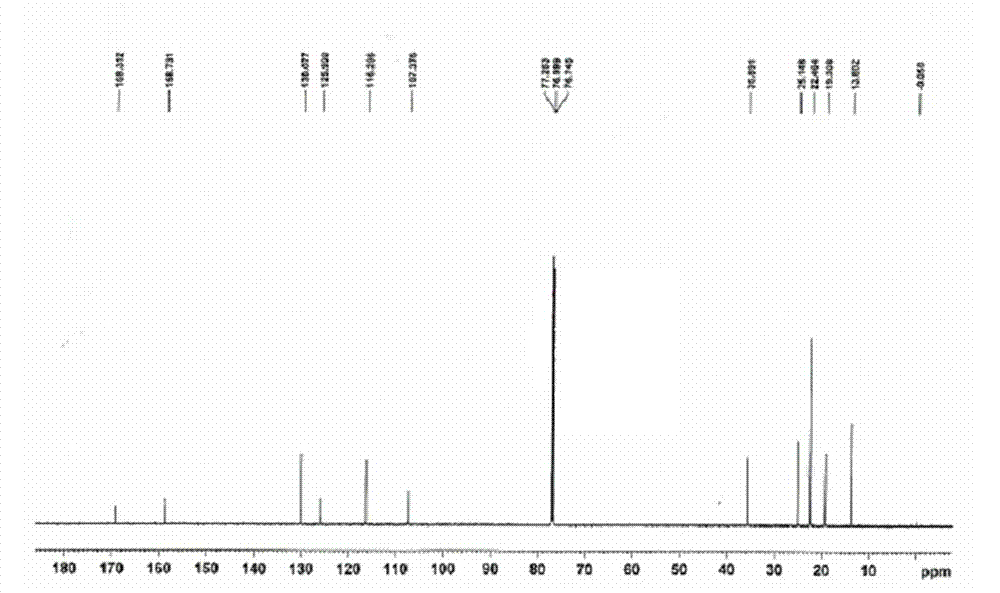
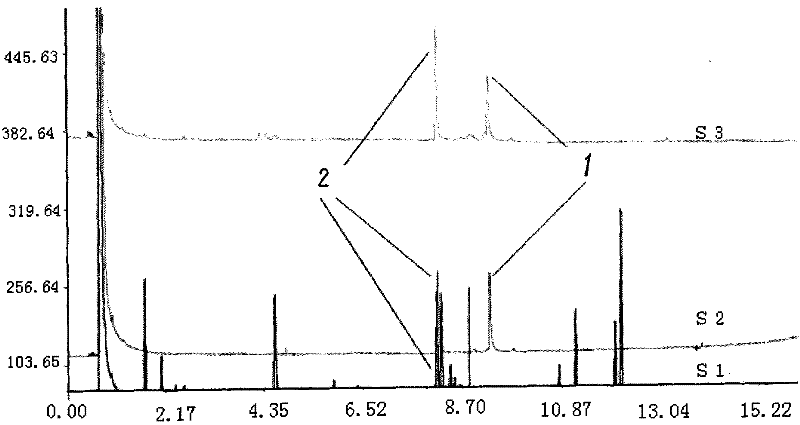
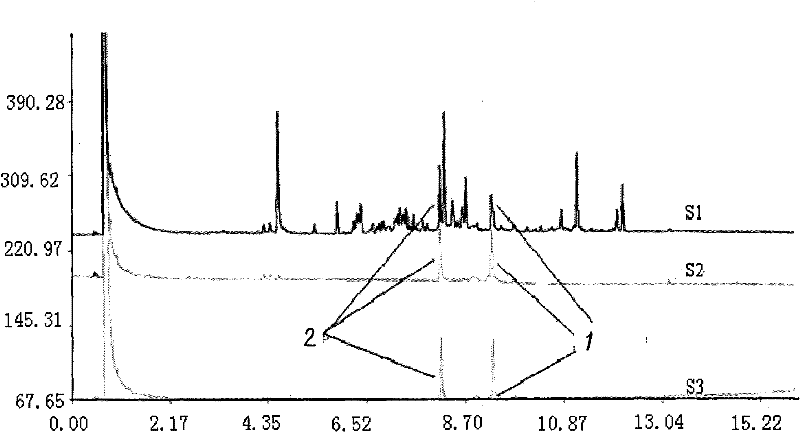
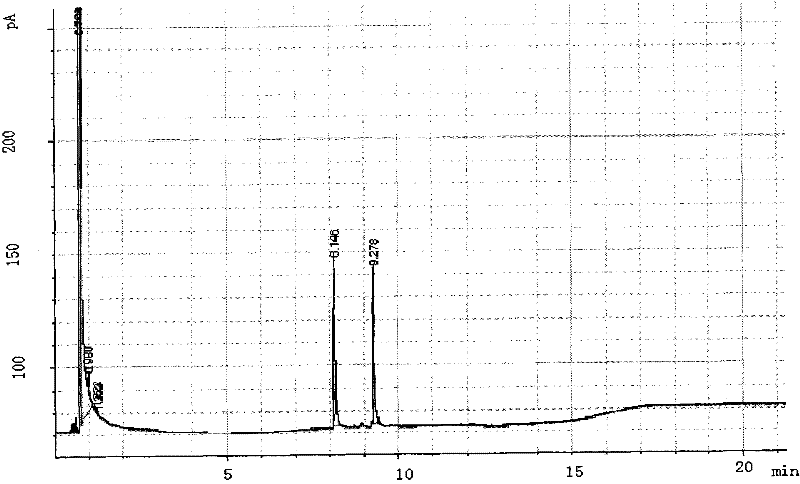
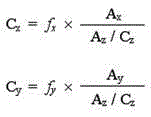Patents
Literature
53 results about "Z-ligustilide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
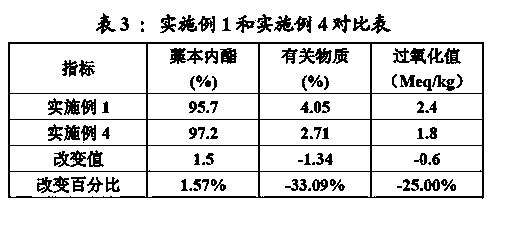
Quality detection method of traditional Chinese medicine preparation
ActiveCN111044624AReasonable quality inspectionEasy to separateComponent separationBiotechnologyGallic acid ester
The invention relates to a quality detection method of a traditional Chinese medicine preparation. The method comprises the following steps: respectively taking gallic acid, albiflorin, paeoniflorin,ferulic acid, liquiritin, beta-ecdysterone, senkyunolide I, glycyrrhizin, cinnamic acid, cinnamyl aldehyde, paeonol, ammonium glycyrrhizinate and ligustilide as reference substances, and establishinga fingerprint spectrum of the traditional Chinese medicine preparation by adopting high performance liquid chromatography; identifying Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba,moutan bark, ginseng, cassia bark, licorice root and the root of bidentate achyranthes of the traditional Chinese medicine preparation by adopting thin-layer chromatography. The traditional Chinese medicine preparation is prepared by using the following raw materials: Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba, cassia bark, moutan bark, zedoray rhizome, ginseng, licorice root and the root of bidentate achyranthes. With the two detection ways, comprehensive quality evaluation can be conducted on the meridian-warming decoction traditional Chinese medicine preparation. Themethod is simple, convenient, high in accuracy and high in reproducibility; a scientific basis can be provided for quality detection and evaluation of the meridian-warming decoction traditional Chinese medicine preparation, and the product quality is effectively controlled.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Six-flavor hematinic capsule, its quality control method and application thereof
ActiveCN103381217ARealize authenticationAccurate measurementSenses disorderComponent separationBlurred visionLithospermum
The invention discloses a six-flavor hematinic capsule, its quality control method and an application thereof. The six-flavor hematinic capsule is prepared by adding auxiliary materials into raw materials consisting of Chinese angelica, Ligusticum wallichii, Radix Astragali, prepared rehmannia root, lithospermum and white peony root. According to the quality control method of the six-flavor hematinic preparation, whether the six-flavor hematinic capsule contains white peony root, Chinese angelica, Radix Astragali, prepared rehmannia root, lithospermum, Ligusticum wallichii and components is identified by thin layer chromatography; by high-performance liquid chromatography, in vitro dissolution behavior of the six-flavor hematinic capsule is determined, and effective component groups are identified by fingerprint; verbascoside and calycosin glucoside are used as reference substances to simultaneously determine contents in the six-flavor hematinic capsule; and the content of a volatile component ligustilide in the six-flavor hematinic capsule is determined by a gas chromatographic method. The method provided by the invention is simple to operate, is accurate and advanced, has good linear relation, reappearance, precision, stability and recovery rate, can be adopted to effectively control product quality and guarantee curative effect of the product. The invention also provides an application in the preparation of a medicine for treating eye damage and blurred vision.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
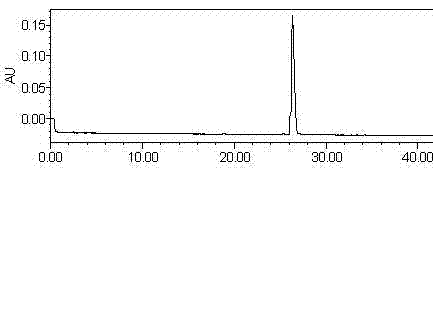
Method for simultaneously and quantitatively detecting ligustilide and senkyunolide A
The invention discloses a method for simultaneously and quantitatively detecting ligustilide and senkyunolide A. The method comprises the following steps: according to an HPLC (high performance liquid chromatography) method in which butylphthalide serves as a substitution reference substance and octadecyl silane bonded silica gel serves as a filling agent, respectively determining the peak area Ax and the peak area Ay of the ligustilide (X) and the senkyunolide A (Y) in a to-be-detected sample by using a single wavelength in a range of 275-284nm; and according to the concentration Cz and the peak area Az of the substitution reference substance, namely the butylphthalide (Z), calculating the concentration C of the detected components, namely the ligustilide and the senkyunolide A, according to a formula by using correction factors f, wherein a correction factor fx is within 0.20-0.25, and a correction factor fy is within 0.46-0.54. A detection result obtained by using the method provided by the invention is stable, and is in accordance with a detection result obtained by using a method in which the ligustilide and the senkyunolide A, freshly prepared, serve as reference substances, and the problems that the dual-wavelength detection needs to be simultaneously adopted, the requirements on HPLC equipment are high and the universality is poor in the prior art are solved.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Chinese angelica medicinal material detection method
The invention belongs to the field of traditional Chinese medicine quality detection and relates to a Chinese angelica medicinal material detection method. The Chinese angelica medicinal material detection method comprises determination of ligustilide and ferulic acid content of a Chinese angelica medicinal material and determination of pesticide residue content of the Chinese angelica medicinal material. The Chinese angelica medicinal material detection method has high detection accuracy and simple and fast processes, can finish multiple detection indexes, has good accuracy and utility, is conducive to Chinese angelica medicinal material quality control and is conducive to improvement of drug use safety and stability.
