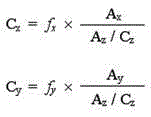Method for simultaneously and quantitatively detecting ligustilide and senkyunolide A
A technology for ligustilide and ligustilide, which is applied in the field of simultaneous quantitative detection of ligustilide and ligustilide A, and can solve the problems of quantitative detection, poor stability of pure products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
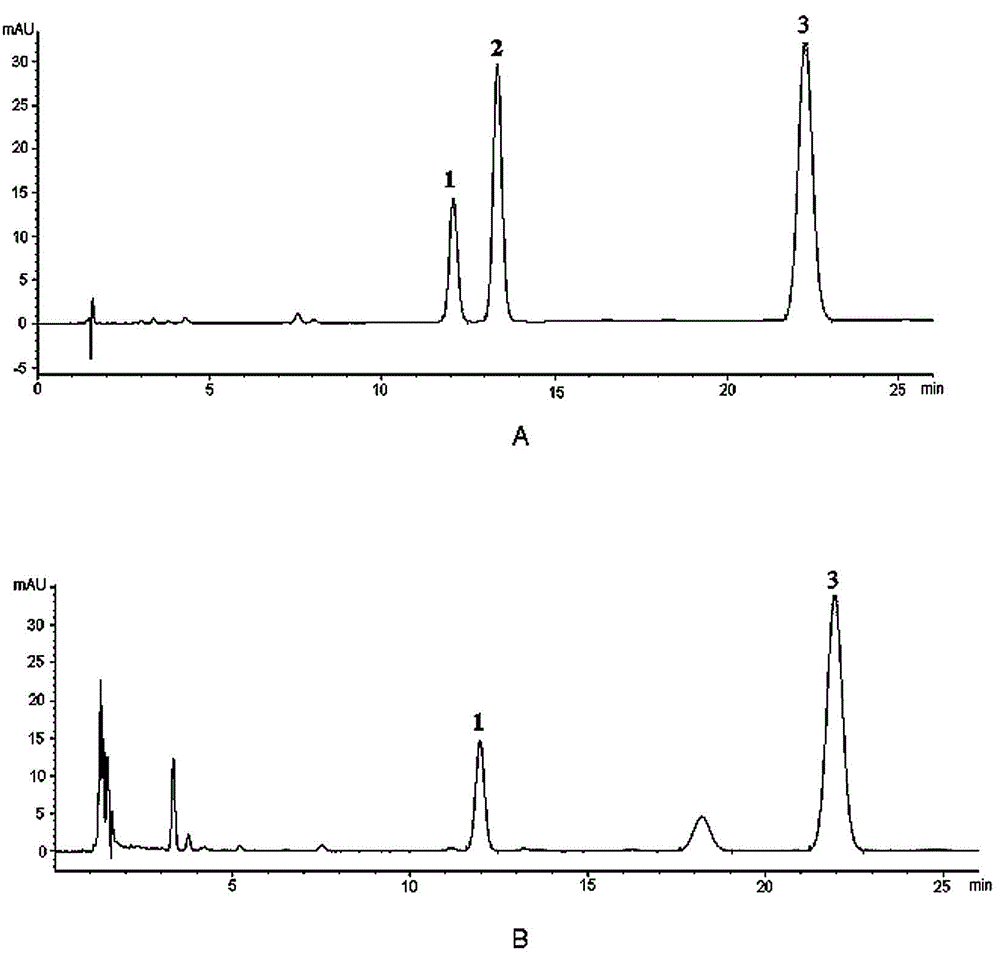
[0028] Quantitative detection of ligustilide and ligustilide A in Chuanxiong:
[0029] According to the high-performance liquid chromatography specified in Appendix VI D of the Chinese Pharmacopoeia 2010 edition.
[0030] 1.1 Chromatographic conditions and system suitability test
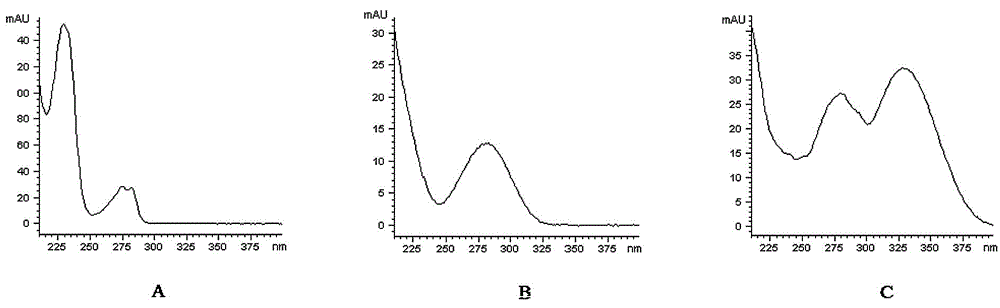
[0031] Column: Agilent Eclipse XDB-C 18 (4.6mm×150mm, 5μm); mobile phase: methanol-water (50 : 50); the flow rate is 1.0ml / min; the column temperature is 35°C, and the detection wavelength is 280nm; the number of theoretical plates should not be less than 3000 based on the peak of ligustilide.
[0032] 1.2 Preparation of solution
[0033]1.2.1 Reference substance solution Accurately weigh 49.85 mg of butylphthalide reference substance, put it in a 25ml measuring bottle, add absolute ethanol to dissolve it and adjust the volume to the mark, and shake well to obtain the butylphthalide reference substance stock solution; precisely weigh Put 22.54mg of ligustilide reference substance into a 25ml b...
Embodiment 2
[0080] Quantitative detection of ligustilide and ligustilide A in Angelica sinensis:
[0081] Determined according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 Edition).
[0082] 2.1 Chromatographic conditions and system suitability test
[0083] Octadecylsilane bonded silica gel as filler; methanol-water (30 :70) is the mobile phase; the flow rate is 1.5ml / min; the column temperature is 40°C, and the detection wavelength is 280nm; the number of theoretical plates should not be less than 3000 based on the ligustilide peak.
[0084] 2.2 Preparation of solution
[0085] The preparation of reference substance solution and need testing solution is the same as in Example 1.
[0086] 2.3 Linear relationship inspection
[0087] Precisely draw 1, 2, 5, 10, 15, and 20 μl of the above-mentioned mixed reference solution, and measure the peak areas of butylphthalide, ligustilide A, and ligustilide respectively according to 1.1 chromatographi...
Embodiment 3
[0106] Quantitative detection of ligustilide and ligustilide A in Chuanxiong:
[0107] Determined according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 Edition).
[0108] 3.1 Chromatographic conditions and system suitability test
[0109] Octadecylsilane bonded silica gel as filler; methanol-water (70 : 30) is the mobile phase; the flow rate is 0.6ml / min; the column temperature is 15°C, and the detection wavelength is 275nm; the number of theoretical plates should not be less than 3000 based on the ligustilide peak.
[0110] 3.2 Preparation of solution
[0111] The preparation of reference substance solution and need testing solution is the same as in Example 1.
[0112] 3.3 Linear relationship inspection
[0113] Precisely draw 1, 2, 5, 10, 15, and 20 μl of the above-mentioned mixed reference solution, and measure the peak areas of butylphthalide, ligustilide A, and ligustilide respectively according to 3.1 chromatographic cond...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com