Owner:LONGXI ZHONGTIAN PHARM CO LTD
A pure-component Chinese medicinal preparation for treating headache and its preparation process
A pure-component Chinese medicinal preparation for treating headache and its preparation process, using Gastrodia elata and Ligusticum chuanxiong as basic formulation, adopting modern flash extraction technique, combining macroporous resin, normal / reversed-phase silica gel chromatography technique and high-speed counter-current chromatography preparation technique, extracting a volatile oil fraction containing butylphthalide as main component, an alkaloid fraction containing ligustrazine as main component, an organic acid fraction containing ferulic acid as main component, a lactone fraction containing ligustilide as main component, a phenols fraction containing gastrodine as main component and a polysaccharide fraction containing polysaccharides from Ligusticum chuanxiong as main component. The Chinese medicinal preparation provided by the invention has definite curative effect, definite composition, controllable quality, and simple operation method.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Cyclodextrin clathrate and preparation method thereof
InactiveCN103751793AOrganic active ingredientsPharmaceutical non-active ingredientsChemistryHydroxypropyl-beta-cyclodextrin
The invention provides a cyclodextrin clathrate containing ligustilide. The cyclodextrin clathrate contains ligustilide and hydroxypropyl-beta-cyclodextrin. Ligustilide is coated with hydroxypropyl-beta-cyclodextrin so that the cyclodextrin clathrate is formed. A weight ratio of ligustilide to hydroxypropyl-beta-cyclodextrin is in a range of 10-20wt%. The cyclodextrin clathrate further comprises povidone K29 / 32. A use amount of povidone K29 / 32 is 10-50wt% of that of hydroxypropyl-beta-cyclodextrin. The cyclodextrin clathrate containing ligustilide can be processed to form tablets, capsules, powder or a powder injection and can be used for preparation of drugs for treating dysmenorrhoea.
Owner:SHANGHAI TIANLONG PHARMA
Novel Therapeutic Methods for Treating Inflammation and Immune System Disorders
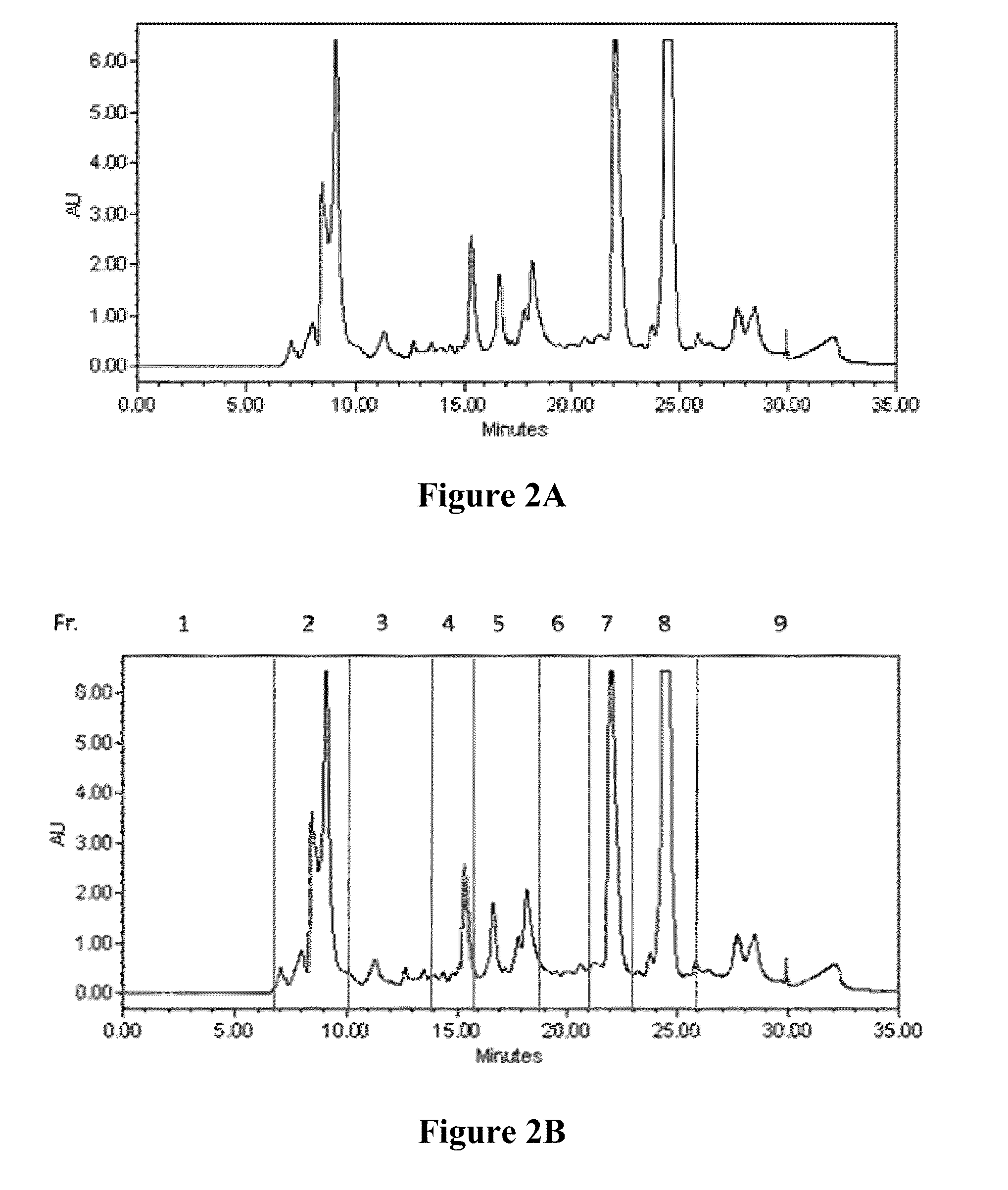
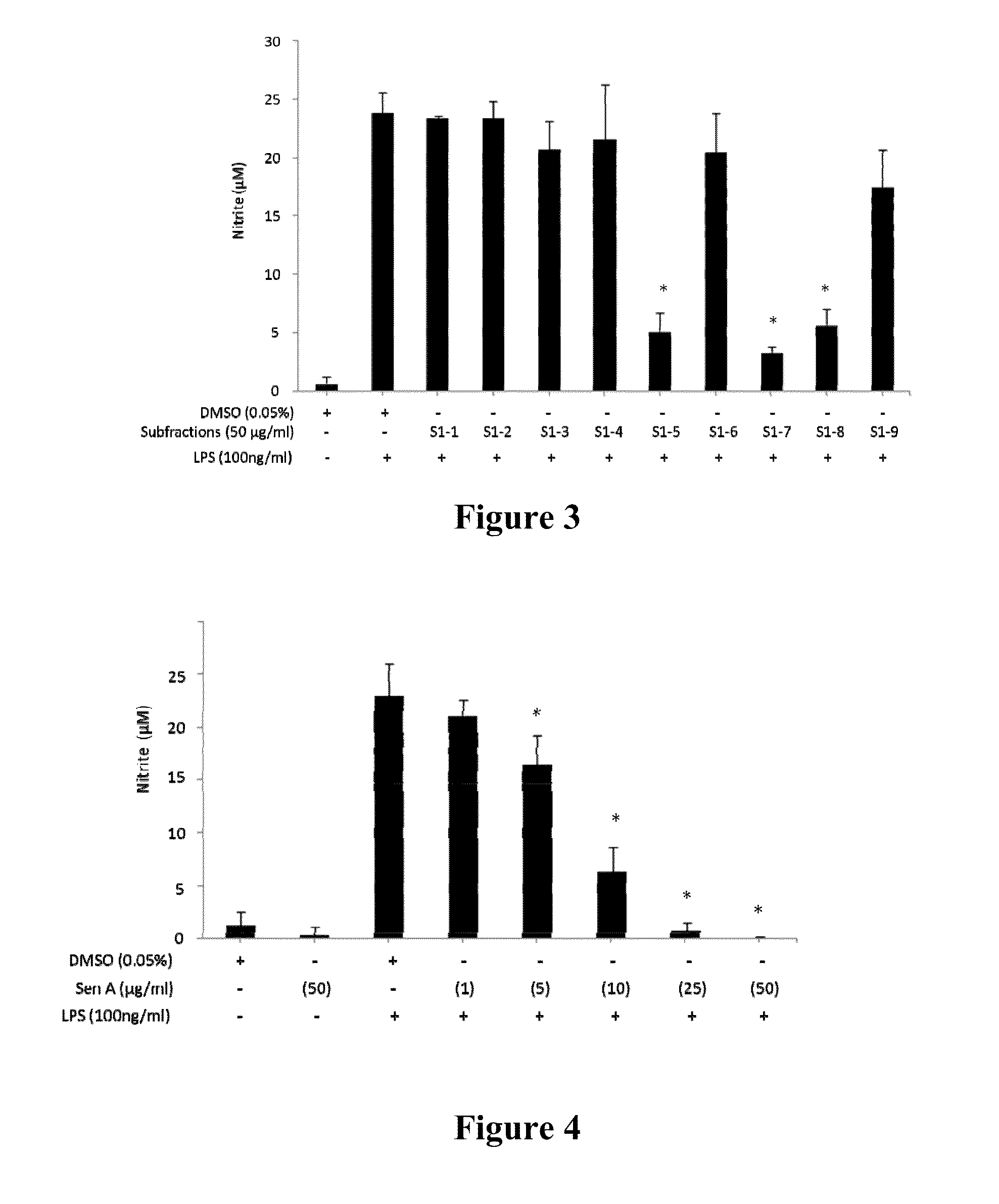
The subject invention provides novel and advantageous materials and methods for treating neuroinflammation, neurodegenerative disease, and cerebrovascular disease by modulating TNF-α and / or nitric oxide production. Specifically exemplified herein is the therapeutic use of senkyunolide A (Sen A) and Z-ligustilide (Z-Lig), compounds isolated from traditional Chinese medicinal material Ligusticum chuanxiong (LCX).
Owner:BAGI RES +1
Ligustilide cyclodextrin or its derivatives clathrate, its preparation method and pharmaceutical formulation
InactiveCN1732923AHigh inclusion rateGood water solubilityOrganic active ingredientsPharmaceutical delivery mechanismOrally disintegrating tabletCyclodextrin derivative
Owner:钱忠明 +2
Method for preparing Ligusticum wallichii lactone extract having anti-liquid-accumulation activity, and use
InactiveCN102824382ASimple extraction and separation processThe chemical composition is simple and clearOrganic active ingredientsDigestive systemAcetic acidReflux
The invention relates to a method for preparing Ligusticum wallichii lactone extract having anti-liquid-accumulation activity and a use, especially to a Ligusticum wallichii extract containing 30 to 90 % of ligustilide. The extract is prepared by the following method comprising smashing the Ligusticum wallichii medicinal materials, immersing in 70 to 99 % ethanol, e.g. 75 to 98 %, extracting by reflux for 1 to 4 times, combining extract, condensing, extracting by using petroleum ether, recovering the solvent of the combined extract till dryness to get a petroleum ether extract; extracting a part of the extract by using the petroleum ether, adding normal phase silica gel to stir, going through a normal phase silica gel column, eluting by using the petroleum ether and an ethyl acetate solution with a volume ratio of 50 : 0.5-5 as eluents, collecting the eluates, and condensing to get the Ligusticum wallichii lactone extract.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Angelica sinensis effective component extracting method and extracts of angelica sinensis
InactiveCN109867598APromote precipitationIncrease profitCarboxylic compound separation/purificationPlant ingredientsNatural productAlcohol
The invention relates to the field of processing of natural products, in particular to an angelica sinensis effective component extracting method and extracts of angelica sinensis. According to the angelica sinensis effective component extracting method, in the same production technology, multiple kinds of effective components in the angelica sinensis are extracted separately, the limitation of single target product extraction and separation is gotten rid of, the utilization rate of an angelica sinensis raw material is greatly improved, the extraction cost is reduced, moreover, the technologyis simple, operation is simple and convenient, and the equipment requirements are low. Enzymolysis is used for assisting in extraction, the ferulic acid extraction efficiency nearly doubles, moreover,on the premise of controlling parameters of aqueous extraction and alcohol extraction, for extraction of flavone and ligustilide, the solvent dosage is reduced, good yields are obtained, and the angelica sinensis effective component extracting method has significant economic values.
Owner:北京国康本草物种生物科学技术研究院有限公司
Method for evaluating quality level through detection of contents of active ingredients with calcium-antagonistic function in Chinese angelica medicine material
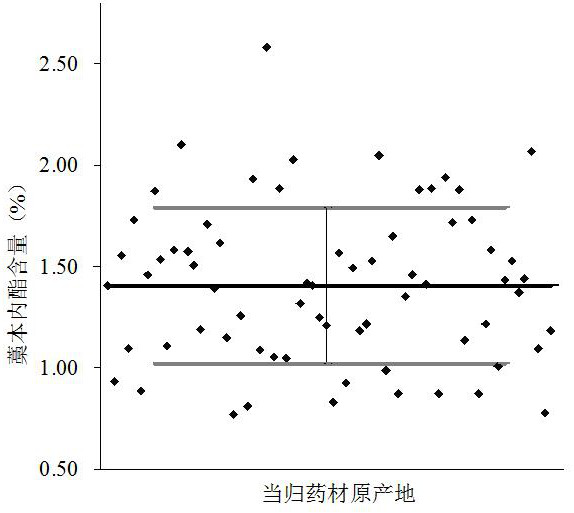
ActiveCN110057958AQuick evaluationThe method is simple and fastComponent separationQuality levelZ-ligustilide
The invention discloses a method for evaluating the quality level through detection of the contents of active ingredients with a calcium-antagonistic function in a Chinese angelica medicine material.The method includes steps: (1) detecting contents of Z-ligustilide and levistilide A in the Chinese angelica medicine material through an ultra-performance liquid chromatography, wherein the mg numberof Z-ligustilide in 1g of the Chinese angelica medicine material is expressed by X1, and the mg number of levistilide A in 1g of the Chinese angelica medicine material is expressed by X2; and (2) enabling the relation between the intracellular calcium-antagonistic function and Z-ligustilide and levistilide A to satisfy the following function: Y=(31.2579X1+381.352X2-248.979X1X2+18.4822)*100%, wherein Y is an evaluation value of the intracellular calcium-antagonistic function in the Chinese angelica medicine material for short, the range interval of Y is 0-100%, and the Chinese angelica medicine material is divided into seven quality levels from level 1 to level 7. According to the method, the quality level of Chinese angelica can be simply and rapidly evaluated.
Owner:NANKAI UNIV
Use of ligustilide in preparing anti-thrombotic medicines
ActiveCN102813687ASimple extraction and separation processThe chemical composition is simple and clearOrganic active ingredientsBlood disorderEthyl acetateSilica gel
The invention relates to use of ligustilide in preparing anti-thrombotic medicines, in particular to a rhizoma ligustici wallichii extract. The rhizoma ligustici wallichii extract contains 30-90 percent of ligustilide. The extract is prepared by adopting a method comprising the following steps of: crushing a rhizoma ligustici wallichii medicinal material; then, soaking by using 70-99 percent of alcohol (for example 75-98 percent), and performing reflux extraction for 1-4 times; merging extracting solutions; concentrating; extracting by using petroleum ether; recovering solvents from the merged extracting solutions until dry to obtain petroleum ether extracting extractum; adding part of petroleum ether extracting extractum to positive-phase silica gel and mixing to exceed a positive-phase silica gel column; performing elution by taking the petroleum ether and an ethyl acetate solution as eluents; collecting the eluents; concentrating to obtain the rhizoma ligustici wallichii extract, wherein the volume ratio of the petroleum ether to the ethyl acetate solution is 50:(0.5-5) [for example 50:(0.5-3)].
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
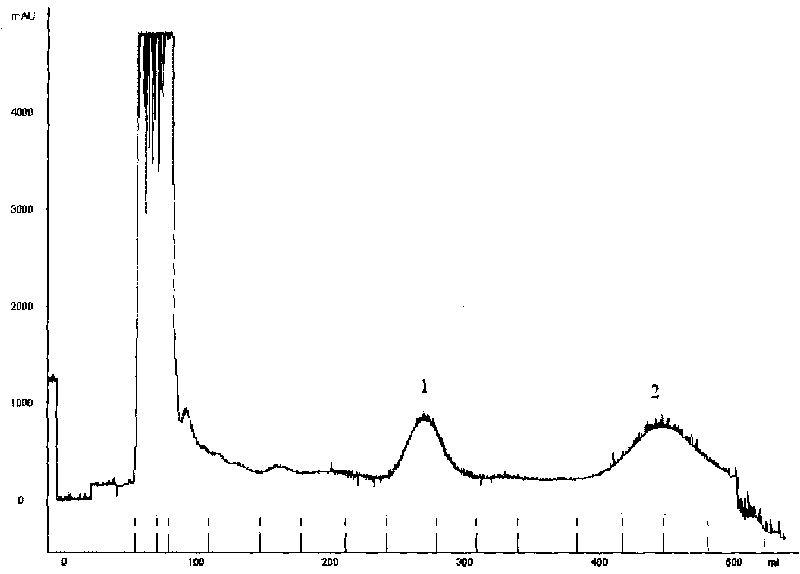
Method for preparing high purity z-ligustilide and cnidilide A
ActiveCN101759674AHigh recovery rateOvercome irreversible adsorptionOrganic chemistryPlant ingredientsAlkaneStationary phase
The invention discloses a method for preparing high purity z-ligustilide and cnidilide A, which is characterized by adopting high-speed countercurrent chromatography and comprises the following steps: preparing the crude extract of rhizoma ligustici wallichii or angelica as a sample injection substance; preparing a solvent system forming stationary phase and mobile phase; filling the stationary phase into a countercurrent chromatography column; then rotating a host machine, pumping the mobile phase into the column, or simultaneously pumping the stationary phase and the mobile phase into the column and then rotating the host machine; and injecting samples from an injection valve, receiving target components according to a map of a detector and obtaining the z-ligustilide and cnidilide A through separation. The solvent system is formed by four components (A, B, C and D). The component A is n-alkane, the component B is fatty ether, the component C is fatty alcohol or fatty ketone and the component D is water. The z-ligustilide and cnidilide A with purity higher than 95% can be obtained by the method. The method is simple and convenient, easy to operate, high in recovery and easy to promote and use.
Owner:NAT ENG RES CENT FOR TRADITIONAL CHINESE MEDICINE
Cyclodextrin inclusion compound containing ligustilide, and preparation method thereof
The invention provides a cyclodextrin inclusion compound containing ligustilide, comprising ligustilide and hydroxypropyl-beta-cyclodextrin, wherein the ligustilide is included in the hydroxypropyl-beta-cyclodextrin to form an inclusion compound, the percentages by weight of the ligustilide and the hydroxypropyl-beta-cyclodextrin are 10-20%, and the cyclodextrin inclusion compound further contains povidone K29 / 32 which accounts for 10-50% of the hydroxypropyl-beta-cyclodextrin by weight. The cyclodextrin inclusion compound containing the ligustilide can be prepared into tablets, capsules, powder or powder injections as medicaments for treating dysmenorrhea.
Owner:上海港太药业发展有限公司
Butylphthalide preparation composite, preparation method therefor, and application thereof
The invention discloses a butylphthalide preparation composite and a preparation method therefor. The butylphthalide preparation composite comprises butylphthalide and ligustilide; accessories that are pharmaceutically acceptable can be further added into the pharmaceutical composite, so as to prepare soft capsules, liquid hard capsules, pills, dropping pills, etc.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
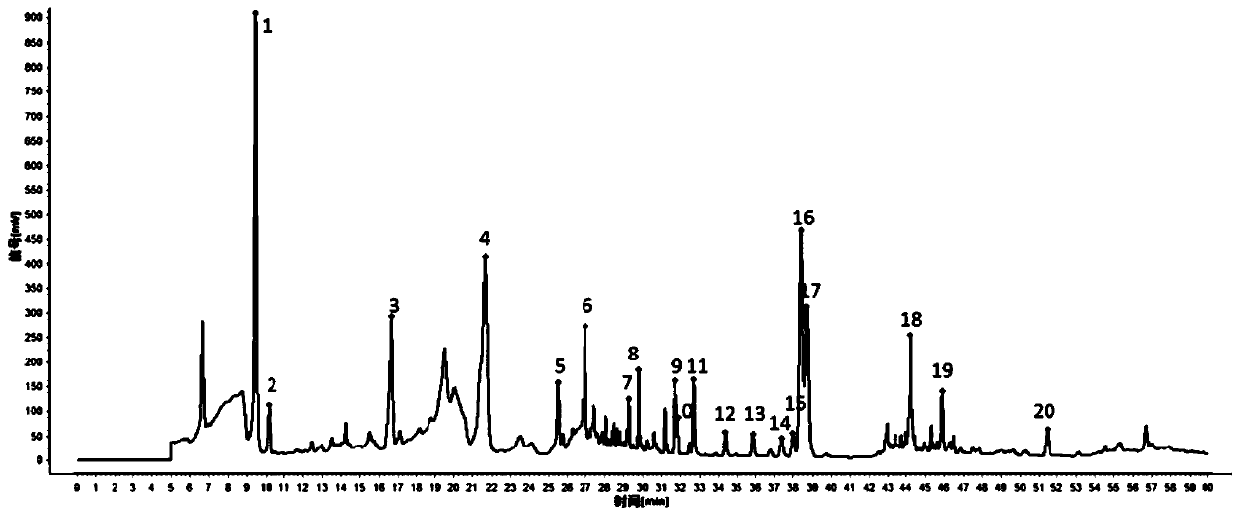
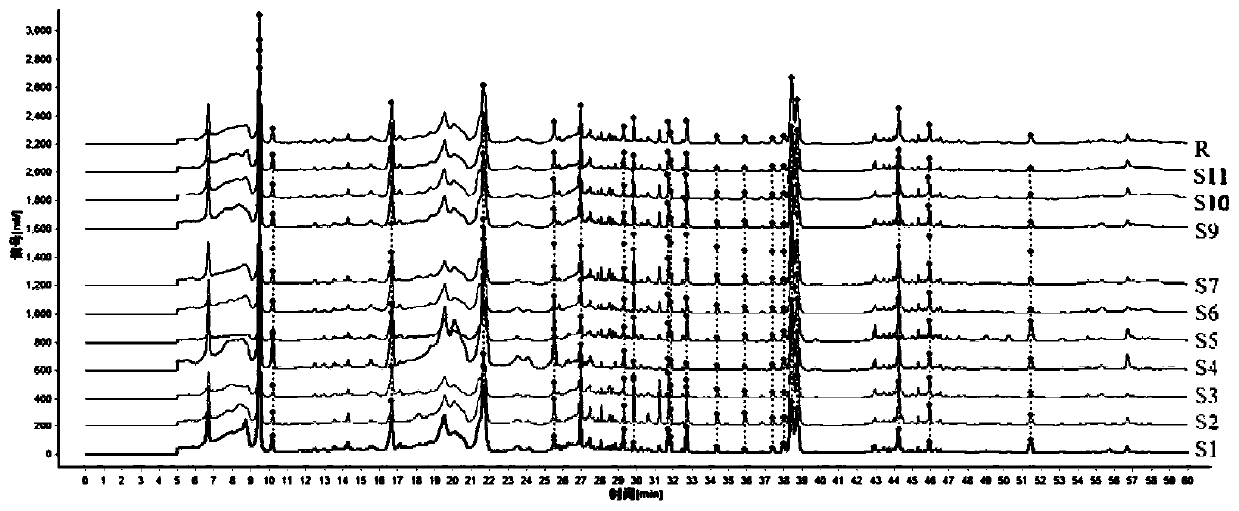
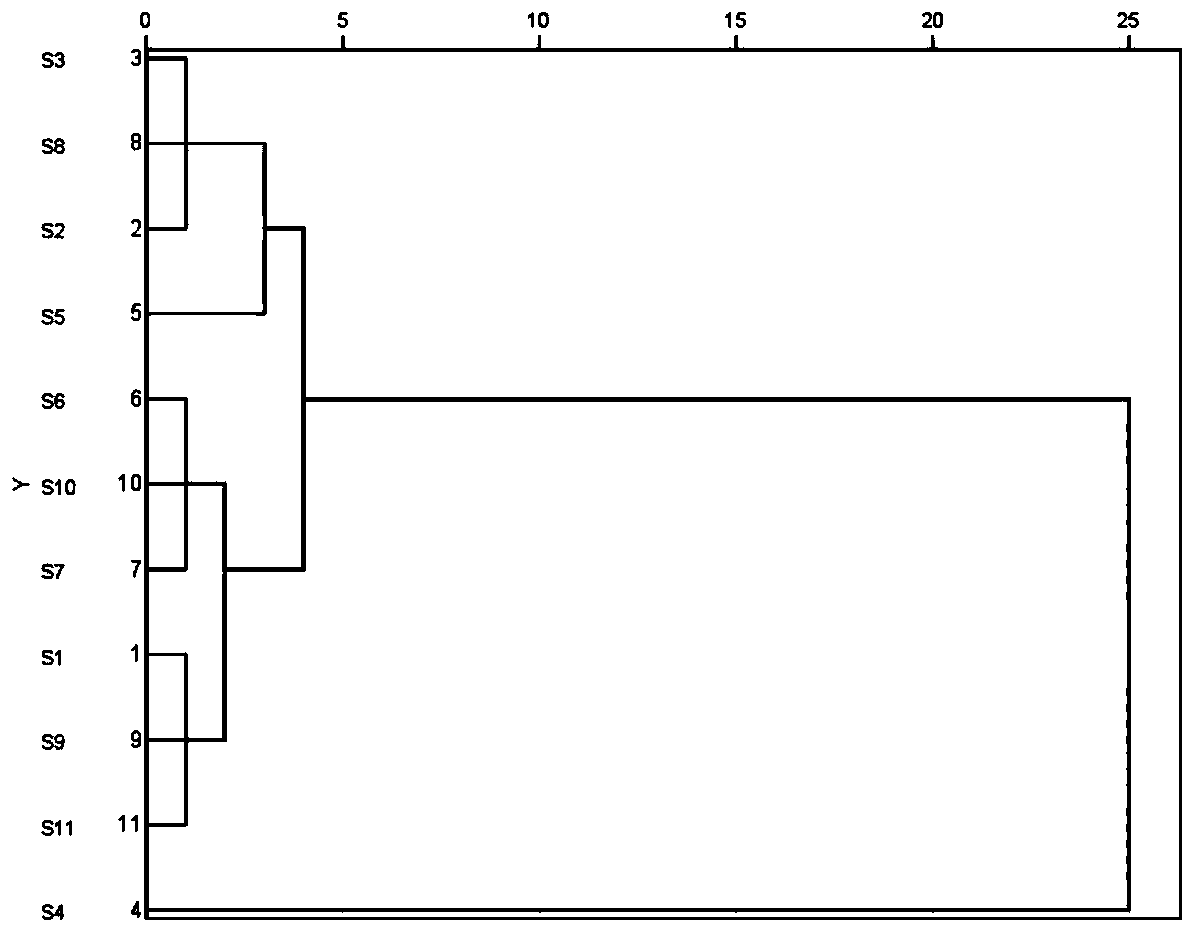
Fingerprint spectrum detection method for aboveground part of ligusticum wallichii
InactiveCN111257456AHigh similarityImprove consistencyComponent separationPattern recognitionZ-ligustilide
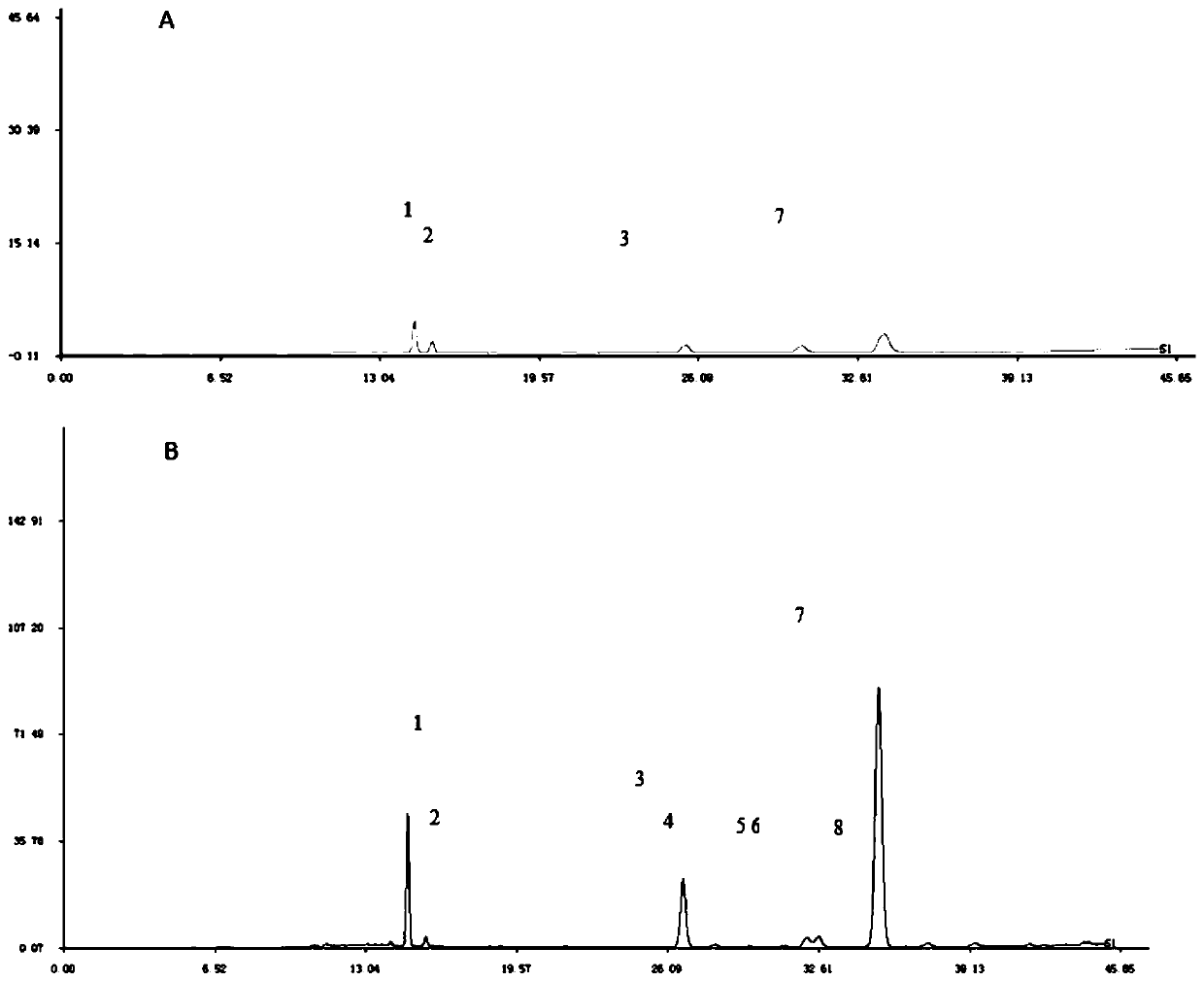
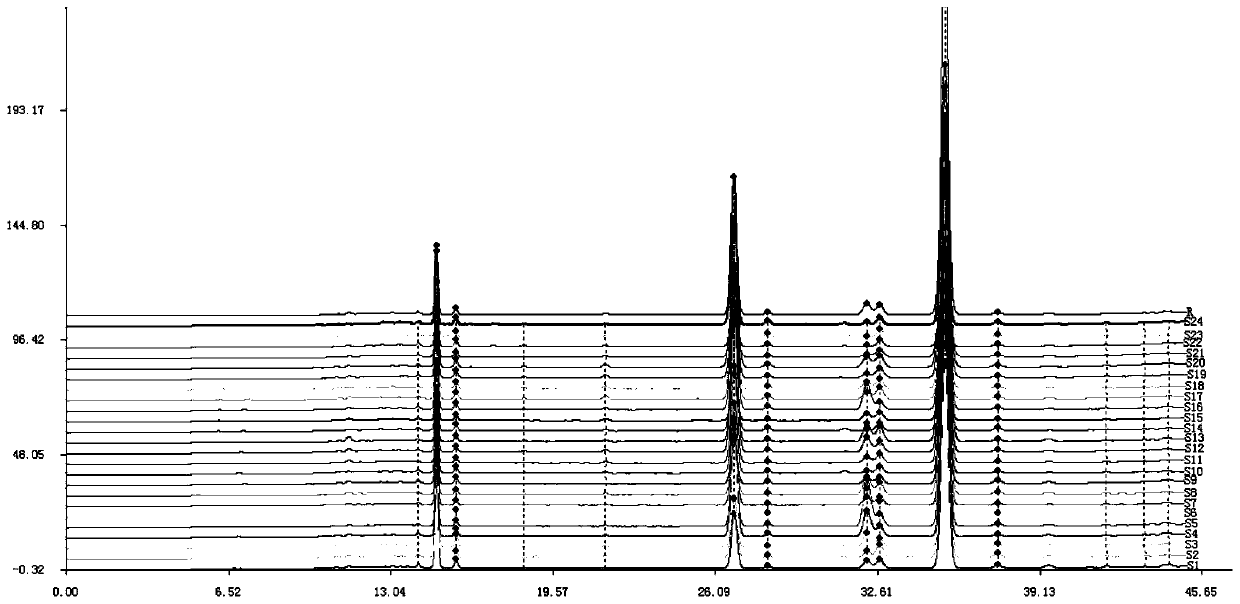
The invention discloses a fingerprint spectrum detection method for an aboveground part of ligusticum wallichii. The fingerprint spectrum detection method for the aboveground part (stems and leaves) of the ligusticum wallichii fills the blank of quality detection of the aboveground parts of the ligusticum wallichii, and a standard quality control system is established for the aboveground part of the ligusticum wallichii. According to the fingerprint spectrum of the aboveground part of the ligusticum wallichii, 20 common peaks are calibrated, and two components, namely ligustilide and butenylphthalide, are identified. Compared with a control spectrum, the fingerprint spectrum similarity of the aboveground part of the ligusticum wallichii in different batches is 0.942-0.990, and the similarity is good such that the chemical components of the aboveground part of the ligusticum wallichii in different producing areas have good consistency, and the method can be used for controlling the quality of the aboveground part of the ligusticum wallichii.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV +1
Fast production of high-purity radix ligustici sinensis lactone
InactiveCN100351247CImprove efficiencyFast separationOrganic chemistryLactone formationColumn chromatography
Production of high-purity oshaic internal ester is carried out by separating out the high-purity oshaic internal ester from oshaic internal ester crude oil by reduced pressure column chromatography. Its advantages include fast separation, simple operation and recovery and re-utilization.
Owner:钱忠明 +2
Angelica sinensis and astragalus membranaceus blood-enriching granules and preparation method thereof
PendingCN112656834AAvoid lostHigh retention rateSkeletal disorderGranular deliveryAngelica Sinensis RootZ-ligustilide
The invention discloses Angelica sinensis and astragalus membranaceus blood-enriching granules and a preparation method thereof. The preparation method comprises the following steps: (1) soaking radix astragali decoction pieces and wine radix angelicae sinensis in water, then carrying out reflux extraction, and collecting aromatic water in the extraction process, so as to obtain an extract solution and aromatic water; (2) concentrating the extract solution under reduced pressure to obtain a concentrated solution; (3) uniformly mixing the concentrated solution with aromatic water, and performing spray drying to obtain spray-dried powder; and (4) uniformly mixing the spray-dried powder with auxiliary materials, and performing dry granulation to obtain the angelica sinensis and astragalus membranaceus blood-enriching granules. The aromatic water is collected by adopting a condensing device in the extraction process, so that the volatilized ligustilide is retained, and the aromatic water is directly added back into the concentration device for drying and granulating, so that the loss of the volatile component ligustilide is avoided to a greater extent, the retention rate of the ligustilide is increased from 0-1% to more than 40%, and the quality of the ligustilide is improved. Effective utilization of water-soluble components and volatile components is effectively guaranteed, and it is guaranteed that the angelica sinensis and astragalus membranaceus blood-enriching soup granules effectively exert the clinical effect.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD
Method for indirectly measuring ligustilide content in traditional Chinese medicinal materials or Chinese patent medicines by chemical conversion method
ActiveCN111796048AEffective quality controlImprove reliabilityComponent separationZ-ligustilidePharmaceutical drug
The invention discloses a method for indirectly measuring the accurate content of ligustilide in traditional Chinese medicinal materials or Chinese patent medicines by a chemical conversion method. The method comprises the steps of extracting and / or converting ligustilide in traditional Chinese medicinal materials or Chinese patent medicine powder; converting ligustilide with an unstable chemicalstructure in traditional Chinese medicinal materials or Chinese patent medicines into cyclopropylligustilide with a stable structure, directly measuring the content of cyclopropylligustilide through an HPLC external standard method, and then indirectly calculating the content of ligustilide in traditional Chinese medicinal materials or Chinese patent medicines through equimolar weight. The methodis used for quantitatively detecting the ligustilide content in medicines, the result is reliable, the accuracy is high, the stability and reproducibility of the method are good, and the direct targetof effectively controlling the medicine quality is achieved through application of the method.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Fingerprint spectrum detection method for ethyl acetate part of ligusticum wallichii
ActiveCN110907555AImprove the quality evaluation systemGuaranteed clinical efficacyComponent separationClinical efficacyEthyl acetate
The invention discloses a fingerprint spectrum detection method for an ethyl acetate part of ligusticum wallichii. The fingerprint spectrum detection method adopts high performance liquid chromatography for detection. Through the detection method provided by the invention, the senkyunolide I, the senkyunolide H, the senkyunolide A and the ligustilide in the ligusticum wallichii can be measured atthe same time, the quality of effective parts with the analgesic effect can be controlled, the method is simple, convenient and reliable, a quality evaluation system of the ligusticum wallichii is perfected, the clinical curative effect of the ligusticum wallichii is guaranteed, and practical application and popularization value is achieved.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Fingerprint of Fushiming Capsules and Its Application in Quality Control and Component Analysis
ActiveCN110196299BGood analysis and evaluation abilityEffective treatmentComponent separationHplc fingerprintDaidzin
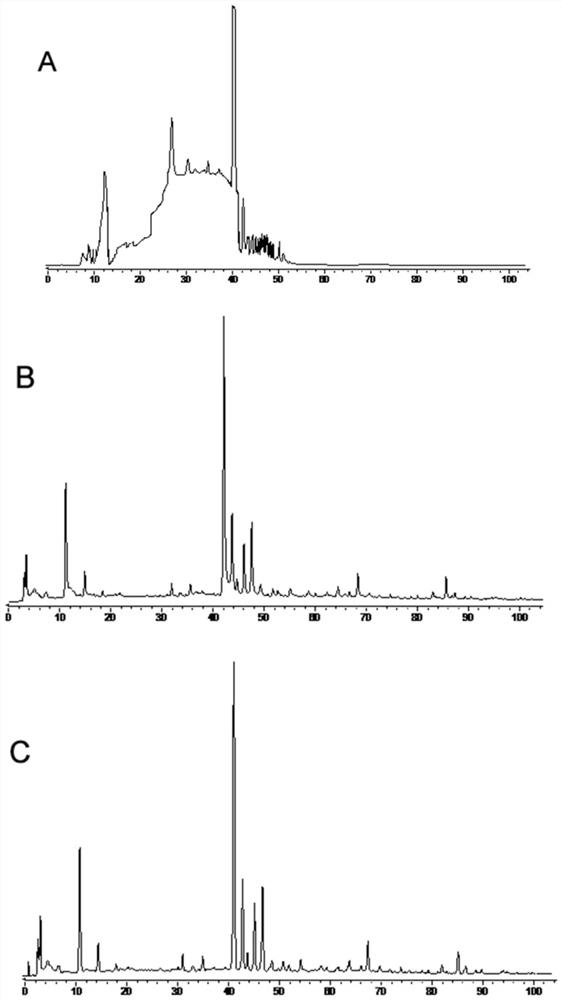
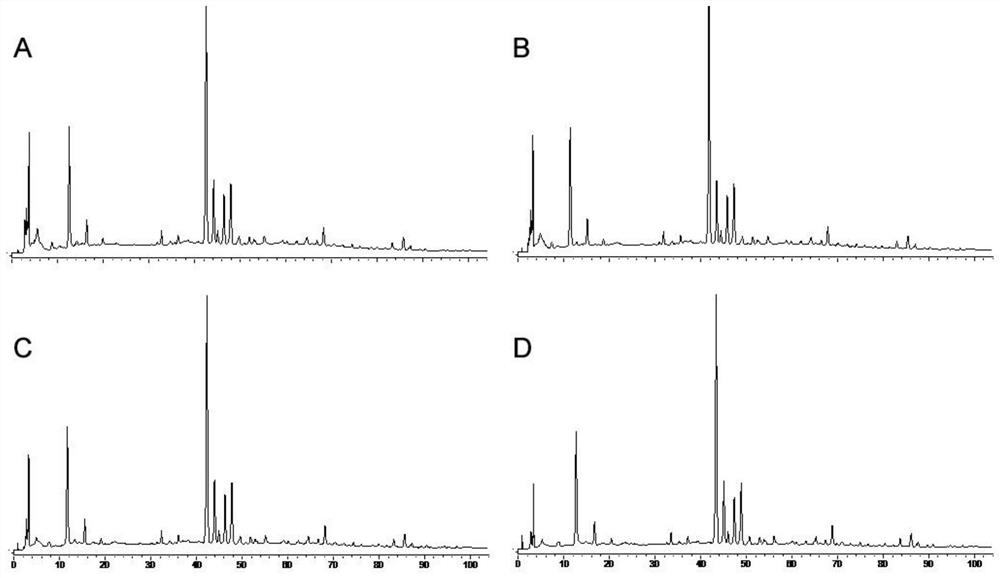
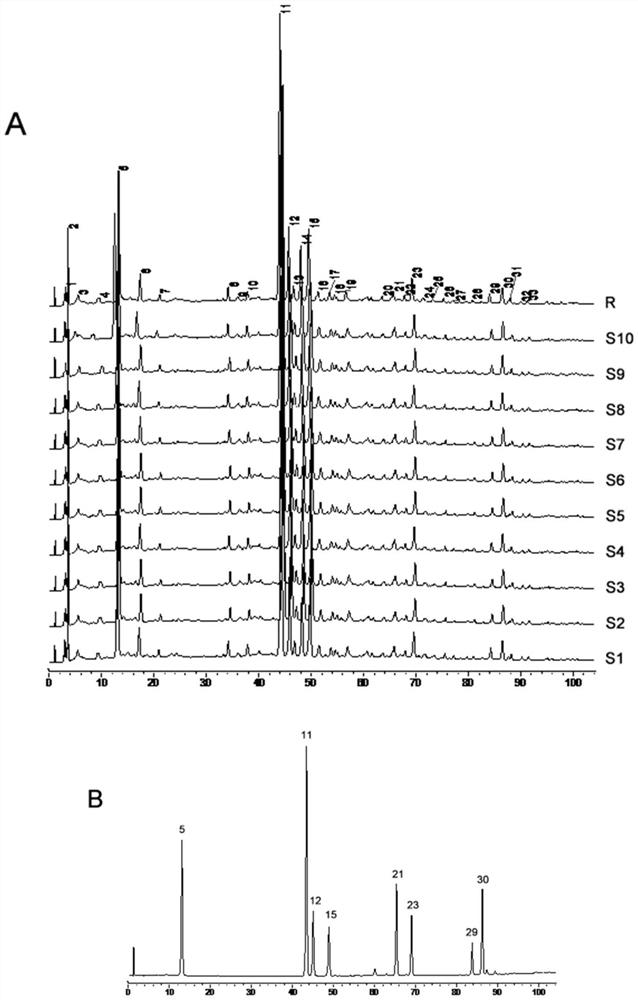
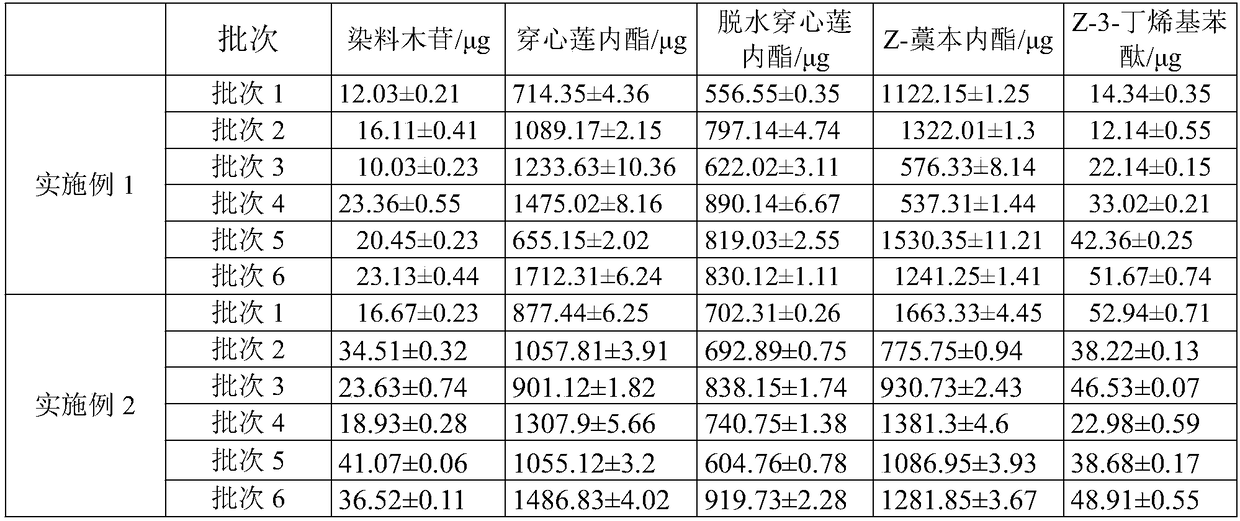
The invention relates to the fingerprint of Fushiming capsules and its application in quality control and component analysis. The invention optimizes the HPLC mobile phase. The chromatograms of 10 batches of Fushiming capsule samples are analyzed and 33 chromatographic peaks are determined as: The fingerprints of Diushiming Capsules have common peaks, and the peak area of the common peaks accounts for more than 90% of the total peak area. The common pattern of the HPLC fingerprints of Diushiming Capsules is determined, and 33 common peaks are demarcated, and the similarity is 0.994~ 1.000; and the contents of 5-HMF, puerarin, 3'-methoxypuerarin, daidzin, formononetin, daidzein, aurisin and ligustilide in 10 batches of preparations were determined. The HPLC fingerprint method and the content determination method of Fushiming capsule established by the invention have good analysis and evaluation ability, and have the advantages of accuracy, simplicity, stability and reliability. Therefore, this method can be used as an effective evaluation method for the quality of this preparation.
Owner:XIAN LEJIAN BIOTECH CO LTD
Quality control method for gynecological Qianjin tablets
PendingCN109470788AIncrease the content of core ingredientsThe effect is consistent and stableComponent separationMedicineZ-ligustilide
The invention discloses a quality control method for gynecological Qianjin tablets. The method comprises the following steps: S1, selecting angelica sinensis, radix codonopsis and common andrographisherb, respectively grinding into fine powder for later use; S2, selecting zanthoxylum dissitum, radix rosae laevigatae, spatholobus stems, Chinese mahonia stems and philippine flemingia roots, performing water extraction, and concentrating filtrate into clear paste of which CF is 1.08 / 85 DEG C; S3, uniformly mixing the fine powder with the clear paste, and controlling the content of genistin in amixture, the content of at least one of Z-ligustilide and Z-3-butylidenephthalide, and both the total amounts of andrographolide and dehydrated andrographolide to reach the standard content; then drying, performing tabletting, and wrapping the tablets to obtain the gynecological Qianjin tablets. The quality control method disclosed by the invention increases the content of various core componentsin the existing pharmacopoeia standards, and the gynecological Qianjin tablets prepared by using the control method have more stable effect consistency among different batches, and compared with the prior art, the gynecological Qianjin tablets have better clinical treatment effects.
Owner:ZHUZHOU QIANJIN PHARMA
Method for determining ligustilide in natural medicinal preparation
The invention discloses a method for detecting ligustilide in a natural medicinal preparation. The method comprises the following steps: taking 2-(1-pentanone)-1,5-cyclohexadiene formic acid, namely the hydrolysis product of the ligustilide, as a reference substance, determining the content of ligustilide in the natural medicinal preparation, fully hydrolyzing the ligustilide preparation in an acetonitrile solution containing alkali metal hydroxide, determining the content of the 2-(1-pentanone)-1,5-cyclohexadiene formic acid in the sample by use of high performance liquid chromatography, and converting the content of 2-(1-pentanone)-1.5-cyclohexadiene formic acid to the content of ligustilide according to a molar ratio 1:1. The method has the advantages of good stability, low cost, and capability of determining the content of ligustilide in the natural medicinal preparation simply, conveniently and accurately.
Owner:SHINEWAY PHARMA GRP LTD
Preparation method of Shiyao angelica sinensis extract for tobacco perfuming
ActiveCN112273709APromote improvementImprove retouching effectTobacco treatmentBiotechnologyAngelica Sinensis Root
The invention discloses a preparation method of a Shiyao angelica sinensis extract for tobacco perfuming. The preparation method comprises the following steps of: S1, taking a mixture of Shiyao angelica sinensis and desertliving cistanche, and crushing and sieving the mixture; S2, extracting the sieved mixture with 100% ethanol, filtering to obtain filter residues and a first filtrate, and distilling the first filtrate under normal pressure to obtain a distillate; S3, leaching the filter residues with a solanic acid solution, filtering to obtain a second filtrate, and concentrating the secondfiltrate under reduced pressure to obtain a concentrated solution; S4, mixing the distillate and the concentrated solution, and storing the mixed solution in an inert gas environment to obtain the Shiyao angelica sinensis extract. The Shiyao angelica sinensis extraction rate is high, and the extracted ligustilide is high in stability.
Owner:HUBEI CHINA TOBACCO IND +1
A kind of detection method of Jingfukang preparation
ActiveCN101744936BIncrease the means of determining the content of ligustilideReliable test resultsAnthropod material medical ingredientsComponent separationFluid phaseGas liquid chromatographic
The invention provides a new quality index of a Jingfukang preparation and a detection method. The content of ligustilide is adopted as a detection index for the quality of the Jingfukang preparation. The detection is carried out by adopting a GC (gas chromatogram) internal standard method and an HPLC (high performance liquid chromatography) external standard method. The new quality index is as follows: the content of the ligustilide per 5g Jingfukang preparation is from 0.1 to 1.0mg; the content of the ligustilide per 5g Jingfukang preparation is not lower than 0.1mg, and the content of puerarin is not lower than 8.0mg. The invention has the following positive effects: the suggestion that the ligustilide is adopted as the quality control index of the Jingfukang preparation is put forward for the first time; the content of the ligustilide in the Jingfukang preparation can be detected by adopting a liquid chromatography and the GC internal standard method; the detecting result is reliable, and the repeatability is good; therefore, the invention provides the new quality control index and the detection method for safety production and better clinical application of the Jingfukang preparation.
Owner:CHENGDE TRADITIONAL CHINESE MEDICINE GROUP
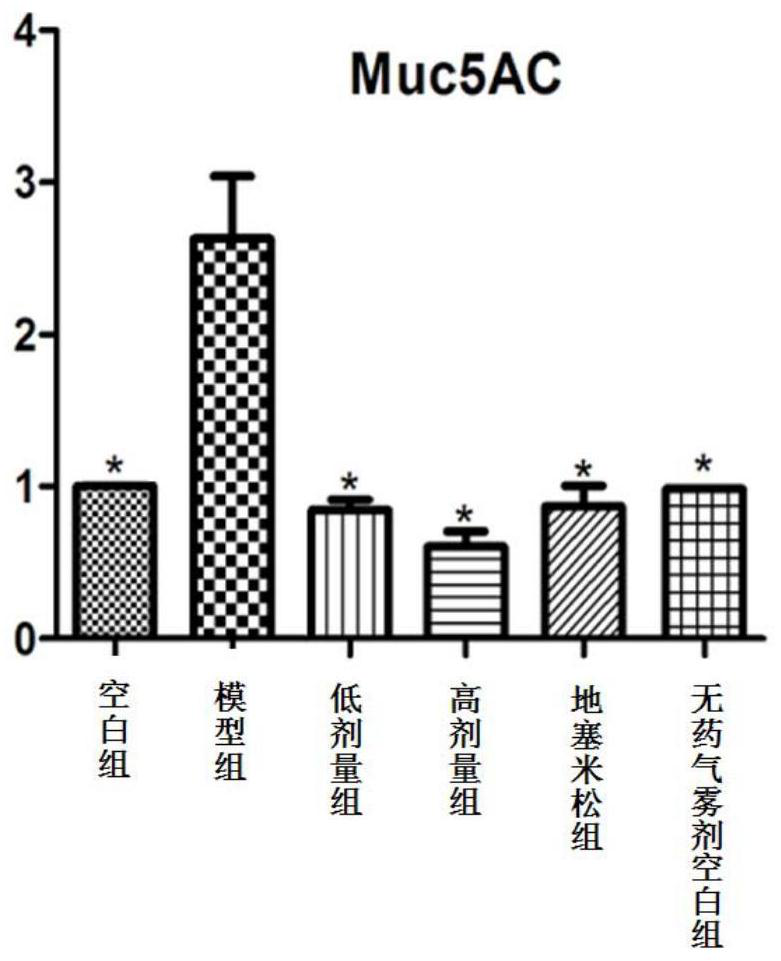
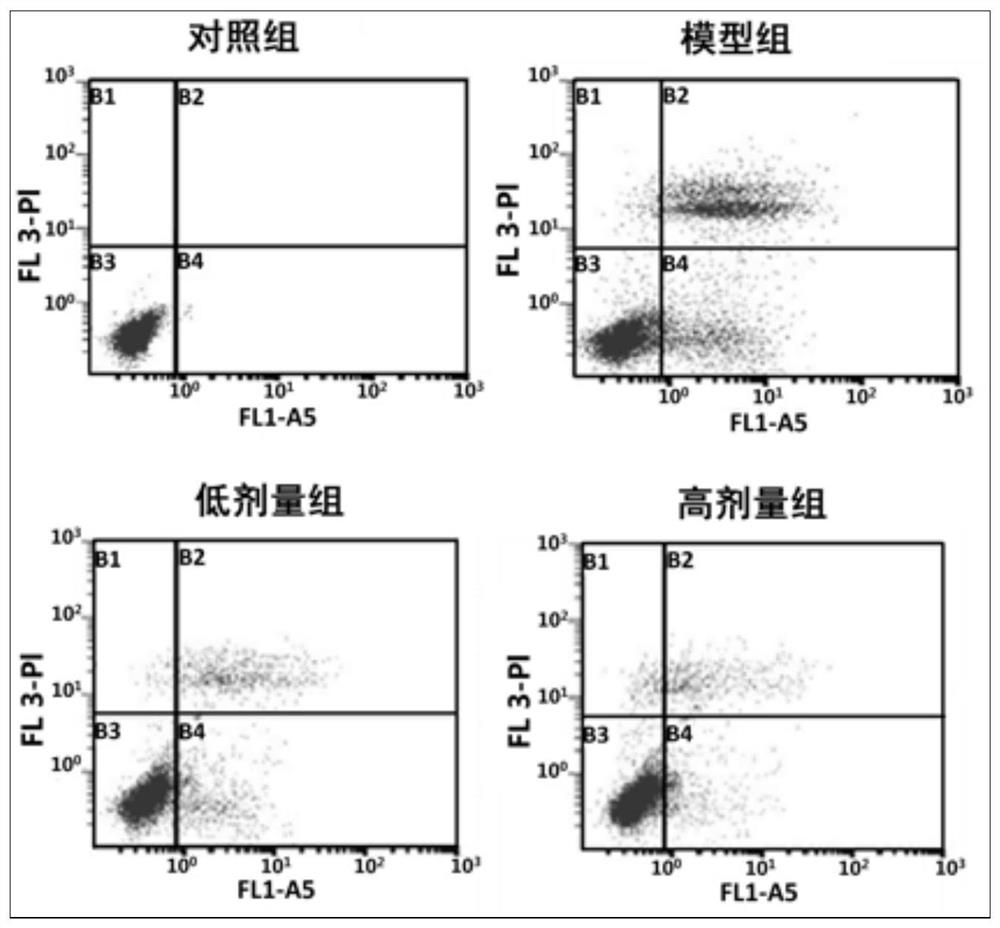
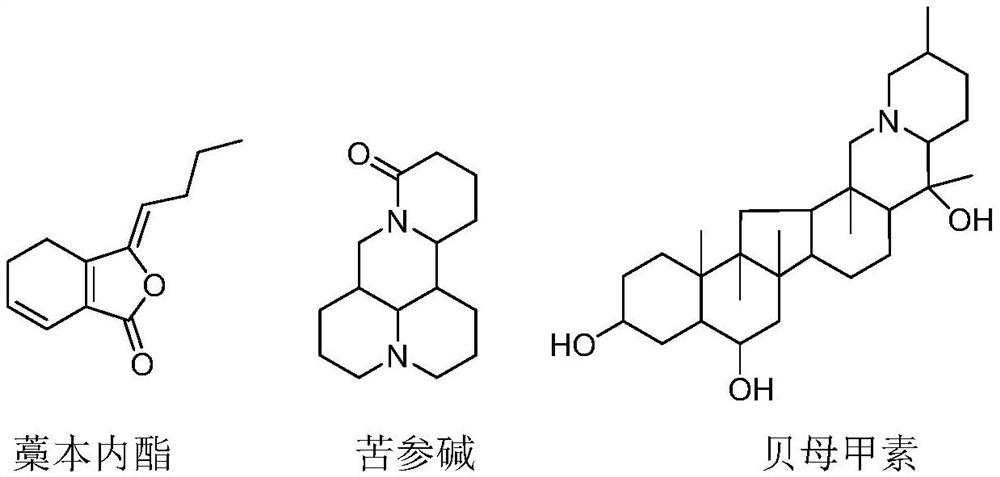
A kind of aerosol for treating chronic obstructive pulmonary disease
ActiveCN107669689BInhibition of secretionInhibition of hypersecretionOrganic active ingredientsDispersion deliveryInflammatory factorsAerosol drugs
The invention provides an aerosol for treating chronic obstructive pulmonary disease, which is composed of active ingredients, additives, and propellants, wherein the active ingredients are composed of three compounds: ligustilide, matrine, and peiminin in proportion. The mass ratio of the three active components is 1% to 99% for each component, and the range of the active component to the mass of the aerosol is 1 to 99%. There are two types of aerosol dosage forms: solution type and mixed type. The above-mentioned aerosol drug, through the COPD animal model, has proved that it has various effects such as inhibiting the secretion of relevant inflammatory factors, inhibiting the expression of mucus hypersecretion-related proteins, and inhibiting epithelial cell apoptosis and necrosis. It can directly reach the affected area and has a quick effect. The curative effect is good, the animal experiment proves that it has good anti-COPD effect, and can be applied in the preparation of medicines for treating chronic obstructive pulmonary disease.
Owner:ZHEJIANG UNIV
Processing extraction method of radix bupleuri decoction pieces and radix bupleuri composition
InactiveCN106176851AIncrease contentSimple structureAntibacterial agentsOrganic active ingredientsChemistryMedicinal herbs
The invention provides a processing extraction method of radix bupleuri decoction pieces. The method comprises the steps that radix bupleuri is uniformly mixed with vinegar, put into a pot to be stir-fried till the vinegar is completely absorbed and radix bupleuri is slightly dry and then taken out, water of which the weight is 10 times the weight of radix bupleuri is added, soaking is conducted for 0.5 h, decocting is conducted for one hour, and filtering is conducted; water of which the weight is 8 times the weight of the medicinal material is added into medicine residues, decocting is conducted for one hour, and medicine liquid is filtered; decoction solutions obtained in two times are mixed and concentrated till the relative density is 1.05 at 60 DEG C, ethyl alcohol is added to enable the alcohol volume percent to reach 75%, stirring, standing and filtering are sequentially conducted, filtrate is concentrated till the relative density is 1.25 at 65 DEG C, ethyl alcohol is recycled, and concentrated liquor is obtained; refining is conducted through silica gel, the pre-selected silica gel is contained in a resin column, the concentrated liquor is diluted with purified water and passes through a reverse-phase silica gel column, impurities in liquid are removed, all effluent is collected, concentrating continues to be conducted, concentrated liquor is dried and granulated, and then radix bupleuri granules are obtained. The radix bupleuri granules prepared through the method are high in saikoside content, and a radix bupleuri composition is prepared from the radix bupleuri granules, ligustilide and quercetin through compatibility.
Owner:NANJING ZHENGKUAN MEDICAL TECH
A kind of Pseudomonas compound microbial bacterial agent and its application in improving yield and quality of Angelica sinensis
ActiveCN113403236BHas ACC deaminase activityImprove the immunityBiocidePlant growth regulatorsBiotechnologyAngelica Sinensis Root
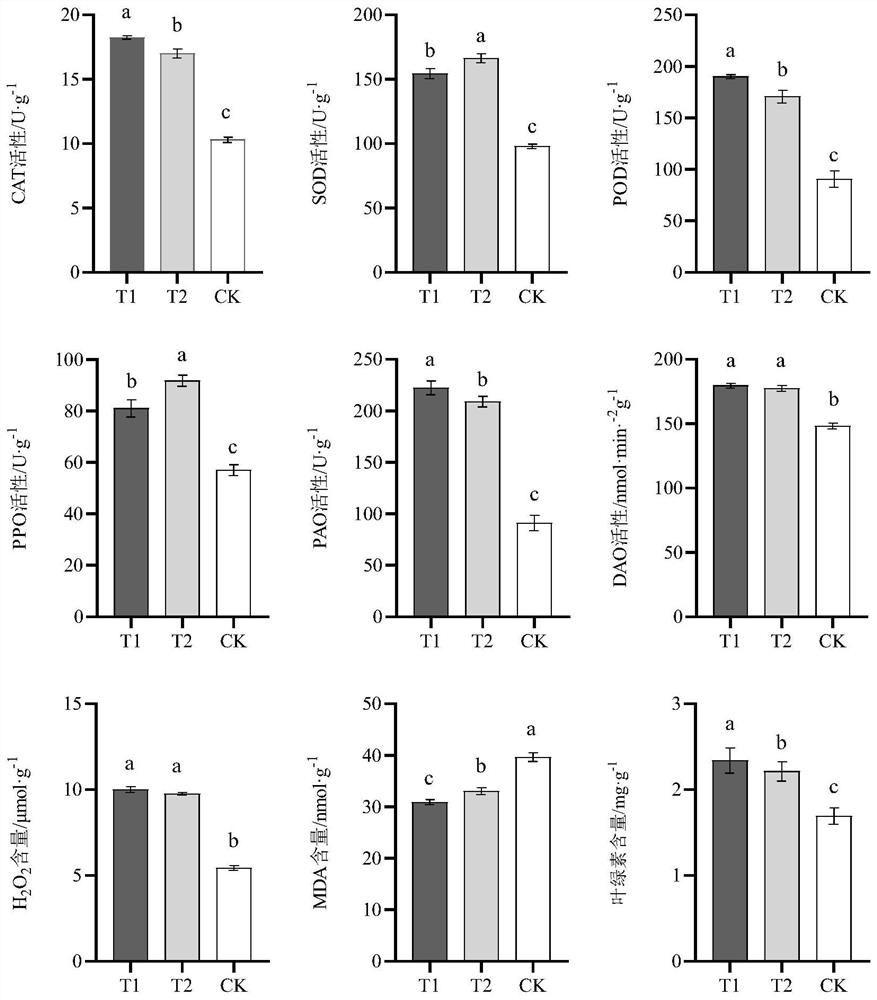
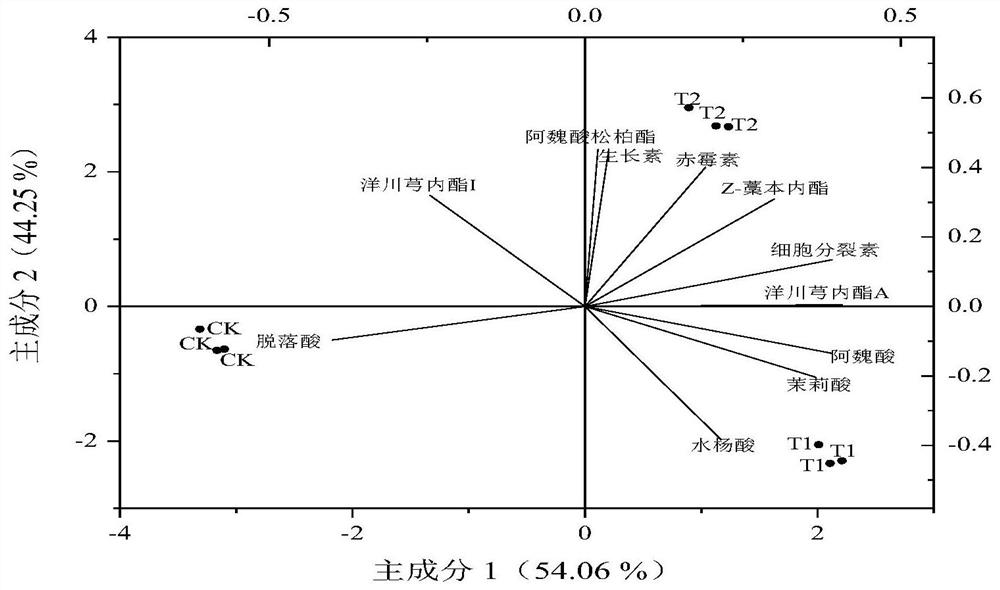
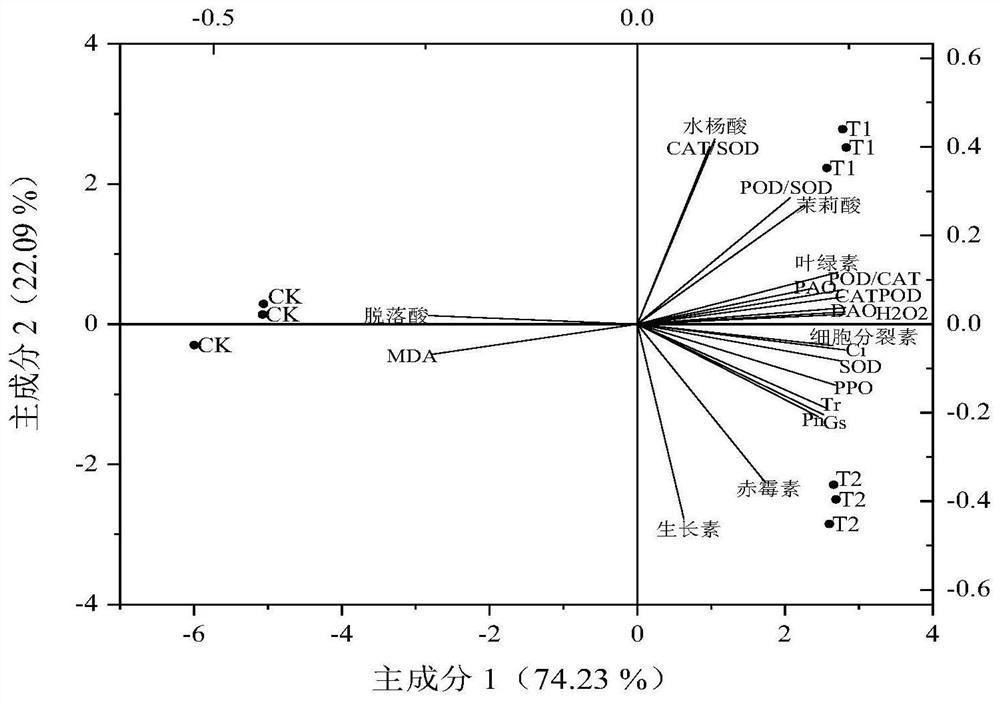
The invention belongs to the field of microorganisms and biotechnology, and in particular relates to a compound bacterial agent of microorganisms and its application in increasing production and quality of Angelica sinensis against diseases. The compound microbial agent is composed of Pseudomonas fluorescens, Pseudomonas alcaligenes and Pseudomonas psychrophilus; it has ACC deaminase activity, SOD activity, POD activity, NEX neutral xylan Enzyme activity, chitinase activity, and laccase activity; can promote the growth of the roots of the underground medicinal organs of Angelica sinensis, increase the diameter of the reed head, the length of the reed head, the length of the main root, and the average weight per plant, not only increasing the production but also improving the quality of commercial Angelica sinensis. Appearance properties; can increase the content of ferulic acid, Z-ligustilide, ligustilide A, endogenous jasmonic acid, salicylic acid, etc., which can significantly improve the quality of Angelica sinensis, and can be used to prepare special microorganisms for Angelica sinensis Fertilizer; it can prevent and control plant diseases caused by pathogenic fungi of the genus Fusarium, and can be used to prepare pesticides for preventing and controlling plant diseases.
Owner:GANSU ACAD OF SCI INST OF BIOLOGY +1
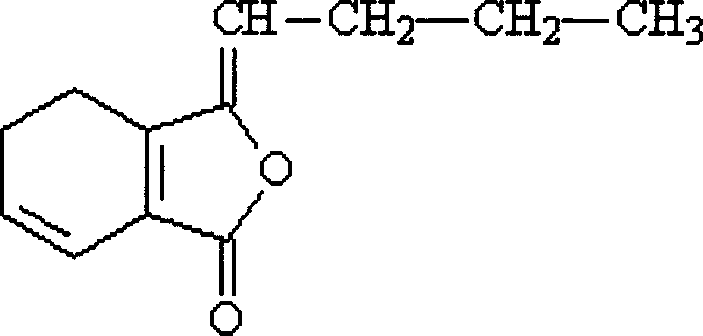
6,7-dihydroligustilide and alkylidene phthalide synthesis method
InactiveCN100430387CGood removal effectEasy to operateOrganic chemistryPtru catalystGrignard reagent
The invention discloses a new synthetic method of several perfumes and medical intermediate compound, which comprises the following steps: heating 4-cyclohexene-1, 2-bianhydrides to produce 1-cyclohexene-1, 2-bianhydrides under the palladium catalyst; making Grignard reagent of alkyl and 1-cyclohexene-1, 2-bianhydrides or ortho-phthalic anhydride react to produce 6, 7-dihydro-ligustilide and phthalic monoalkyl benzene. The invention is simple to convenient, which improves the obtaining rate.
Owner:LANZHOU UNIVERSITY
Method for preparing high purity z-ligustilide and cnidilide A
ActiveCN101759674BHigh recovery rateOvercome irreversible adsorptionOrganic chemistryPlant ingredientsStationary phaseAlkane
The invention discloses a method for preparing high purity z-ligustilide and cnidilide A, which is characterized by adopting high-speed countercurrent chromatography and comprises the following steps: preparing the crude extract of rhizoma ligustici wallichii or angelica as a sample injection substance; preparing a solvent system forming stationary phase and mobile phase; filling the stationary phase into a countercurrent chromatography column; then rotating a host machine, pumping the mobile phase into the column, or simultaneously pumping the stationary phase and the mobile phase into the column and then rotating the host machine; and injecting samples from an injection valve, receiving target components according to a map of a detector and obtaining the z-ligustilide and cnidilide A through separation. The solvent system is formed by four components (A, B, C and D). The component A is n-alkane, the component B is fatty ether, the component C is fatty alcohol or fatty ketone and thecomponent D is water. The z-ligustilide and cnidilide A with purity higher than 95% can be obtained by the method. The method is simple and convenient, easy to operate, high in recovery and easy to promote and use.
Owner:NAT ENG RES CENT FOR TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com